 Found 103 hits with Last Name = 'chang' and Initial = 'hh'
Found 103 hits with Last Name = 'chang' and Initial = 'hh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584094
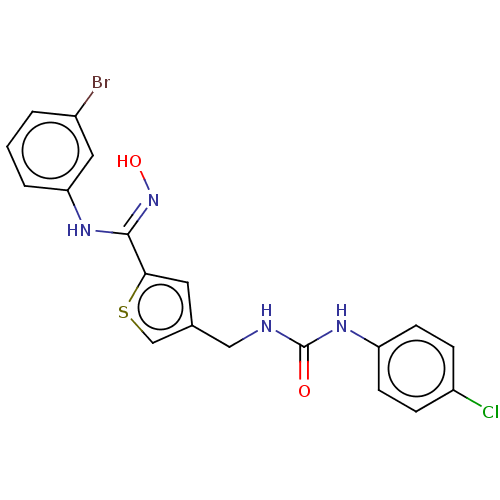
(CHEMBL5077002)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2ccc(Cl)cc2)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584075
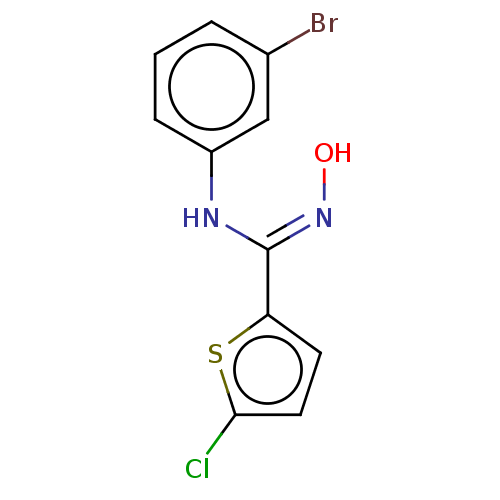
(CHEMBL5076745) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584093

(CHEMBL5087302)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2ccc(F)cc2)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584087

(CHEMBL5093619)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2ccc(cc2)C#N)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584085
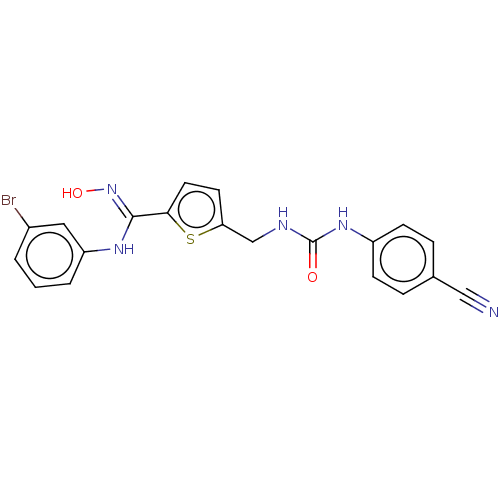
(CHEMBL5074542)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2ccc(cc2)C#N)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584073

(CHEMBL5081219) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584074
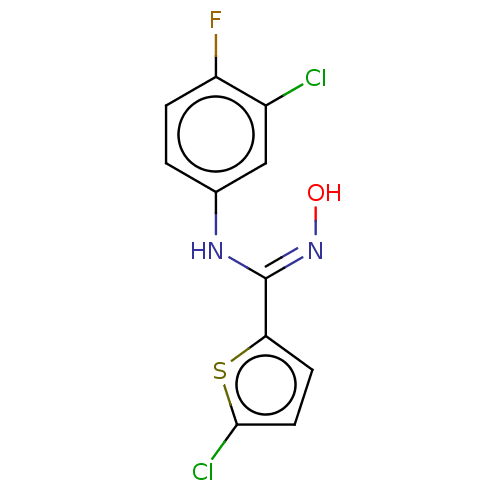
(CHEMBL5075428) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584076
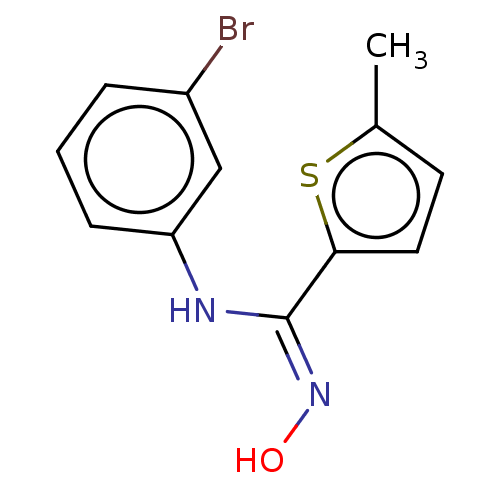
(CHEMBL5083059) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584086
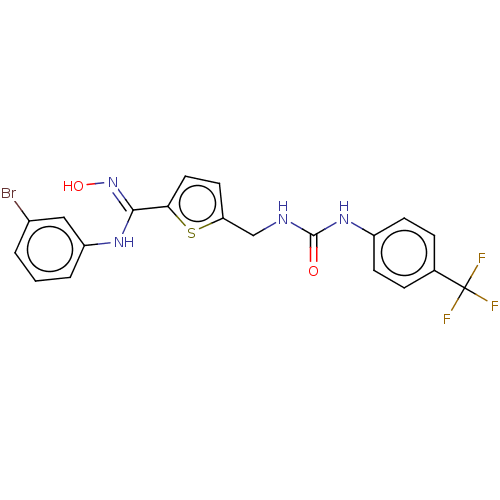
(CHEMBL5094947)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2ccc(cc2)C(F)(F)F)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584084
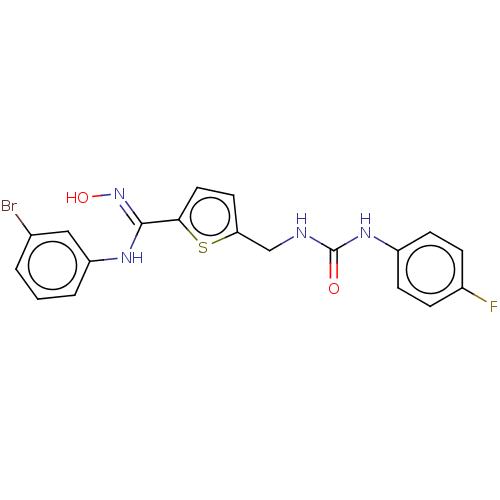
(CHEMBL5080101)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2ccc(F)cc2)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584077
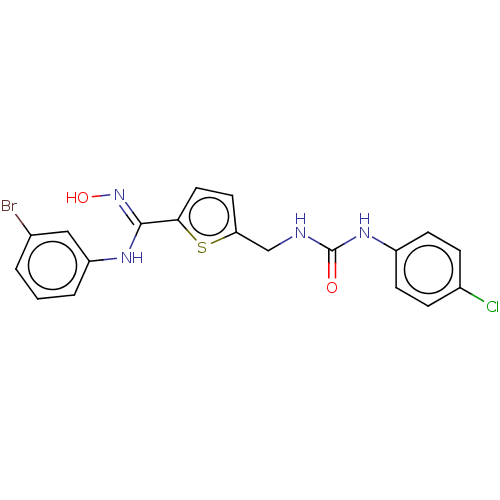
(CHEMBL5091205)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2ccc(Cl)cc2)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584090

(CHEMBL5094353)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2cccc(F)c2)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584080

(CHEMBL5078610)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2cccc(F)c2)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584078

(CHEMBL5093621)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2cccc(Cl)c2)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584081

(CHEMBL5070676)Show SMILES Cc1cccc(NC(=O)NCc2ccc(s2)C(\Nc2cccc(Br)c2)=N\O)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584089

(CHEMBL5078852)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2cccc(c2)C#N)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584079
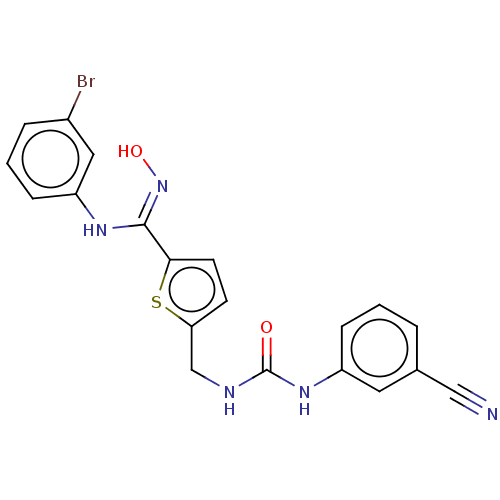
(CHEMBL5080294)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2cccc(c2)C#N)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584082

(CHEMBL5094486)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1ccc(CNC(=O)Nc2ccccc2F)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584091
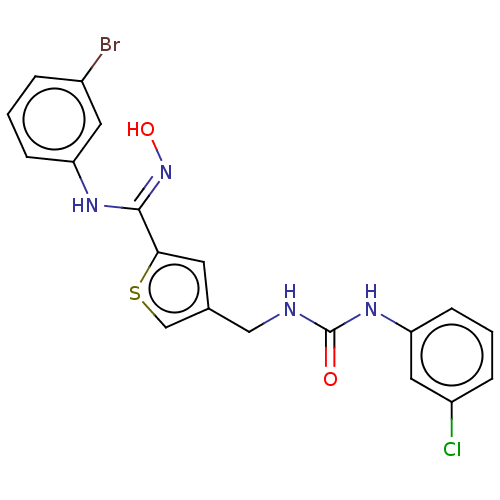
(CHEMBL5087813)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2cccc(Cl)c2)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584088
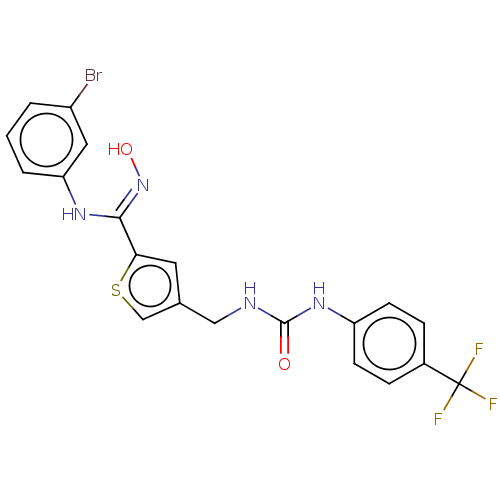
(CHEMBL5082408)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2ccc(cc2)C(F)(F)F)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584072
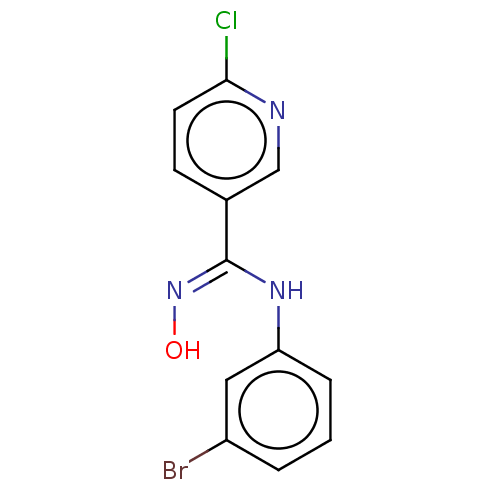
(CHEMBL5071322) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584092
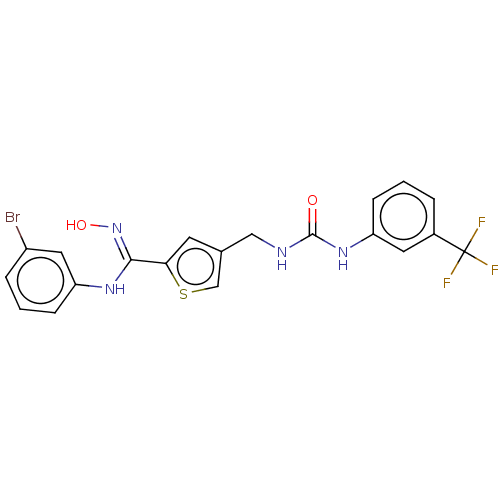
(CHEMBL5089919)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2cccc(c2)C(F)(F)F)cs1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50580117

(CHEMBL5076176)Show SMILES Cn1c(Nc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)nc2n(C)c(=O)n(Cc3cc4c(Cl)cccc4[nH]3)c(=O)c12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal His-tagged human MTHFD2 (36 to 350 residues) expressed in insect cells using tetrahydrofolate as substrate preincubated for ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00663
BindingDB Entry DOI: 10.7270/Q2B56PKM |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584083
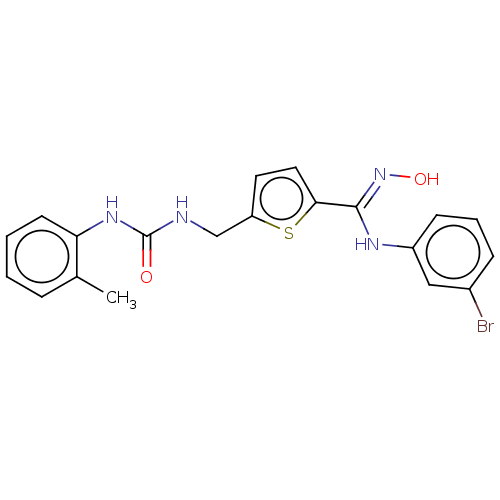
(CHEMBL5078069)Show SMILES Cc1ccccc1NC(=O)NCc1ccc(s1)C(\Nc1cccc(Br)c1)=N\O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50382181

(CHEMBL1894768 | cid_684847)Show InChI InChI=1S/C16H12N2O2S/c1-2-4-11(5-3-1)13-9-21-16(18-13)17-12-6-7-14-15(8-12)20-10-19-14/h1-9H,10H2,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50580113
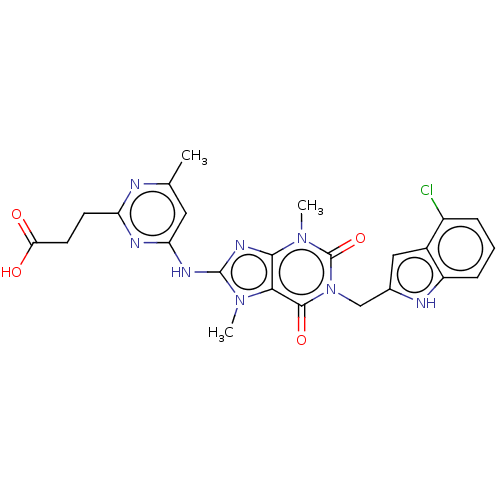
(CHEMBL5090659)Show SMILES Cc1cc(Nc2nc3n(C)c(=O)n(Cc4cc5c(Cl)cccc5[nH]4)c(=O)c3n2C)nc(CCC(O)=O)n1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal His-tagged human MTHFD2 (36 to 350 residues) expressed in insect cells using tetrahydrofolate as substrate preincubated for ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00663
BindingDB Entry DOI: 10.7270/Q2B56PKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584071
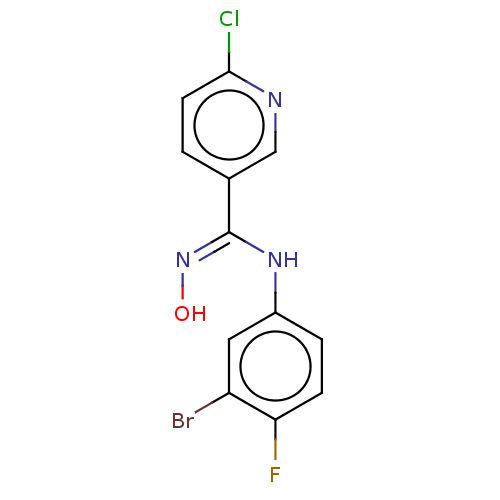
(CHEMBL5074528) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 742 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50580121
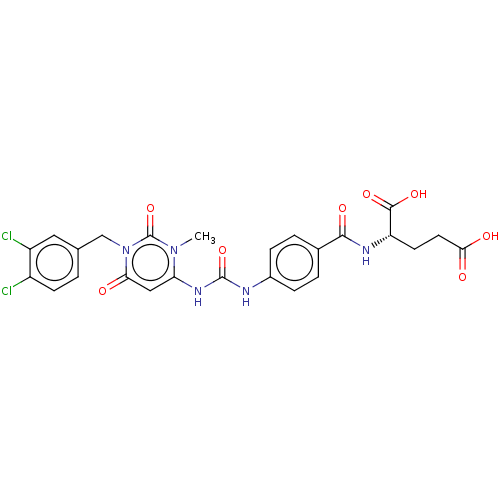
(CHEMBL5085594)Show SMILES Cn1c(NC(=O)Nc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc(=O)n(Cc2ccc(Cl)c(Cl)c2)c1=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal His-tagged human MTHFD2 (36 to 350 residues) expressed in insect cells using tetrahydrofolate as substrate preincubated for ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00663
BindingDB Entry DOI: 10.7270/Q2B56PKM |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50580116

(CHEMBL5093943)Show SMILES Cn1c(Nc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)nc2n(C)c(=O)n(Cc3ccc(Cl)c(Cl)c3)c(=O)c12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal His-tagged human MTHFD2 (36 to 350 residues) expressed in insect cells using tetrahydrofolate as substrate preincubated for ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00663
BindingDB Entry DOI: 10.7270/Q2B56PKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50382182
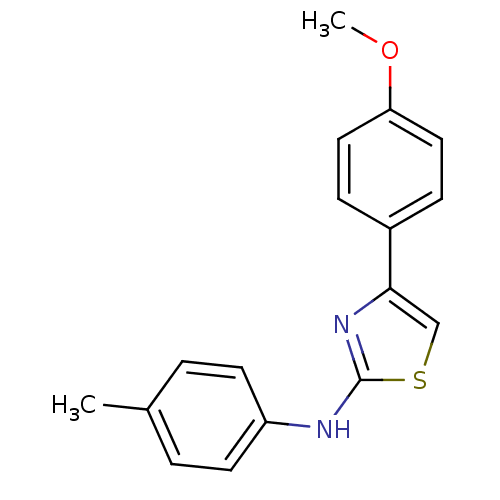
(CHEMBL2023858)Show InChI InChI=1S/C17H16N2OS/c1-12-3-7-14(8-4-12)18-17-19-16(11-21-17)13-5-9-15(20-2)10-6-13/h3-11H,1-2H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50382192
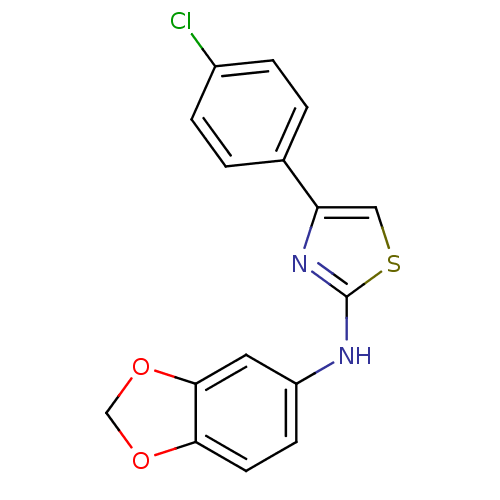
(CHEMBL2023847)Show InChI InChI=1S/C16H11ClN2O2S/c17-11-3-1-10(2-4-11)13-8-22-16(19-13)18-12-5-6-14-15(7-12)21-9-20-14/h1-8H,9H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584069
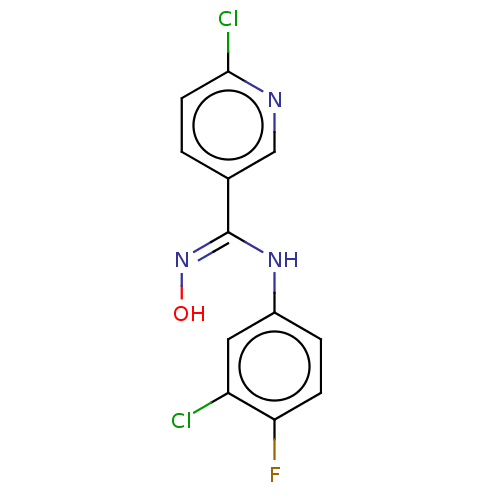
(CHEMBL5074663) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50382177
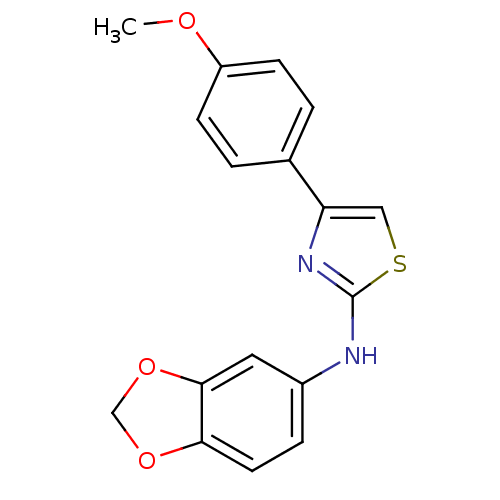
(CHEMBL2023862)Show InChI InChI=1S/C17H14N2O3S/c1-20-13-5-2-11(3-6-13)14-9-23-17(19-14)18-12-4-7-15-16(8-12)22-10-21-15/h2-9H,10H2,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
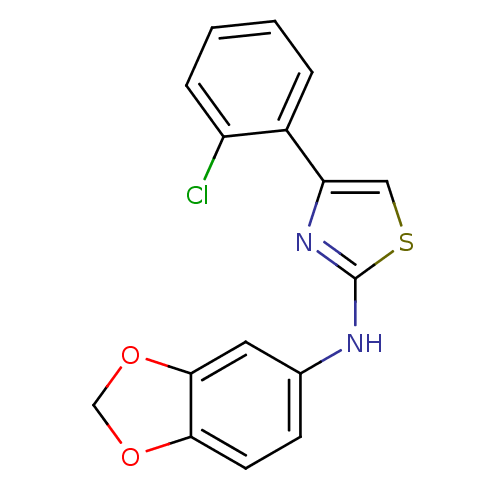
(Homo sapiens (Human)) | BDBM50382180
(CHEMBL2023859)Show InChI InChI=1S/C16H11ClN2O2S/c17-12-4-2-1-3-11(12)13-8-22-16(19-13)18-10-5-6-14-15(7-10)21-9-20-14/h1-8H,9H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50580115

(CHEMBL5084848)Show SMILES Cn1c(Nc2ccc(cc2)C(=O)N[C@@H](CC(O)=O)C(O)=O)nc2n(C)c(=O)n(Cc3ccc(Cl)c(Cl)c3)c(=O)c12 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal His-tagged human MTHFD2 (36 to 350 residues) expressed in insect cells using tetrahydrofolate as substrate preincubated for ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00663
BindingDB Entry DOI: 10.7270/Q2B56PKM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50382178

(CHEMBL2023861)Show InChI InChI=1S/C17H14N2O3S/c1-20-13-4-2-3-11(7-13)14-9-23-17(19-14)18-12-5-6-15-16(8-12)22-10-21-15/h2-9H,10H2,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50382179

(CHEMBL2023860)Show InChI InChI=1S/C17H14N2O3S/c1-20-14-5-3-2-4-12(14)13-9-23-17(19-13)18-11-6-7-15-16(8-11)22-10-21-15/h2-9H,10H2,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50382190

(CHEMBL2023850)Show InChI InChI=1S/C16H13ClN2OS/c1-19(13-6-8-14(20)9-7-13)16-18-15(10-21-16)11-2-4-12(17)5-3-11/h2-10,20H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
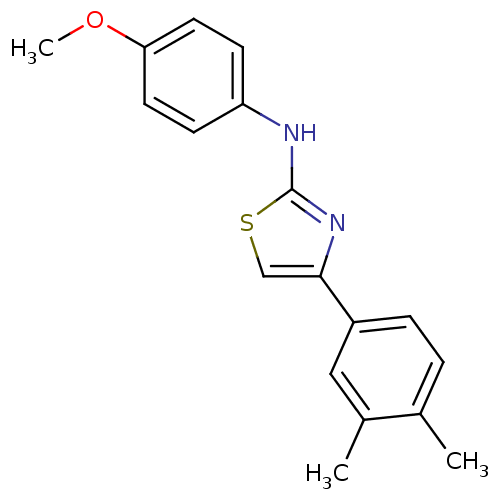
(Homo sapiens (Human)) | BDBM50382184
(CHEMBL2023857)Show InChI InChI=1S/C18H18N2OS/c1-12-4-5-14(10-13(12)2)17-11-22-18(20-17)19-15-6-8-16(21-3)9-7-15/h4-11H,1-3H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50584072

(CHEMBL5071322) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
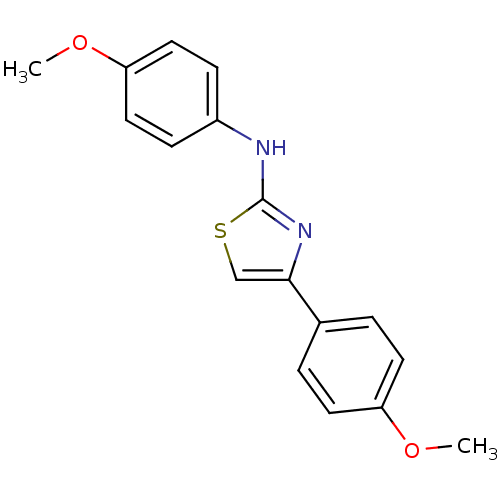
(Homo sapiens (Human)) | BDBM50382183
(CHEMBL1622194)Show InChI InChI=1S/C17H16N2O2S/c1-20-14-7-3-12(4-8-14)16-11-22-17(19-16)18-13-5-9-15(21-2)10-6-13/h3-11H,1-2H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50382191
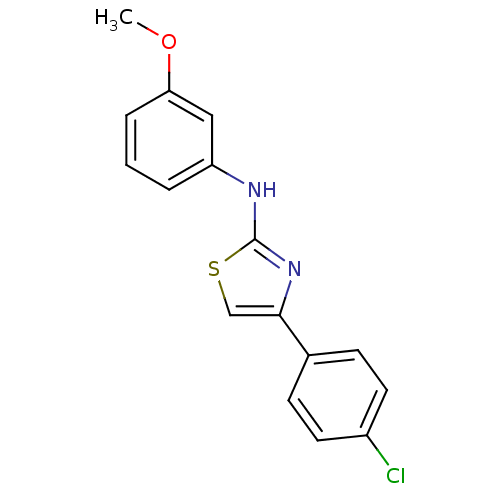
(CHEMBL2023848)Show InChI InChI=1S/C16H13ClN2OS/c1-20-14-4-2-3-13(9-14)18-16-19-15(10-21-16)11-5-7-12(17)8-6-11/h2-10H,1H3,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50584070
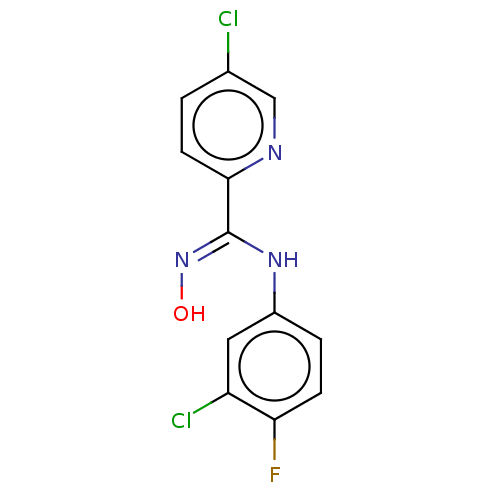
(CHEMBL5080559) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate by methylene blue reagent base... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50584073

(CHEMBL5081219) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50584075

(CHEMBL5076745) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50584093

(CHEMBL5087302)Show SMILES O\N=C(/Nc1cccc(Br)c1)c1cc(CNC(=O)Nc2ccc(F)cc2)cs1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human TDO2 protein assessed as reduction in N-formylkynurenine formation using L-tryptophan as substrate incubated for 1 hr... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114043
BindingDB Entry DOI: 10.7270/Q2SX6J39 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM50580112
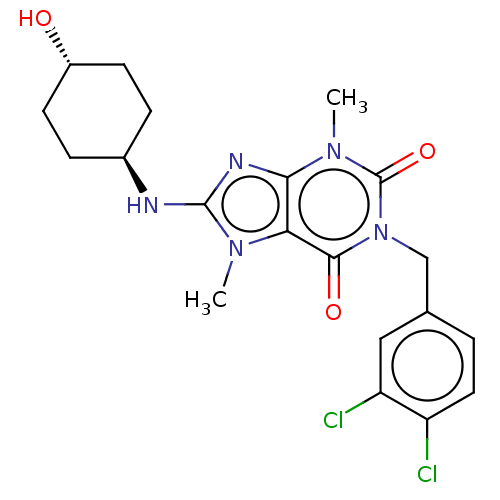
(CHEMBL5077748)Show SMILES Cn1c(N[C@H]2CC[C@H](O)CC2)nc2n(C)c(=O)n(Cc3ccc(Cl)c(Cl)c3)c(=O)c12 |r,wU:4.3,wD:7.7,(14.78,-9.97,;14.31,-8.5,;15.22,-7.26,;16.76,-7.27,;17.54,-5.94,;16.78,-4.61,;17.56,-3.28,;19.1,-3.29,;19.89,-1.97,;19.86,-4.63,;19.08,-5.95,;14.32,-6,;12.85,-6.47,;11.52,-5.7,;11.52,-4.16,;10.18,-6.47,;8.84,-5.7,;10.18,-8.01,;8.85,-8.78,;7.52,-8.01,;7.51,-6.46,;6.18,-5.7,;4.85,-6.47,;3.52,-5.7,;4.85,-8.01,;3.51,-8.78,;6.18,-8.78,;11.52,-8.78,;11.52,-10.32,;12.84,-8.02,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal His-tagged human MTHFD2 (36 to 350 residues) expressed in insect cells using tetrahydrofolate as substrate preincubated for ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00663
BindingDB Entry DOI: 10.7270/Q2B56PKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50382175
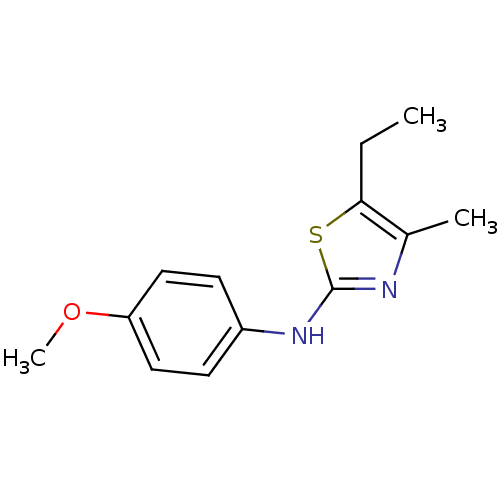
(CHEMBL2023948)Show InChI InChI=1S/C13H16N2OS/c1-4-12-9(2)14-13(17-12)15-10-5-7-11(16-3)8-6-10/h5-8H,4H2,1-3H3,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50382189
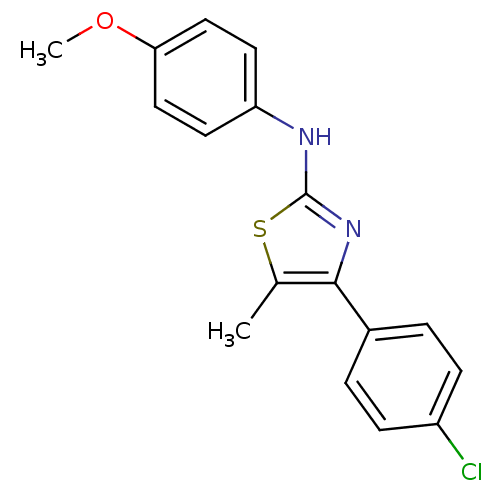
(CHEMBL2023851)Show InChI InChI=1S/C17H15ClN2OS/c1-11-16(12-3-5-13(18)6-4-12)20-17(22-11)19-14-7-9-15(21-2)10-8-14/h3-10H,1-2H3,(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of COX2 by fluorescence assay |
Bioorg Med Chem Lett 22: 3567-70 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.013
BindingDB Entry DOI: 10.7270/Q2RB75MP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data