 Found 157 hits with Last Name = 'chao' and Initial = 'mw'
Found 157 hits with Last Name = 'chao' and Initial = 'mw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM24346
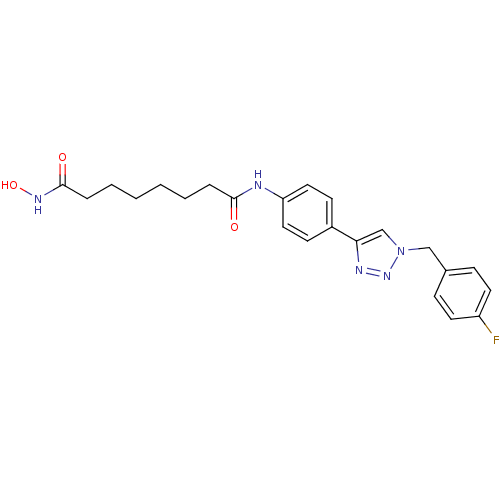
(N-(4-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(cc1)-c1cn(Cc2ccc(F)cc2)nn1 Show InChI InChI=1S/C23H26FN5O3/c24-19-11-7-17(8-12-19)15-29-16-21(26-28-29)18-9-13-20(14-10-18)25-22(30)5-3-1-2-4-6-23(31)27-32/h7-14,16,32H,1-6,15H2,(H,25,30)(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111725
BindingDB Entry DOI: 10.7270/Q2V1286G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50551233

(CHEMBL4790152)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM110036
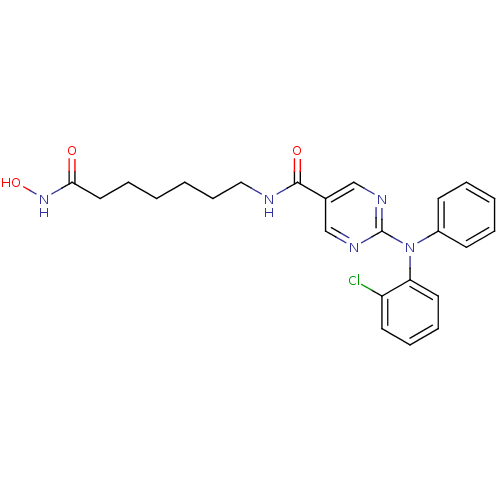
(US8609678, 2-((2-chlorophenyl)(phenyl)amino)-N-(7-...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1Cl Show InChI InChI=1S/C24H26ClN5O3/c25-20-12-7-8-13-21(20)30(19-10-4-3-5-11-19)24-27-16-18(17-28-24)23(32)26-15-9-2-1-6-14-22(31)29-33/h3-5,7-8,10-13,16-17,33H,1-2,6,9,14-15H2,(H,26,32)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) assessed as release of 7-amino-4-methoxy-coumarin using FTS as substrate preincubated for 10 mins followed by su... |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111725
BindingDB Entry DOI: 10.7270/Q2V1286G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50519393
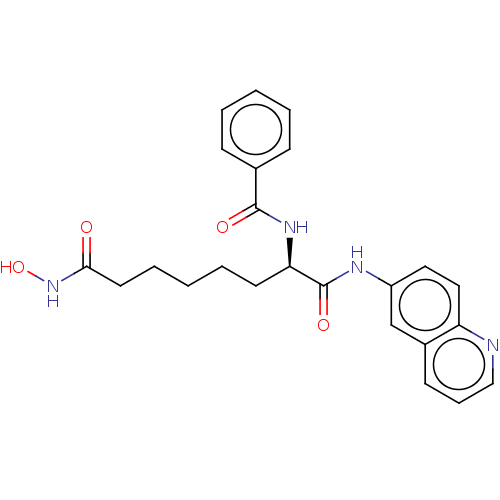
(CHEMBL4564170)Show SMILES ONC(=O)CCCCC[C@@H](NC(=O)c1ccccc1)C(=O)Nc1ccc2ncccc2c1 |r| Show InChI InChI=1S/C24H26N4O4/c29-22(28-32)12-6-2-5-11-21(27-23(30)17-8-3-1-4-9-17)24(31)26-19-13-14-20-18(16-19)10-7-15-25-20/h1,3-4,7-10,13-16,21,32H,2,5-6,11-12H2,(H,26,31)(H,27,30)(H,28,29)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111725
BindingDB Entry DOI: 10.7270/Q2V1286G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG-His-tagged HDAC1 (1 to 482 residues) expressed in Sf21 cells using RHK-K(Ac)-AMC as substrate incubat... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50551231

(CHEMBL4798549)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC3 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50519399

(CHEMBL4468150)Show SMILES ONC(=O)CCCCC[C@@H](NC(=O)c1ccccc1)C(=O)Nc1ccccc1 |r| Show InChI InChI=1S/C21H25N3O4/c25-19(24-28)15-9-3-8-14-18(21(27)22-17-12-6-2-7-13-17)23-20(26)16-10-4-1-5-11-16/h1-2,4-7,10-13,18,28H,3,8-9,14-15H2,(H,22,27)(H,23,26)(H,24,25)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111725
BindingDB Entry DOI: 10.7270/Q2V1286G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50551227

(CHEMBL4782478)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NCc4ccc(cc4)C(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145168
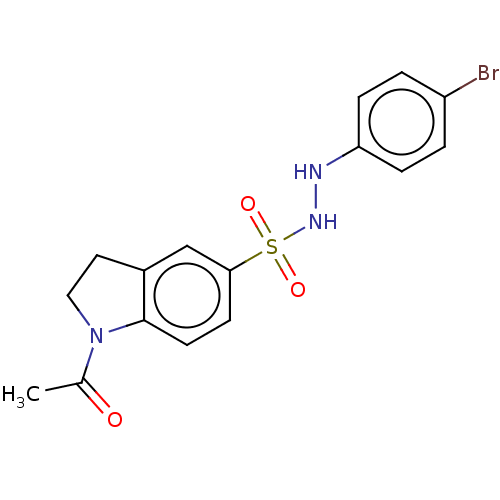
(CHEMBL3764319)Show SMILES CC(=O)N1CCc2cc(ccc12)S(=O)(=O)NNc1ccc(Br)cc1 Show InChI InChI=1S/C16H16BrN3O3S/c1-11(21)20-9-8-12-10-15(6-7-16(12)20)24(22,23)19-18-14-4-2-13(17)3-5-14/h2-7,10,18-19H,8-9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145157
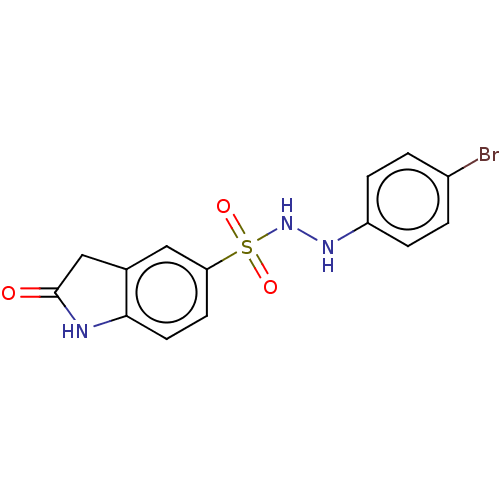
(CHEMBL3763166)Show InChI InChI=1S/C14H12BrN3O3S/c15-10-1-3-11(4-2-10)17-18-22(20,21)12-5-6-13-9(7-12)8-14(19)16-13/h1-7,17-18H,8H2,(H,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50519391

(CHEMBL4514330)Show SMILES CC(C)c1cc(C(=O)N(C)c2ccc(NC(=O)CCCCCCC(=O)NO)cc2)c(O)cc1O Show InChI InChI=1S/C25H33N3O6/c1-16(2)19-14-20(22(30)15-21(19)29)25(33)28(3)18-12-10-17(11-13-18)26-23(31)8-6-4-5-7-9-24(32)27-34/h10-16,29-30,34H,4-9H2,1-3H3,(H,26,31)(H,27,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using RHK-K(Ac)-AMC a... |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111725
BindingDB Entry DOI: 10.7270/Q2V1286G |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50551231

(CHEMBL4798549)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HSP90a (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50551232
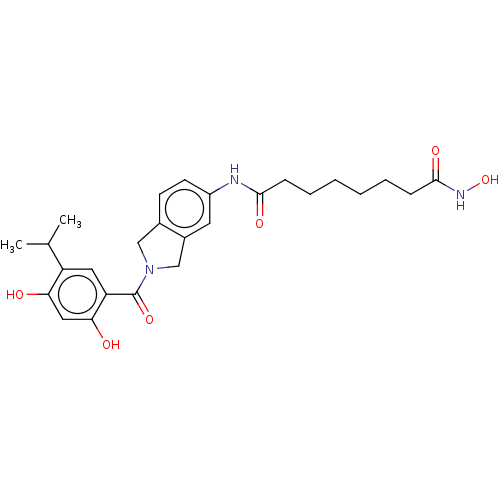
(CHEMBL4790559)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HSP90a (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50551234

(CHEMBL4789155)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HSP90a (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145166
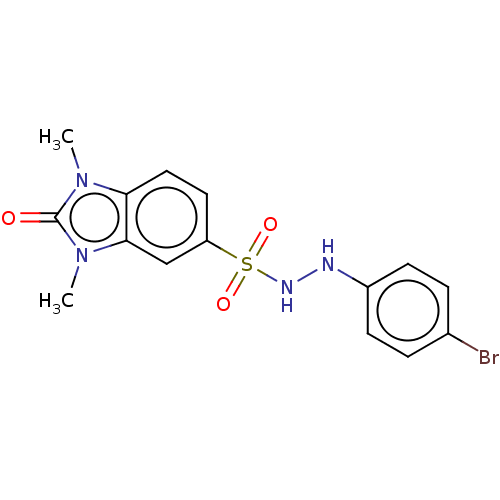
(CHEMBL3763493)Show SMILES Cn1c2ccc(cc2n(C)c1=O)S(=O)(=O)NNc1ccc(Br)cc1 Show InChI InChI=1S/C15H15BrN4O3S/c1-19-13-8-7-12(9-14(13)20(2)15(19)21)24(22,23)18-17-11-5-3-10(16)4-6-11/h3-9,17-18H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111725
BindingDB Entry DOI: 10.7270/Q2V1286G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145159
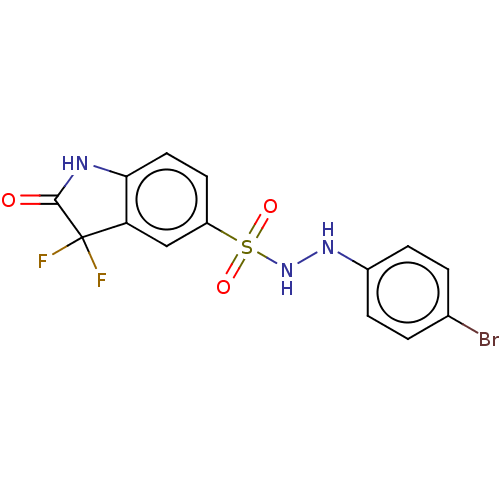
(CHEMBL3765205)Show SMILES FC1(F)C(=O)Nc2ccc(cc12)S(=O)(=O)NNc1ccc(Br)cc1 Show InChI InChI=1S/C14H10BrF2N3O3S/c15-8-1-3-9(4-2-8)19-20-24(22,23)10-5-6-12-11(7-10)14(16,17)13(21)18-12/h1-7,19-20H,(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145164
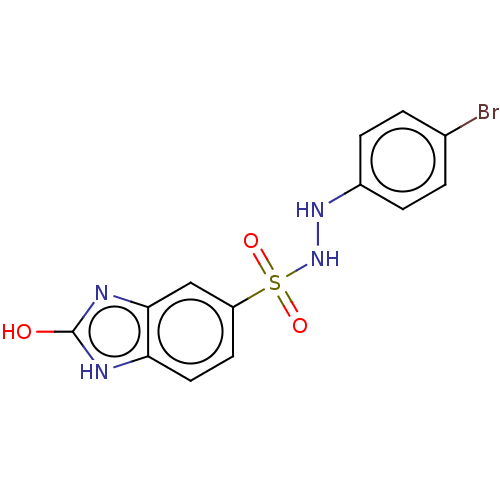
(CHEMBL3765698)Show InChI InChI=1S/C13H11BrN4O3S/c14-8-1-3-9(4-2-8)17-18-22(20,21)10-5-6-11-12(7-10)16-13(19)15-11/h1-7,17-18H,(H2,15,16,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50551227

(CHEMBL4782478)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NCc4ccc(cc4)C(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HSP90a (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM20800
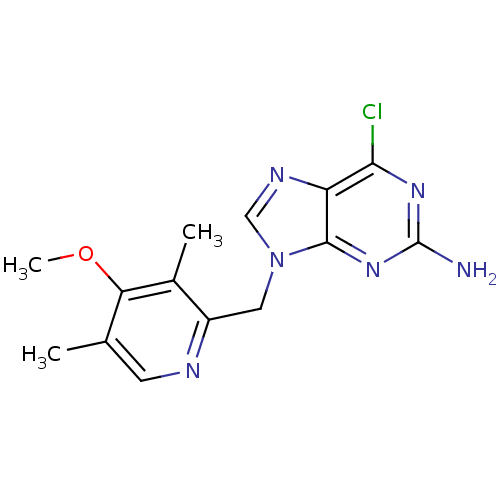
(2-amino-6-halopurine analogue, 20 | 6-chloro-9-[(4...)Show InChI InChI=1S/C14H15ClN6O/c1-7-4-17-9(8(2)11(7)22-3)5-21-6-18-10-12(15)19-14(16)20-13(10)21/h4,6H,5H2,1-3H3,(H2,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HSP90a (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145169

(CHEMBL3763974)Show InChI InChI=1S/C16H16BrN3O3S/c17-12-3-5-13(6-4-12)18-19-24(22,23)15-9-7-14(8-10-15)20-11-1-2-16(20)21/h3-10,18-19H,1-2,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50551229

(CHEMBL4740842)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145160
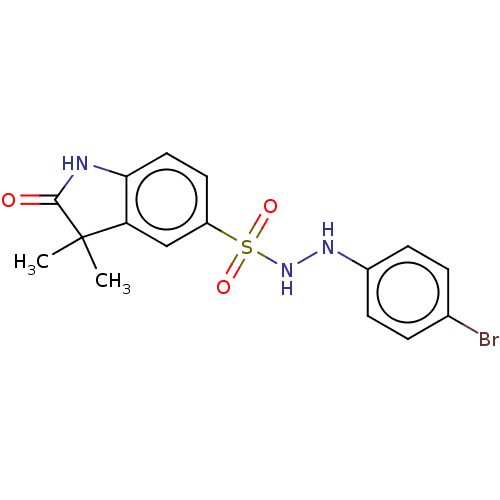
(CHEMBL3764846)Show SMILES CC1(C)C(=O)Nc2ccc(cc12)S(=O)(=O)NNc1ccc(Br)cc1 Show InChI InChI=1S/C16H16BrN3O3S/c1-16(2)13-9-12(7-8-14(13)18-15(16)21)24(22,23)20-19-11-5-3-10(17)4-6-11/h3-9,19-20H,1-2H3,(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
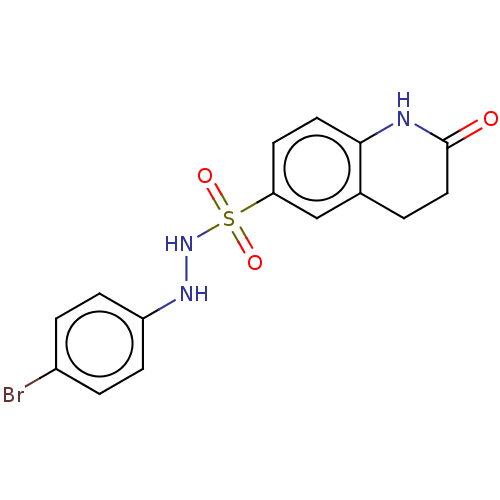
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145161
(CHEMBL3765545)Show InChI InChI=1S/C15H14BrN3O3S/c16-11-2-4-12(5-3-11)18-19-23(21,22)13-6-7-14-10(9-13)1-8-15(20)17-14/h2-7,9,18-19H,1,8H2,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145158
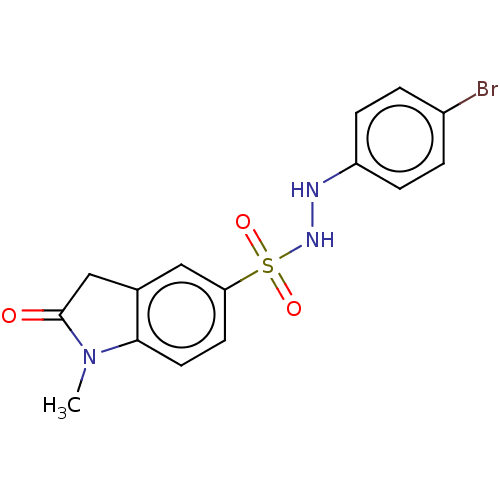
(CHEMBL3764144)Show SMILES CN1C(=O)Cc2cc(ccc12)S(=O)(=O)NNc1ccc(Br)cc1 Show InChI InChI=1S/C15H14BrN3O3S/c1-19-14-7-6-13(8-10(14)9-15(19)20)23(21,22)18-17-12-4-2-11(16)3-5-12/h2-8,17-18H,9H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145170
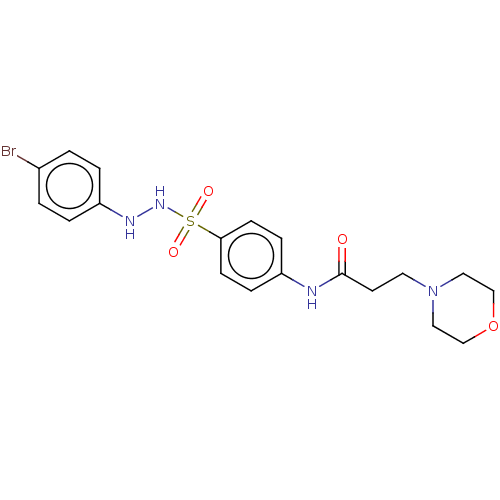
(CHEMBL3765405)Show SMILES Brc1ccc(NNS(=O)(=O)c2ccc(NC(=O)CCN3CCOCC3)cc2)cc1 Show InChI InChI=1S/C19H23BrN4O4S/c20-15-1-3-17(4-2-15)22-23-29(26,27)18-7-5-16(6-8-18)21-19(25)9-10-24-11-13-28-14-12-24/h1-8,22-23H,9-14H2,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145162
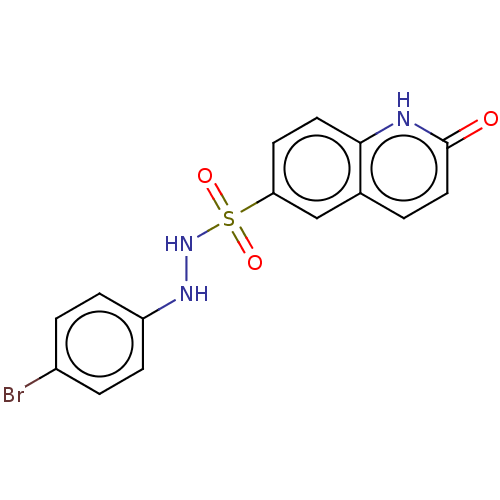
(CHEMBL3764508)Show SMILES Brc1ccc(NNS(=O)(=O)c2ccc3[nH]c(=O)ccc3c2)cc1 Show InChI InChI=1S/C15H12BrN3O3S/c16-11-2-4-12(5-3-11)18-19-23(21,22)13-6-7-14-10(9-13)1-8-15(20)17-14/h1-9,18-19H,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24346

(N-(4-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...)Show SMILES ONC(=O)CCCCCCC(=O)Nc1ccc(cc1)-c1cn(Cc2ccc(F)cc2)nn1 Show InChI InChI=1S/C23H26FN5O3/c24-19-11-7-17(8-12-19)15-29-16-21(26-28-29)18-9-13-20(14-10-18)25-22(30)5-3-1-2-4-6-23(31)27-32/h7-14,16,32H,1-6,15H2,(H,25,30)(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111725
BindingDB Entry DOI: 10.7270/Q2V1286G |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using fluorescence substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50551235
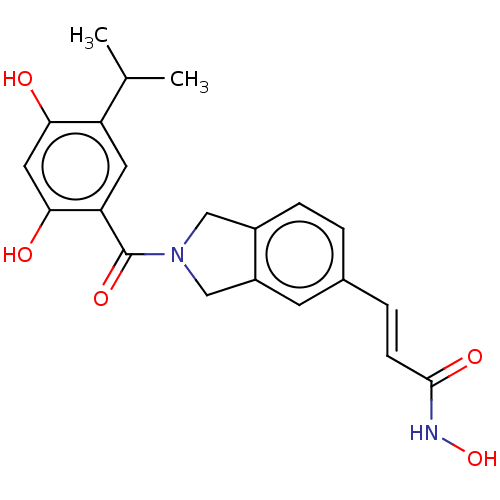
(CHEMBL4747757)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(\C=C\C(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HSP90a (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50551230

(CHEMBL4741850)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HSP90a (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145163
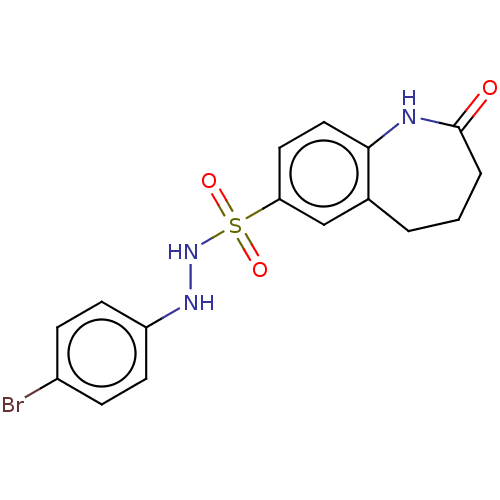
(CHEMBL3763962)Show InChI InChI=1S/C16H16BrN3O3S/c17-12-4-6-13(7-5-12)19-20-24(22,23)14-8-9-15-11(10-14)2-1-3-16(21)18-15/h4-10,19-20H,1-3H2,(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50145165
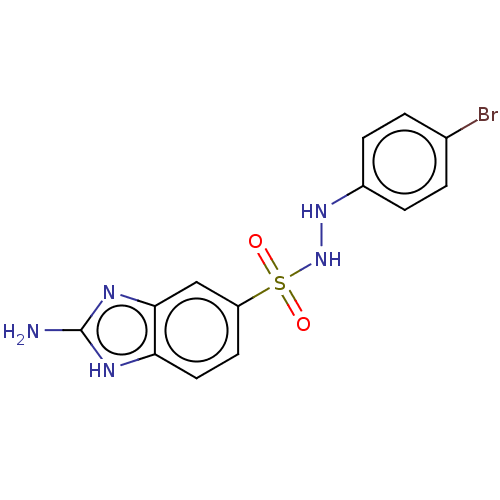
(CHEMBL3765220)Show InChI InChI=1S/C13H12BrN5O2S/c14-8-1-3-9(4-2-8)18-19-22(20,21)10-5-6-11-12(7-10)17-13(15)16-11/h1-7,18-19H,(H3,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of human Indoleamine 2,3-dioxygenase using L-tryptophan as substrate by emission fluorescence analysis |
J Med Chem 59: 419-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01640
BindingDB Entry DOI: 10.7270/Q2FF3V69 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50551228

(CHEMBL4749731)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HSP90a (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50551229

(CHEMBL4740842)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HSP90a (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human K562 nuclear extract |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111725
BindingDB Entry DOI: 10.7270/Q2V1286G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50551233

(CHEMBL4790152)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using fluorescence substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50551232

(CHEMBL4790559)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50551235

(CHEMBL4747757)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(\C=C\C(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50519398
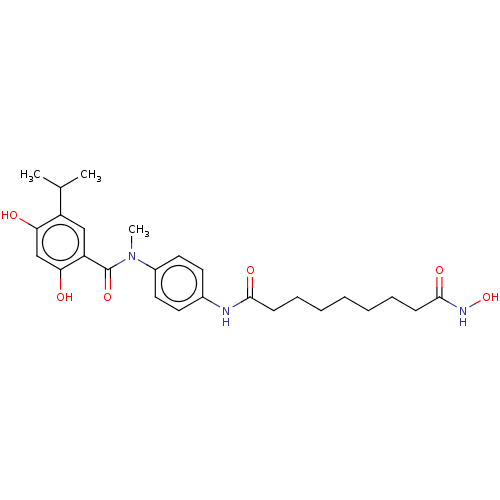
(CHEMBL4448385)Show SMILES CC(C)c1cc(C(=O)N(C)c2ccc(NC(=O)CCCCCCCC(=O)NO)cc2)c(O)cc1O Show InChI InChI=1S/C26H35N3O6/c1-17(2)20-15-21(23(31)16-22(20)30)26(34)29(3)19-13-11-18(12-14-19)27-24(32)9-7-5-4-6-8-10-25(33)28-35/h11-17,30-31,35H,4-10H2,1-3H3,(H,27,32)(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1125 residues) expressed in baculovirus infected insect cells using RHK-K(Ac)-AMC a... |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111725
BindingDB Entry DOI: 10.7270/Q2V1286G |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50551230

(CHEMBL4741850)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50551232

(CHEMBL4790559)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC3 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50551234

(CHEMBL4789155)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC in human HeLa cell nuclear extract using fluorescence substrate incubated for 30 mins by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50551227

(CHEMBL4782478)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NCc4ccc(cc4)C(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC8 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50519391

(CHEMBL4514330)Show SMILES CC(C)c1cc(C(=O)N(C)c2ccc(NC(=O)CCCCCCC(=O)NO)cc2)c(O)cc1O Show InChI InChI=1S/C25H33N3O6/c1-16(2)19-14-20(22(30)15-21(19)29)25(33)28(3)18-12-10-17(11-13-18)26-23(31)8-6-4-5-7-9-24(32)27-34/h10-16,29-30,34H,4-9H2,1-3H3,(H,26,31)(H,27,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
Taipei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His-fusion tagged and N-terminal Streptavidin2-tagged human HDAC8 (1 to 377 residues) expressed in insect cells ... |
Eur J Med Chem 185: (2020)
Article DOI: 10.1016/j.ejmech.2019.111725
BindingDB Entry DOI: 10.7270/Q2V1286G |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50551233

(CHEMBL4790152)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HSP90a (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50551234

(CHEMBL4789155)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 432 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50551233

(CHEMBL4790152)Show SMILES CC(C)c1cc(C(=O)N2Cc3ccc(NC(=O)CCCCCCCC(=O)NO)cc3C2)c(O)cc1O | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 494 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC3 using RHK-K(Ac)-AMC as substrate by fluorescence based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112086
BindingDB Entry DOI: 10.7270/Q2HX1H96 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data