

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA helicase IV (Escherichia coli (strain K12)) | BDBM50177710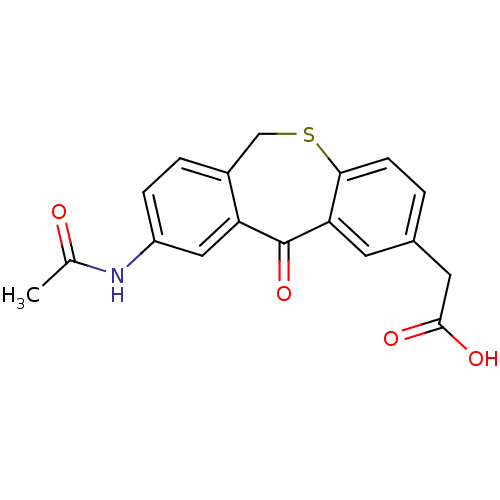 ((3-acetylamino-5-oxo-5,11-dihydro-10-thia-dibenzo[...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATPase activity of Helicase4 from Escherichia coli in presence of ATP (5x Km(ATP)) and dT25 (5x KDNA) | Bioorg Med Chem Lett 16: 923-7 (2006) Article DOI: 10.1016/j.bmcl.2005.10.110 BindingDB Entry DOI: 10.7270/Q2MS3SB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50295258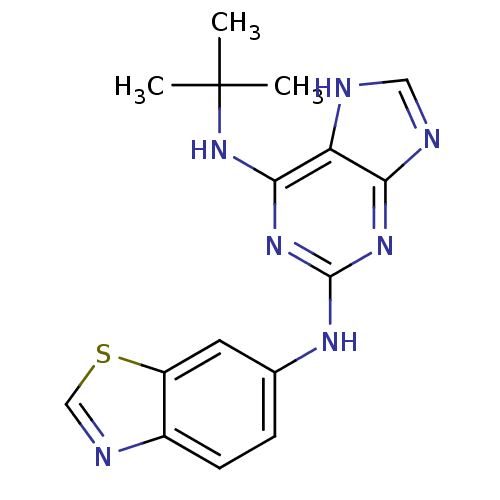 (CHEMBL571444 | N2-(benzo[d]thiazol-6-yl)-N6-tert-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ATPase activity of human topoisomerase 2 alpha | Bioorg Med Chem Lett 19: 4014-7 (2009) Article DOI: 10.1016/j.bmcl.2009.06.034 BindingDB Entry DOI: 10.7270/Q23J3D1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA helicase IV (Escherichia coli (strain K12)) | BDBM50177713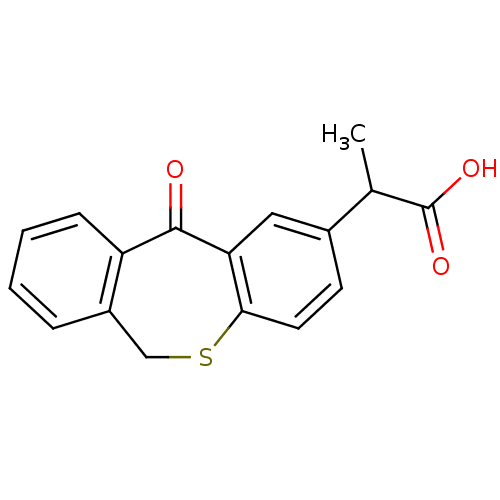 (2-(5-oxo-5,11-dihydro-10-thia-dibenzo[a,d]cyclohep...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATPase activity of Helicase4 from Escherichia coli in presence of ATP (5x Km(ATP)) and dT25 (5x KDNA) | Bioorg Med Chem Lett 16: 923-7 (2006) Article DOI: 10.1016/j.bmcl.2005.10.110 BindingDB Entry DOI: 10.7270/Q2MS3SB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA helicase IV (Escherichia coli (strain K12)) | BDBM50177711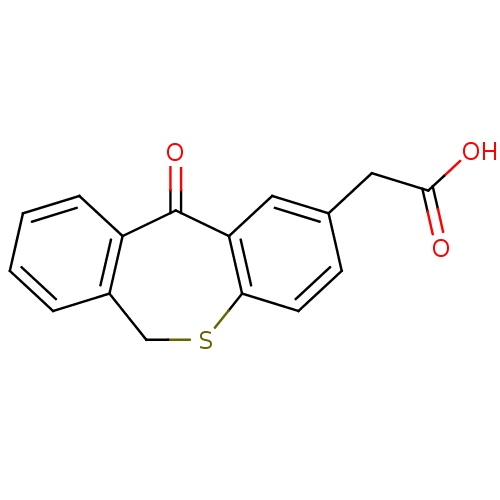 ((5-oxo-5,11-dihydro-10-thia-dibenzo[a,d]cyclohepte...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATPase activity of Helicase4 from Escherichia coli in presence of ATP (5x Km(ATP)) and dT25 (5x KDNA) | Bioorg Med Chem Lett 16: 923-7 (2006) Article DOI: 10.1016/j.bmcl.2005.10.110 BindingDB Entry DOI: 10.7270/Q2MS3SB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA helicase IV (Escherichia coli (strain K12)) | BDBM50177711 ((5-oxo-5,11-dihydro-10-thia-dibenzo[a,d]cyclohepte...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATPase activity of Tyr473Ala mutated Helicase4 in presence of ATP (5x Km(ATP)) and dT25 (5x KDNA) | Bioorg Med Chem Lett 16: 923-7 (2006) Article DOI: 10.1016/j.bmcl.2005.10.110 BindingDB Entry DOI: 10.7270/Q2MS3SB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA helicase IV (Escherichia coli (strain K12)) | BDBM50177712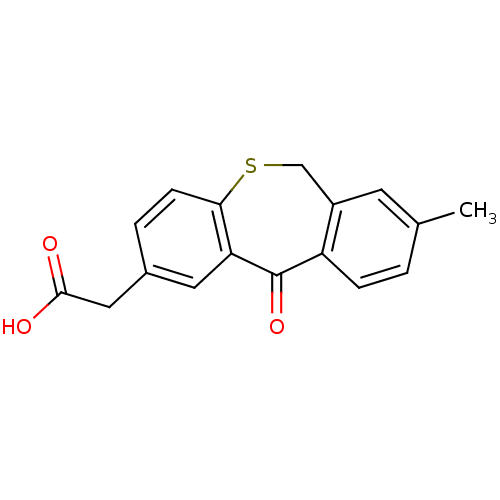 ((2-methyl-5-oxo-5,11-dihydro-10-thia-dibenzo[a,d]c...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATPase activity of Helicase4 from Escherichia coli in presence of ATP (5x Km(ATP)) and dT25 (5x KDNA) | Bioorg Med Chem Lett 16: 923-7 (2006) Article DOI: 10.1016/j.bmcl.2005.10.110 BindingDB Entry DOI: 10.7270/Q2MS3SB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50295259 (CHEMBL549357 | N2-(benzo[d]thiazol-6-yl)-N6-cycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of ATPase activity of human topoisomerase 2 alpha | Bioorg Med Chem Lett 19: 4014-7 (2009) Article DOI: 10.1016/j.bmcl.2009.06.034 BindingDB Entry DOI: 10.7270/Q23J3D1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Mdm4 (Homo sapiens (Human)) | BDBM50382115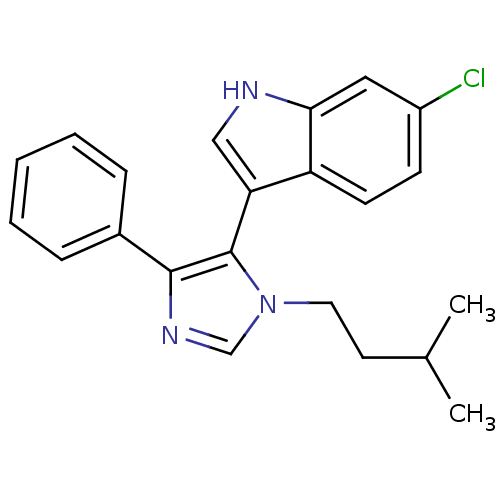 (CHEMBL2024321) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of p53 derived peptide binding to MDM4 using fluorescent dye Cy5 by TR-FRET assay | Bioorg Med Chem Lett 22: 3498-502 (2012) Article DOI: 10.1016/j.bmcl.2012.03.083 BindingDB Entry DOI: 10.7270/Q2D21ZM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Mdm4 (Homo sapiens (Human)) | BDBM50382117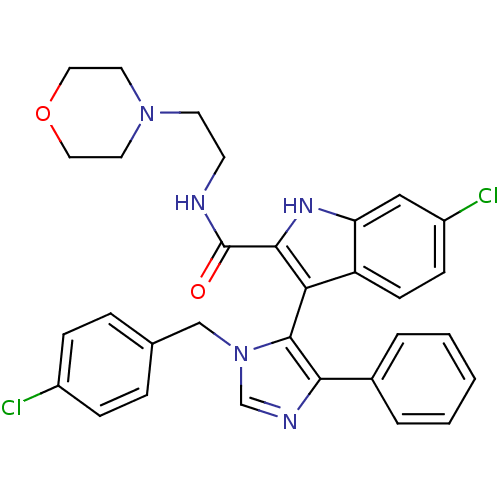 (CHEMBL2024323) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of p53 derived peptide binding to MDM4 using fluorescent dye Cy5 by TR-FRET assay | Bioorg Med Chem Lett 22: 3498-502 (2012) Article DOI: 10.1016/j.bmcl.2012.03.083 BindingDB Entry DOI: 10.7270/Q2D21ZM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Mdm4 (Homo sapiens (Human)) | BDBM50382116 (CHEMBL2024322) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of p53 derived peptide binding to MDM4 using fluorescent dye Cy5 by TR-FRET assay | Bioorg Med Chem Lett 22: 3498-502 (2012) Article DOI: 10.1016/j.bmcl.2012.03.083 BindingDB Entry DOI: 10.7270/Q2D21ZM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Mdm4 (Homo sapiens (Human)) | BDBM50382114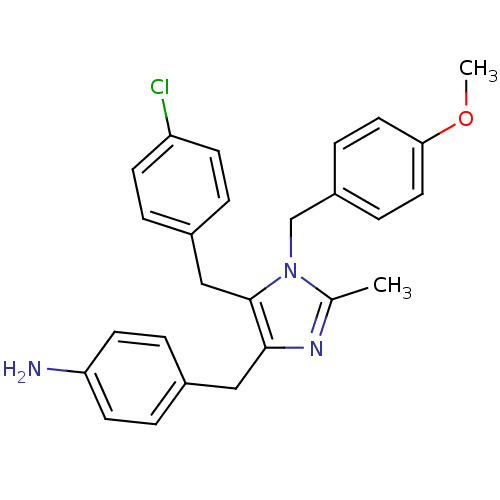 (CHEMBL2024320) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of p53 derived peptide binding to MDM4 using fluorescent dye Cy5 by TR-FRET assay | Bioorg Med Chem Lett 22: 3498-502 (2012) Article DOI: 10.1016/j.bmcl.2012.03.083 BindingDB Entry DOI: 10.7270/Q2D21ZM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||