

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-methylscopolamine ([3H]-NMS) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity by displacing [3H]oxotremorine from mouse cerebral cortex tissue. | J Med Chem 35: 1102-8 (1992) BindingDB Entry DOI: 10.7270/Q28K79QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50403547 (ATROPEN | ATROPINE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-methylscopolamine ([3H]-NMS) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50403547 (ATROPEN | ATROPINE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity by displacing [3H]oxotremorine from mouse cerebral cortex tissue. | J Med Chem 35: 1102-8 (1992) BindingDB Entry DOI: 10.7270/Q28K79QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM31883 (9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor gamma | Bioorg Med Chem Lett 11: 1583-6 (2001) BindingDB Entry DOI: 10.7270/Q20R9NQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity by displacing [3H]oxotremorine from mouse cerebral cortex tissue. | J Med Chem 35: 1102-8 (1992) BindingDB Entry DOI: 10.7270/Q28K79QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-oxotremorine ([3H]-OXO-M) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217008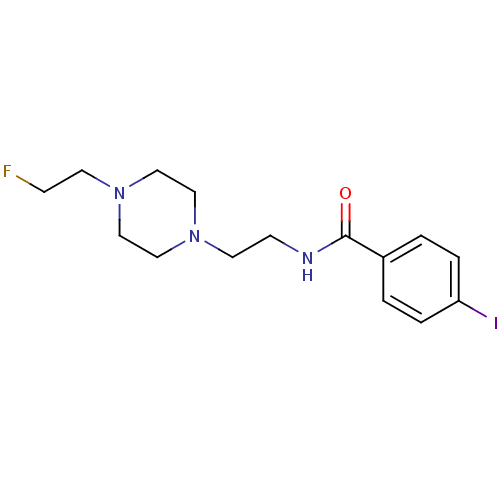 (CHEMBL230904 | N-(1-(2-fluoroethyl)piperidin-4-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity by displacing [3H]oxotremorine from mouse cerebral cortex tissue. | J Med Chem 35: 1102-8 (1992) BindingDB Entry DOI: 10.7270/Q28K79QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity by displacing [3H]oxotremorine from mouse cerebral cortex tissue. | J Med Chem 35: 1102-8 (1992) BindingDB Entry DOI: 10.7270/Q28K79QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-oxotremorine ([3H]-OXO-M) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217012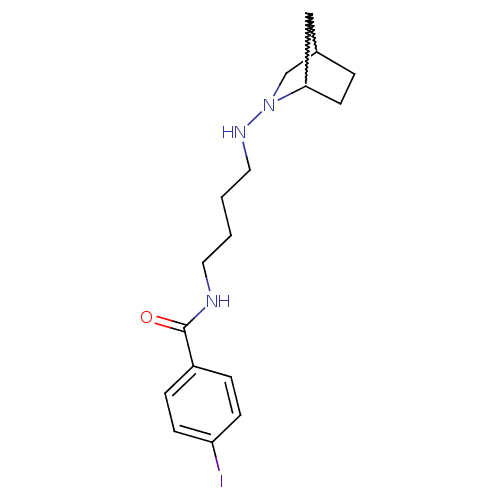 (CHEMBL231120 | N-(4-(2-azanorborn-2-yl)butyl)-4-io...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217015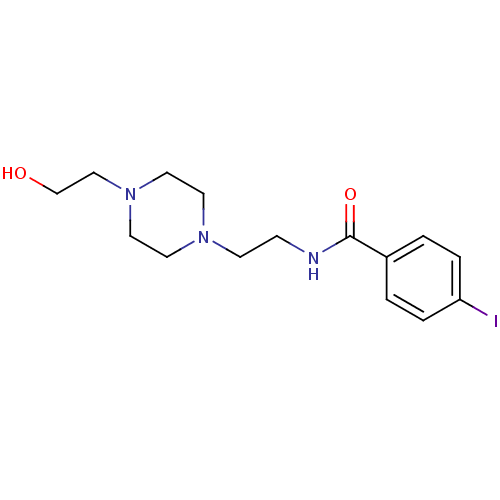 (CHEMBL231119 | N-(1-(2-hydroxyethyl)piperidin-4-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-oxotremorine ([3H]-OXO-M) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM50092055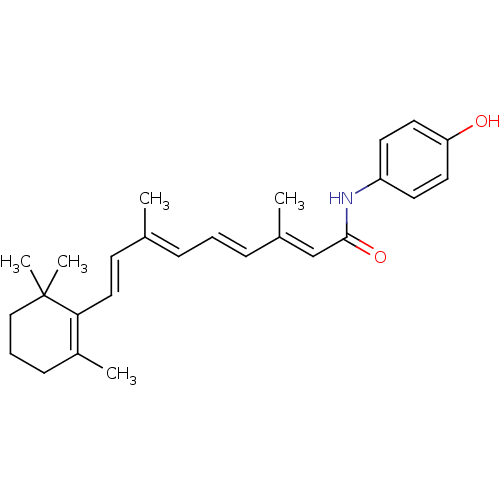 ((2E,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | >400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor gamma | Bioorg Med Chem Lett 11: 1583-6 (2001) BindingDB Entry DOI: 10.7270/Q20R9NQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50004664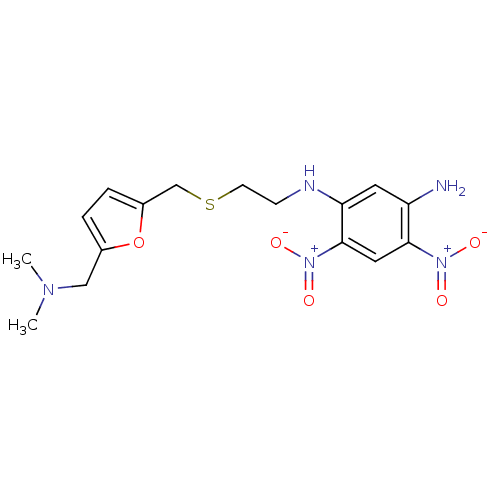 (CHEMBL107892 | N-[2-(5-Dimethylaminomethyl-furan-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-methylscopolamine ([3H]-NMS) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217014 (CHEMBL230903 | N-(2-(diethylamino)ethyl)-5-iodo-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217009 (4-acetamido-N-(4-(2-azanorborn-2-yl)butyl)-5-iodo-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217013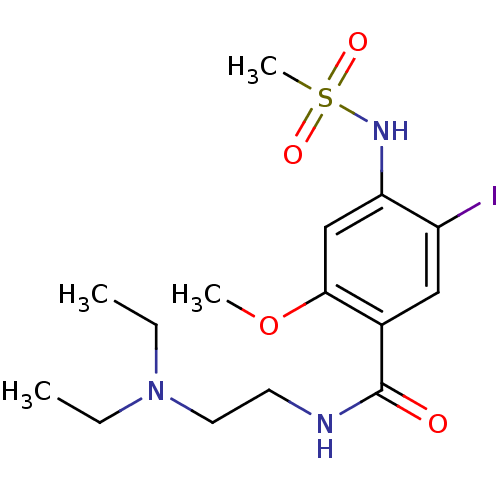 (CHEMBL230692 | N-(2-(diethylamino)ethyl)-5-iodo-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50004661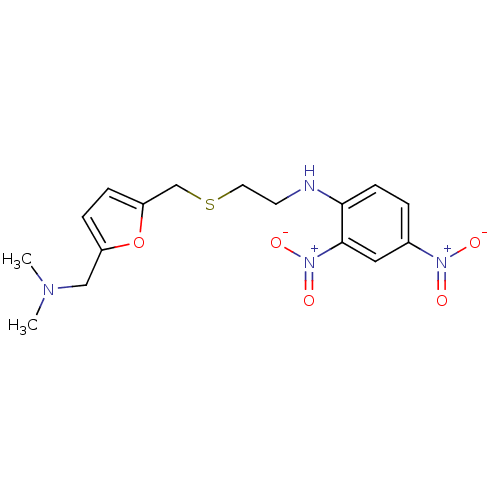 (CHEMBL316973 | [2-(5-Dimethylaminomethyl-furan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-oxotremorine ([3H]-OXO-M) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50004652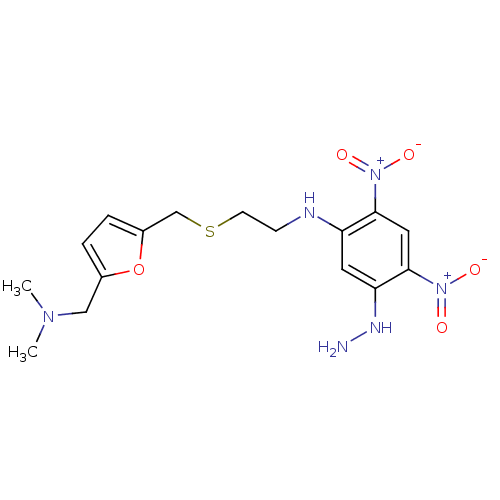 (CHEMBL321605 | [2-(5-Dimethylaminomethyl-furan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-oxotremorine ([3H]-OXO-M) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217018 (CHEMBL395005 | N-(4-(dipropylamino)butyl)-4-iodobe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-methylscopolamine ([3H]-NMS) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity by displacing [3H]methylscopolamine [3H]NMS from mouse cerebral cortex tissue. | J Med Chem 35: 1102-8 (1992) BindingDB Entry DOI: 10.7270/Q28K79QR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217010 (4-acetamido-N-(4-(N-butyl-N-methylamino)butyl)-5-i...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50092740 (4-Acetylamino-N-(2-diethylamino-ethyl)-5-iodo-2-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217011 (4-acetamido-N-(4-(butylamino)butyl)-5-iodo-2-metho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM50100957 ((3E,5E,7E,9E)-1-(4-Hydroxy-phenyl)-4,8-dimethyl-10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Ohio State University Curated by ChEMBL | Assay Description Binding affinity against Retinoic acid receptor gamma | Bioorg Med Chem Lett 11: 1583-6 (2001) BindingDB Entry DOI: 10.7270/Q20R9NQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217016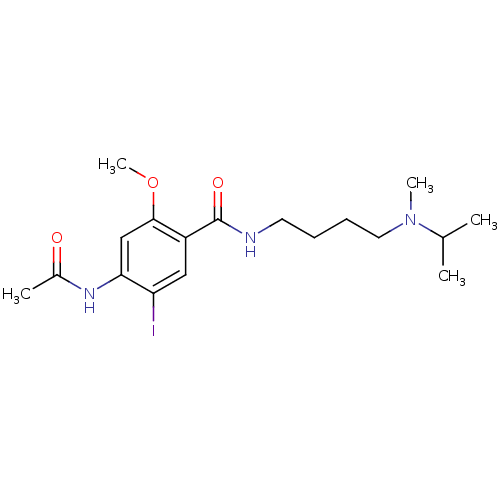 (4-acetamido-N-(4-(N-isopropyl-N-methylamino)butyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Binding affinity of the compound against mouse Muscarinic acetylcholine receptor M2 using heart tissue and [3H]-N-methylscopolamine | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50217017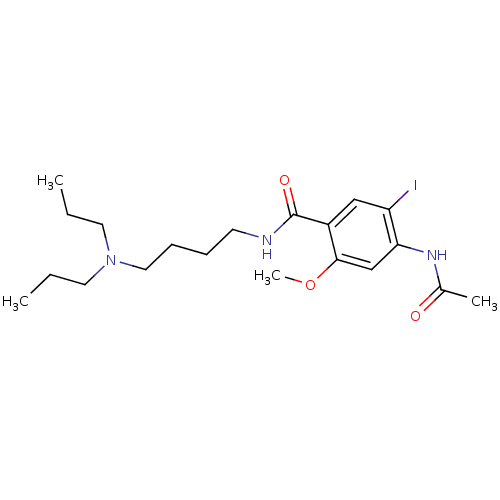 (4-acetamido-N-(4-(dipropylamino)butyl)-5-iodo-2-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Radiopharmaceuticals Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma 1 receptor in guinea pig brain membrane | J Med Chem 50: 3561-72 (2007) Article DOI: 10.1021/jm0701627 BindingDB Entry DOI: 10.7270/Q27H1JBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50004664 (CHEMBL107892 | N-[2-(5-Dimethylaminomethyl-furan-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-methylscopolamine ([3H]-NMS) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50004661 (CHEMBL316973 | [2-(5-Dimethylaminomethyl-furan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-oxotremorine ([3H]-OXO-M) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50004652 (CHEMBL321605 | [2-(5-Dimethylaminomethyl-furan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-methylscopolamine ([3H]-NMS) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Mus musculus) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Carolina Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-oxotremorine ([3H]-OXO-M) from mouse cerebral cortex | J Med Chem 35: 3141-7 (1992) BindingDB Entry DOI: 10.7270/Q200012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2E1 (Homo sapiens (Human)) | BDBM50088436 (BS-749 | CHEBI:76987 | Metacetamol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 9.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pacific University Oregon Curated by ChEMBL | Assay Description Inhibition of human CYP2E1 assessed as chlorzoxazone 6-hydroxylase activity by HPLC analysis | Drug Metab Dispos 40: 1460-5 (2012) Article DOI: 10.1124/dmd.112.045492 BindingDB Entry DOI: 10.7270/Q27H1M9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM443131 (US10654833, Compound 5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hepatikos Therapeutics, LLC US Patent | Assay Description Inhibition of ASK1 kinase activity was determined radiometrically using 33P substrate incorporation (Reaction Biology Corp., Malvern, Pa.). Briefly, ... | US Patent US10654833 (2020) BindingDB Entry DOI: 10.7270/Q2F192R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153906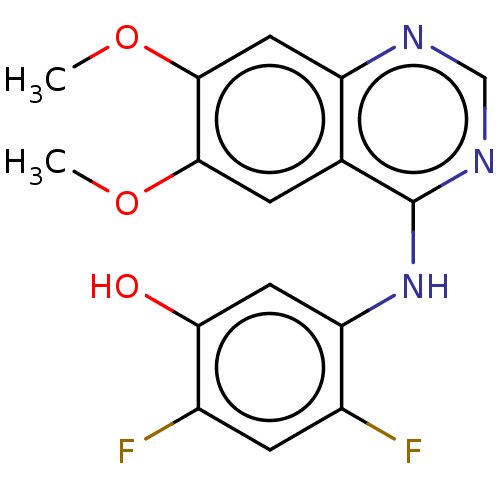 (CHEMBL3775169) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM443190 (US10654833, Compound 102) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hepatikos Therapeutics, LLC US Patent | Assay Description Inhibition of ASK1 kinase activity was determined radiometrically using 33P substrate incorporation (Reaction Biology Corp., Malvern, Pa.). Briefly, ... | US Patent US10654833 (2020) BindingDB Entry DOI: 10.7270/Q2F192R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM4627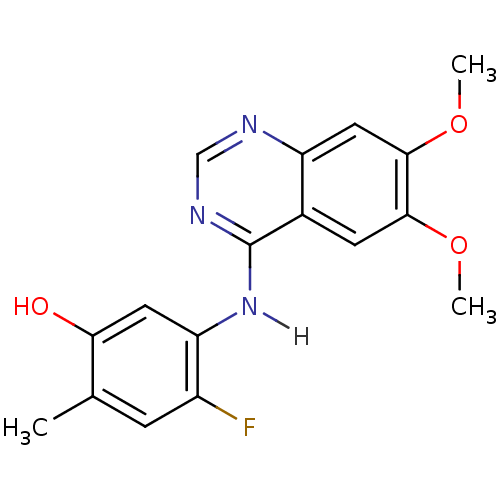 (5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153979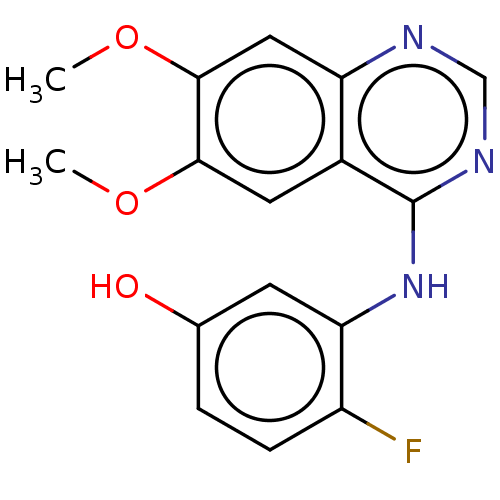 (CHEMBL3774904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM443135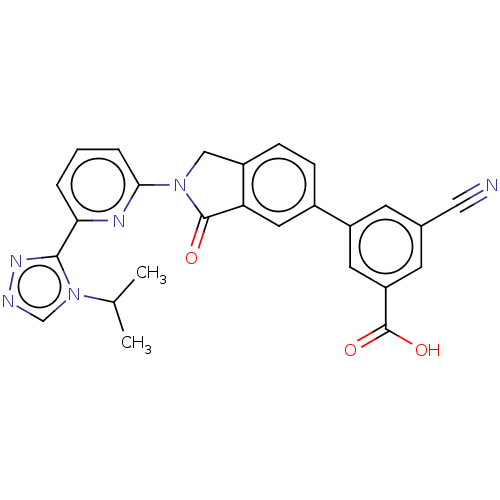 (US10654833, Compound 13) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hepatikos Therapeutics, LLC US Patent | Assay Description Inhibition of ASK1 kinase activity was determined radiometrically using 33P substrate incorporation (Reaction Biology Corp., Malvern, Pa.). Briefly, ... | US Patent US10654833 (2020) BindingDB Entry DOI: 10.7270/Q2F192R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM443199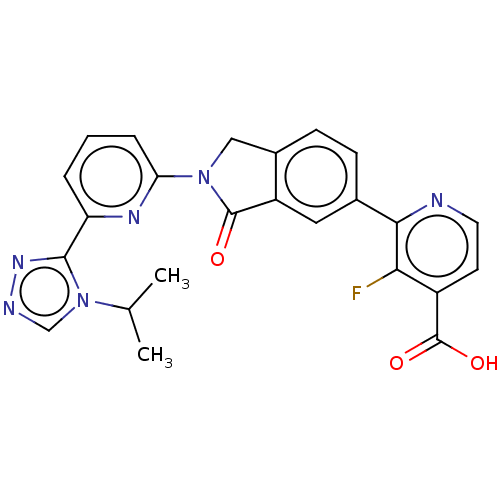 (US10654833, Compound 113) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hepatikos Therapeutics, LLC US Patent | Assay Description Inhibition of ASK1 kinase activity was determined radiometrically using 33P substrate incorporation (Reaction Biology Corp., Malvern, Pa.). Briefly, ... | US Patent US10654833 (2020) BindingDB Entry DOI: 10.7270/Q2F192R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153902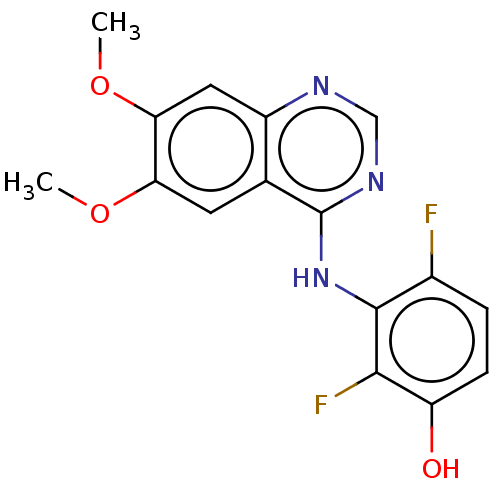 (CHEMBL3775557) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM443174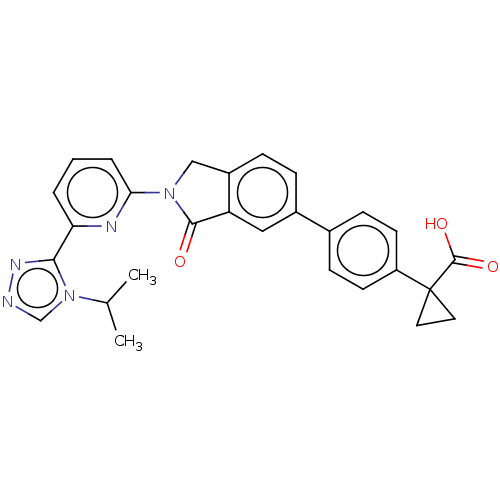 (US10654833, Compound 86) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Hepatikos Therapeutics, LLC US Patent | Assay Description Inhibition of ASK1 kinase activity was determined radiometrically using 33P substrate incorporation (Reaction Biology Corp., Malvern, Pa.). Briefly, ... | US Patent US10654833 (2020) BindingDB Entry DOI: 10.7270/Q2F192R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM443203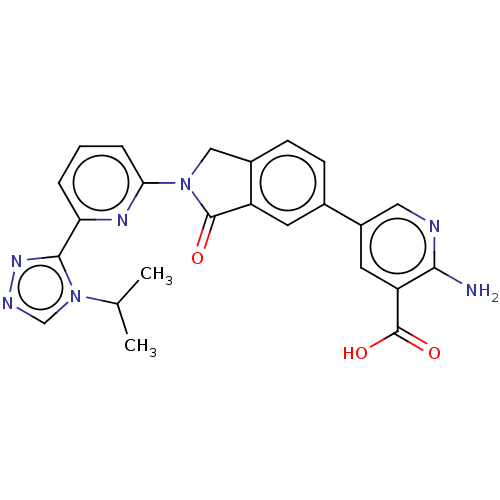 (US10654833, Compound 117) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Hepatikos Therapeutics, LLC US Patent | Assay Description Inhibition of ASK1 kinase activity was determined radiometrically using 33P substrate incorporation (Reaction Biology Corp., Malvern, Pa.). Briefly, ... | US Patent US10654833 (2020) BindingDB Entry DOI: 10.7270/Q2F192R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50153903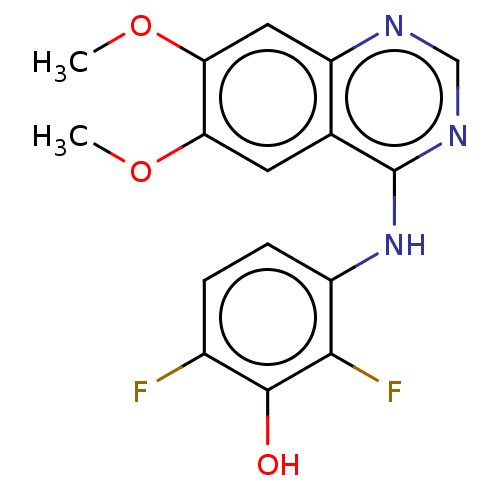 (CHEMBL3775336) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM443200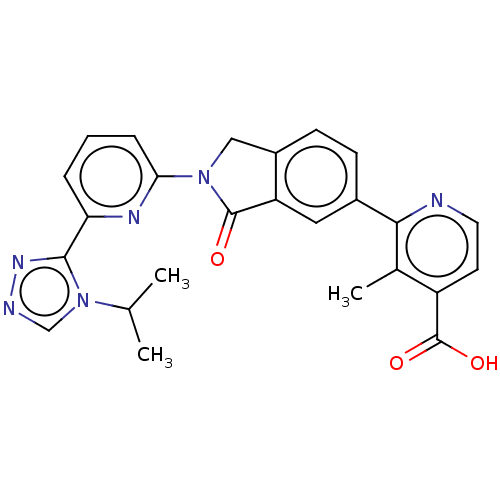 (US10654833, Compound 114) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hepatikos Therapeutics, LLC US Patent | Assay Description Inhibition of ASK1 kinase activity was determined radiometrically using 33P substrate incorporation (Reaction Biology Corp., Malvern, Pa.). Briefly, ... | US Patent US10654833 (2020) BindingDB Entry DOI: 10.7270/Q2F192R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4627 (5-[(6,7-dimethoxyquinazolin-4-yl)amino]-4-fluoro-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human KDR expressed in insect Sf21 cells preincubated for 15 mins followed by substrate addition measured after ... | Eur J Med Chem 112: 20-32 (2016) Article DOI: 10.1016/j.ejmech.2016.01.039 BindingDB Entry DOI: 10.7270/Q27W6F1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 5 (Homo sapiens (Human)) | BDBM443175 (US10654833, Compound 87) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hepatikos Therapeutics, LLC US Patent | Assay Description Inhibition of ASK1 kinase activity was determined radiometrically using 33P substrate incorporation (Reaction Biology Corp., Malvern, Pa.). Briefly, ... | US Patent US10654833 (2020) BindingDB Entry DOI: 10.7270/Q2F192R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1201 total ) | Next | Last >> |