 Found 413 hits with Last Name = 'cheon' and Initial = 'hg'
Found 413 hits with Last Name = 'cheon' and Initial = 'hg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50206821
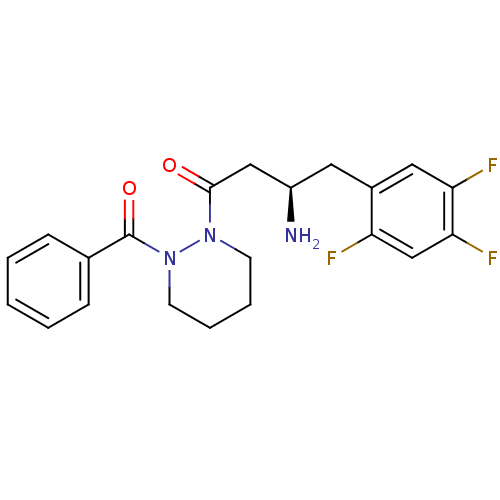
((R)-3-amino-1-(2-benzoylpiperazin-1-yl)-4-(2,4,5-t...)Show SMILES N[C@@H](CC(=O)N1CCCCN1C(=O)c1ccccc1)Cc1cc(F)c(F)cc1F Show InChI InChI=1S/C21H22F3N3O2/c22-17-13-19(24)18(23)11-15(17)10-16(25)12-20(28)26-8-4-5-9-27(26)21(29)14-6-2-1-3-7-14/h1-3,6-7,11,13,16H,4-5,8-10,12,25H2/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney DPP4 |
Bioorg Med Chem Lett 17: 2622-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.111
BindingDB Entry DOI: 10.7270/Q21V5FS4 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Sus scrofa (pig)) | BDBM50206820

((R)-3-amino-1-(2-benzoyl-1,2-diazepan-1-yl)-4-(2,4...)Show SMILES N[C@@H](CC(=O)N1CCCCCN1C(=O)c1ccccc1)Cc1cc(F)c(F)cc1F Show InChI InChI=1S/C22H24F3N3O2/c23-18-14-20(25)19(24)12-16(18)11-17(26)13-21(29)27-9-5-2-6-10-28(27)22(30)15-7-3-1-4-8-15/h1,3-4,7-8,12,14,17H,2,5-6,9-11,13,26H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 56.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of pig kidney DPP4 |
Bioorg Med Chem Lett 17: 2622-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.111
BindingDB Entry DOI: 10.7270/Q21V5FS4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50220217
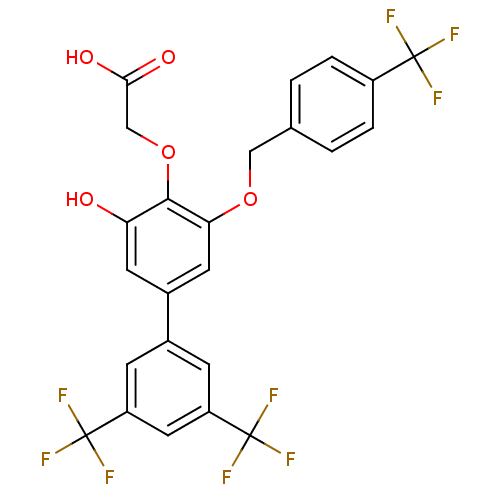
(CHEMBL238138 | [3-hydroxy-3',5'-bis-trifluoromethy...)Show SMILES OC(=O)COc1c(O)cc(cc1OCc1ccc(cc1)C(F)(F)F)-c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H15F9O5/c25-22(26,27)15-3-1-12(2-4-15)10-37-19-8-14(7-18(34)21(19)38-11-20(35)36)13-5-16(23(28,29)30)9-17(6-13)24(31,32)33/h1-9,34H,10-11H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B after 10 mins |
Bioorg Med Chem Lett 17: 5357-60 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.019
BindingDB Entry DOI: 10.7270/Q2BK1D55 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50220217

(CHEMBL238138 | [3-hydroxy-3',5'-bis-trifluoromethy...)Show SMILES OC(=O)COc1c(O)cc(cc1OCc1ccc(cc1)C(F)(F)F)-c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H15F9O5/c25-22(26,27)15-3-1-12(2-4-15)10-37-19-8-14(7-18(34)21(19)38-11-20(35)36)13-5-16(23(28,29)30)9-17(6-13)24(31,32)33/h1-9,34H,10-11H2,(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Inhibition of TC-PTP after 10 mins |
Bioorg Med Chem Lett 17: 5357-60 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.019
BindingDB Entry DOI: 10.7270/Q2BK1D55 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334712
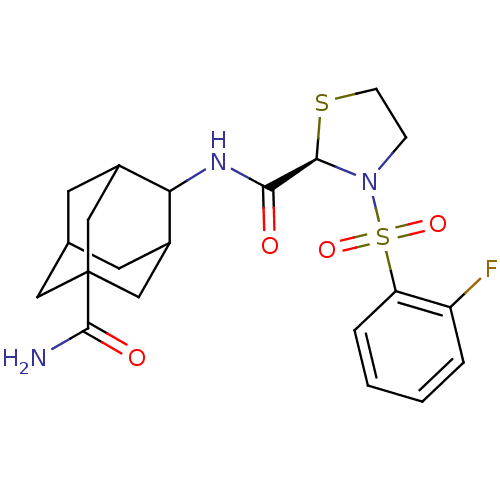
((R,E)-3-(2-Fluoro-benzenesulfonyl)-thiazolidine-2-...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)[C@H]1SCCN1S(=O)(=O)c1ccccc1F)C(C3)C2 |r,wU:13.13,TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(17.46,-12.91,;16.64,-11.61,;17.36,-10.24,;15.1,-11.67,;15.11,-10.14,;13.72,-9.56,;12.68,-10.79,;12.68,-12.38,;14.09,-12.94,;11.18,-12.8,;9.85,-12.03,;8.51,-12.8,;7.18,-12.03,;8.52,-14.34,;9.77,-15.25,;9.29,-16.71,;7.75,-16.71,;7.27,-15.25,;5.93,-14.5,;5.15,-13.16,;6.69,-13.16,;4.61,-15.29,;4.64,-16.82,;3.32,-17.62,;1.97,-16.87,;1.94,-15.33,;3.26,-14.54,;3.25,-13,;12.38,-11.52,;12.37,-10.04,;13.71,-12.01,)| Show InChI InChI=1S/C21H26FN3O4S2/c22-15-3-1-2-4-16(15)31(28,29)25-5-6-30-19(25)18(26)24-17-13-7-12-8-14(17)11-21(9-12,10-13)20(23)27/h1-4,12-14,17,19H,5-11H2,(H2,23,27)(H,24,26)/t12?,13?,14?,17?,19-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334712

((R,E)-3-(2-Fluoro-benzenesulfonyl)-thiazolidine-2-...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)[C@H]1SCCN1S(=O)(=O)c1ccccc1F)C(C3)C2 |r,wU:13.13,TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(17.46,-12.91,;16.64,-11.61,;17.36,-10.24,;15.1,-11.67,;15.11,-10.14,;13.72,-9.56,;12.68,-10.79,;12.68,-12.38,;14.09,-12.94,;11.18,-12.8,;9.85,-12.03,;8.51,-12.8,;7.18,-12.03,;8.52,-14.34,;9.77,-15.25,;9.29,-16.71,;7.75,-16.71,;7.27,-15.25,;5.93,-14.5,;5.15,-13.16,;6.69,-13.16,;4.61,-15.29,;4.64,-16.82,;3.32,-17.62,;1.97,-16.87,;1.94,-15.33,;3.26,-14.54,;3.25,-13,;12.38,-11.52,;12.37,-10.04,;13.71,-12.01,)| Show InChI InChI=1S/C21H26FN3O4S2/c22-15-3-1-2-4-16(15)31(28,29)25-5-6-30-19(25)18(26)24-17-13-7-12-8-14(17)11-21(9-12,10-13)20(23)27/h1-4,12-14,17,19H,5-11H2,(H2,23,27)(H,24,26)/t12?,13?,14?,17?,19-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334708
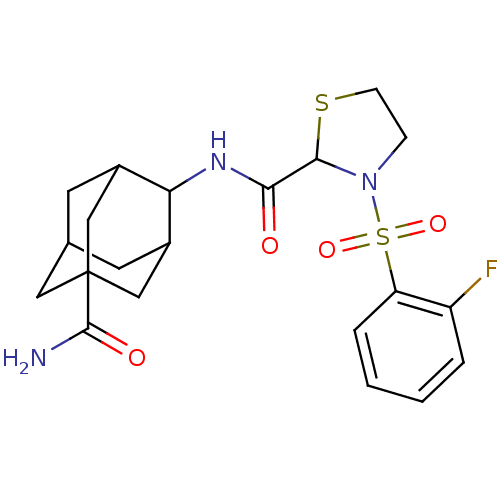
((E)-3-[(2-Fluorophenyl)sulfonyl]-N-[5-(aminocarbon...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)C1SCCN1S(=O)(=O)c1ccccc1F)C(C3)C2 |TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(12.45,-21.42,;11.63,-20.11,;12.35,-18.75,;10.09,-20.17,;10.1,-18.65,;8.71,-18.07,;7.67,-19.3,;7.67,-20.89,;9.08,-21.45,;6.17,-21.31,;4.84,-20.54,;3.51,-21.31,;2.17,-20.54,;3.51,-22.85,;4.76,-23.76,;4.28,-25.22,;2.74,-25.22,;2.27,-23.76,;.92,-23.01,;.14,-21.67,;1.68,-21.67,;-.4,-23.8,;-.37,-25.33,;-1.69,-26.13,;-3.04,-25.38,;-3.06,-23.83,;-1.74,-23.05,;-1.76,-21.51,;7.37,-20.03,;7.36,-18.55,;8.7,-20.52,)| Show InChI InChI=1S/C21H26FN3O4S2/c22-15-3-1-2-4-16(15)31(28,29)25-5-6-30-19(25)18(26)24-17-13-7-12-8-14(17)11-21(9-12,10-13)20(23)27/h1-4,12-14,17,19H,5-11H2,(H2,23,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334708

((E)-3-[(2-Fluorophenyl)sulfonyl]-N-[5-(aminocarbon...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)C1SCCN1S(=O)(=O)c1ccccc1F)C(C3)C2 |TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(12.45,-21.42,;11.63,-20.11,;12.35,-18.75,;10.09,-20.17,;10.1,-18.65,;8.71,-18.07,;7.67,-19.3,;7.67,-20.89,;9.08,-21.45,;6.17,-21.31,;4.84,-20.54,;3.51,-21.31,;2.17,-20.54,;3.51,-22.85,;4.76,-23.76,;4.28,-25.22,;2.74,-25.22,;2.27,-23.76,;.92,-23.01,;.14,-21.67,;1.68,-21.67,;-.4,-23.8,;-.37,-25.33,;-1.69,-26.13,;-3.04,-25.38,;-3.06,-23.83,;-1.74,-23.05,;-1.76,-21.51,;7.37,-20.03,;7.36,-18.55,;8.7,-20.52,)| Show InChI InChI=1S/C21H26FN3O4S2/c22-15-3-1-2-4-16(15)31(28,29)25-5-6-30-19(25)18(26)24-17-13-7-12-8-14(17)11-21(9-12,10-13)20(23)27/h1-4,12-14,17,19H,5-11H2,(H2,23,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50334709

(3-(3-Fluoro-benzenesulfonyl)-thiazolidine-2-carbox...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)C1SCCN1S(=O)(=O)c1cccc(F)c1)C(C3)C2 |TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(54.1,-20.21,;53.28,-18.91,;54,-17.55,;51.74,-18.97,;51.75,-17.44,;50.36,-16.86,;49.32,-18.1,;49.32,-19.68,;50.73,-20.25,;47.82,-20.1,;46.49,-19.33,;45.16,-20.11,;43.82,-19.34,;45.16,-21.65,;46.41,-22.55,;45.93,-24.02,;44.39,-24.02,;43.92,-22.55,;42.57,-21.81,;41.79,-20.46,;43.33,-20.46,;41.25,-22.6,;41.28,-24.13,;39.96,-24.92,;38.61,-24.17,;38.59,-22.63,;37.24,-21.88,;39.91,-21.84,;49.02,-18.83,;49.01,-17.34,;50.35,-19.32,)| Show InChI InChI=1S/C21H26FN3O4S2/c22-15-2-1-3-16(8-15)31(28,29)25-4-5-30-19(25)18(26)24-17-13-6-12-7-14(17)11-21(9-12,10-13)20(23)27/h1-3,8,12-14,17,19H,4-7,9-11H2,(H2,23,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334704

(3-(3,4-Dichloro-benzenesulfonyl)-thiazolidine-2-ca...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)C1SCCN1S(=O)(=O)c1ccc(Cl)c(Cl)c1)C(C3)C2 |TLB:10:9:31.8.3:6.5.30,10:9:30:31.3.4,8:3:9.7.6:30,THB:8:7:30:31.3.4,4:3:9:6.5.30,4:5:9:31.8.3,1:3:9:6.5.30,1:3:9.7.6:30,(18.16,-3.14,;17.33,-1.84,;18.05,-.47,;15.79,-1.9,;15.81,-.37,;14.41,.21,;13.37,-1.03,;13.38,-2.61,;14.78,-3.17,;11.87,-3.03,;10.54,-2.26,;9.21,-3.03,;7.87,-2.27,;9.21,-4.57,;10.46,-5.48,;9.98,-6.95,;8.44,-6.95,;7.97,-5.48,;6.62,-4.73,;5.84,-3.39,;7.38,-3.39,;5.3,-5.52,;5.33,-7.05,;4.01,-7.85,;2.66,-7.1,;1.34,-7.89,;2.64,-5.56,;1.29,-4.81,;3.96,-4.77,;13.07,-1.75,;13.06,-.27,;14.4,-2.24,)| Show InChI InChI=1S/C21H25Cl2N3O4S2/c22-15-2-1-14(7-16(15)23)32(29,30)26-3-4-31-19(26)18(27)25-17-12-5-11-6-13(17)10-21(8-11,9-12)20(24)28/h1-2,7,11-13,17,19H,3-6,8-10H2,(H2,24,28)(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50334705
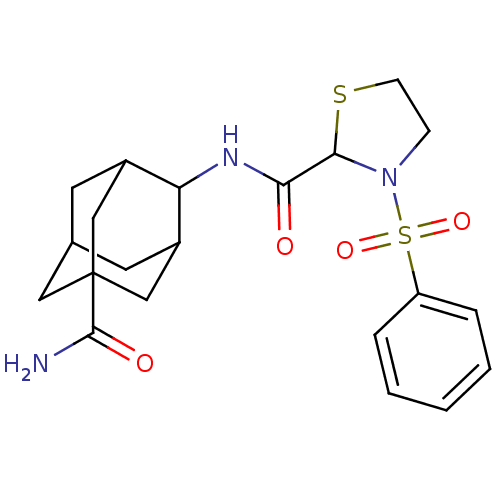
(3-Benzenesulfonyl-thiazolidine-2-carboxylic acid(5...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)C1SCCN1S(=O)(=O)c1ccccc1)C(C3)C2 |TLB:10:9:29.8.3:6.5.28,10:9:28:29.3.4,8:3:9.7.6:28,THB:8:7:28:29.3.4,4:3:9:6.5.28,4:5:9:29.8.3,1:3:9:6.5.28,1:3:9.7.6:28,(22.28,-41.5,;21.46,-40.2,;22.17,-38.83,;19.92,-40.26,;19.93,-38.73,;18.53,-38.15,;17.49,-39.39,;17.5,-40.97,;18.9,-41.53,;16,-41.39,;14.66,-40.62,;13.33,-41.39,;11.99,-40.63,;13.33,-42.93,;14.58,-43.84,;14.1,-45.31,;12.56,-45.31,;12.09,-43.84,;10.74,-43.09,;9.96,-41.75,;11.5,-41.75,;9.42,-43.88,;8.08,-43.13,;6.76,-43.92,;6.78,-45.46,;8.14,-46.21,;9.45,-45.41,;17.19,-40.11,;17.18,-38.63,;18.52,-40.6,)| Show InChI InChI=1S/C21H27N3O4S2/c22-20(26)21-10-13-8-14(11-21)17(15(9-13)12-21)23-18(25)19-24(6-7-29-19)30(27,28)16-4-2-1-3-5-16/h1-5,13-15,17,19H,6-12H2,(H2,22,26)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334706

(3-(Toluene-4-sulfonyl)-thiazolidine-2-carboxylic a...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCSC1C(=O)NC1C2CC3CC1CC(C3)(C2)C(N)=O |TLB:17:18:24.27.25:20.21.22,17:18:22:24.25.26,27:25:18.19.20:22,THB:27:19:22:24.25.26,26:25:18:20.21.22,26:21:18:24.27.25,28:25:18:20.21.22,28:25:18.19.20:22,(21.54,-45.78,;22.86,-44.98,;22.84,-43.44,;24.16,-42.65,;25.5,-43.41,;25.53,-44.94,;24.21,-45.73,;26.82,-42.62,;26.04,-41.28,;27.58,-41.28,;28.17,-43.37,;28.64,-44.83,;30.18,-44.83,;30.66,-43.37,;29.41,-42.46,;29.41,-40.92,;28.07,-40.15,;30.74,-40.15,;32.07,-40.91,;33.57,-40.49,;33.57,-38.91,;34.61,-37.68,;33.26,-38.15,;33.27,-39.64,;34.6,-40.13,;35.99,-39.78,;36,-38.25,;34.98,-41.06,;37.53,-39.72,;38.35,-41.02,;38.25,-38.36,)| Show InChI InChI=1S/C22H29N3O4S2/c1-13-2-4-17(5-3-13)31(28,29)25-6-7-30-20(25)19(26)24-18-15-8-14-9-16(18)12-22(10-14,11-15)21(23)27/h2-5,14-16,18,20H,6-12H2,1H3,(H2,23,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50081724
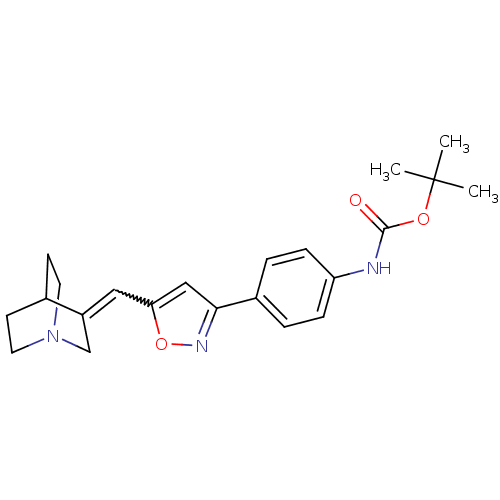
((4-{5-[1-Aza-bicyclo[2.2.2]oct-(3Z)-ylidenemethyl]...)Show SMILES CC(C)(C)OC(=O)Nc1ccc(cc1)-c1cc(C=C2CN3CCC2CC3)on1 |w:17.17,(10.93,-19.04,;11.84,-17.74,;13.3,-17.06,;12.42,-19.09,;11.06,-16.38,;9.51,-16.38,;8.73,-17.71,;8.73,-15.02,;7.17,-15.02,;6.42,-13.66,;4.89,-13.66,;4.12,-14.99,;4.87,-16.32,;6.4,-16.34,;2.59,-14.98,;1.7,-13.72,;.23,-14.2,;-1.12,-13.4,;-2.49,-14.18,;-2.49,-15.7,;-3.82,-16.48,;-3.08,-14.7,;-4.56,-15.11,;-3.82,-13.4,;-5.14,-14.18,;-5.14,-15.7,;.21,-15.72,;1.66,-16.21,)| Show InChI InChI=1S/C22H27N3O3/c1-22(2,3)27-21(26)23-18-6-4-16(5-7-18)20-13-19(28-24-20)12-17-14-25-10-8-15(17)9-11-25/h4-7,12-13,15H,8-11,14H2,1-3H3,(H,23,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
The compound was tested for its binding affinity against M1 human recombinant muscarinic receptor in CHO cells. |
Bioorg Med Chem Lett 9: 2795-800 (1999)
BindingDB Entry DOI: 10.7270/Q26H4GM1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334714
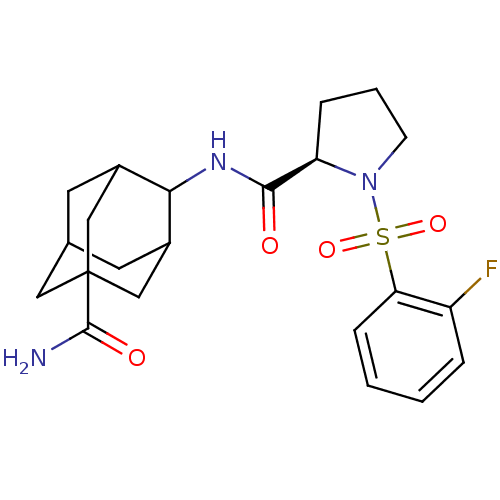
((R)-1-(2-Fluoro-benzenesulfonyl)-pyrrolidine-2-car...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)[C@H]1CCCN1S(=O)(=O)c1ccccc1F)C(C3)C2 |r,wU:13.13,TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(29.87,-8.72,;29.05,-7.42,;29.76,-6.06,;27.51,-7.48,;27.52,-5.95,;26.12,-5.37,;25.08,-6.61,;25.09,-8.19,;26.49,-8.76,;23.59,-8.61,;22.25,-7.85,;20.92,-8.62,;19.58,-7.85,;20.92,-10.16,;22.17,-11.07,;21.69,-12.53,;20.15,-12.53,;19.68,-11.07,;18.33,-10.32,;17.55,-8.98,;19.09,-8.98,;17.01,-11.11,;17.04,-12.64,;15.73,-13.43,;14.37,-12.68,;14.35,-11.14,;15.67,-10.35,;15.65,-8.81,;24.78,-7.34,;24.78,-5.85,;26.11,-7.83,)| Show InChI InChI=1S/C22H28FN3O4S/c23-16-4-1-2-6-18(16)31(29,30)26-7-3-5-17(26)20(27)25-19-14-8-13-9-15(19)12-22(10-13,11-14)21(24)28/h1-2,4,6,13-15,17,19H,3,5,7-12H2,(H2,24,28)(H,25,27)/t13?,14?,15?,17-,19?,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334694
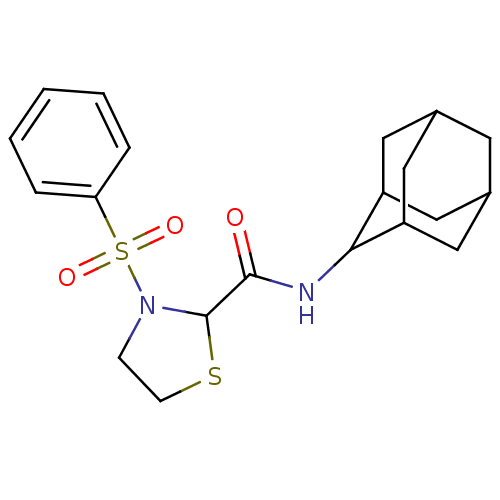
(3-Benzenesulfonyl-thiazolidine-2-carboxylic acid a...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)C1SCCN1S(=O)(=O)c1ccccc1 |TLB:2:3:5.12.6:10.8.9,12:11:9:5.6.7,THB:2:3:9:5.6.7,12:6:3.11.10:9,7:6:3:10.8.9,7:8:3:5.12.6,(22.03,-3.14,;23.37,-3.9,;24.7,-3.13,;26.04,-3.9,;27.23,-2.62,;28.56,-3.11,;29.96,-2.77,;29.97,-1.24,;28.57,-.66,;27.23,-1.14,;27.53,-1.9,;27.54,-3.48,;28.94,-4.05,;23.37,-5.44,;24.62,-6.35,;24.14,-7.82,;22.6,-7.82,;22.13,-6.35,;20.78,-5.6,;20,-4.26,;21.54,-4.26,;19.46,-6.4,;18.12,-5.64,;16.8,-6.43,;16.82,-7.97,;18.18,-8.72,;19.49,-7.92,)| Show InChI InChI=1S/C20H26N2O3S2/c23-19(21-18-15-9-13-8-14(11-15)12-16(18)10-13)20-22(6-7-26-20)27(24,25)17-4-2-1-3-5-17/h1-5,13-16,18,20H,6-12H2,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50081729

(4-{5-[1-Aza-bicyclo[2.2.2]oct-(3Z)-ylidenemethyl]-...)Show SMILES N#Cc1ccc(cc1)-c1cc(\C=C2/CN3CCC2CC3)on1 |(17.18,-15.18,;15.63,-15.18,;14.07,-15.18,;13.29,-16.5,;11.75,-16.48,;11,-15.15,;11.78,-13.82,;13.3,-13.82,;9.48,-15.14,;8.57,-13.89,;7.11,-14.36,;5.77,-13.56,;4.39,-14.34,;4.39,-15.86,;3.06,-16.64,;1.75,-15.86,;1.75,-14.34,;3.06,-13.56,;2.31,-15.27,;3.8,-14.86,;7.1,-15.89,;8.56,-16.37,)| Show InChI InChI=1S/C18H17N3O/c19-11-13-1-3-15(4-2-13)18-10-17(22-20-18)9-16-12-21-7-5-14(16)6-8-21/h1-4,9-10,14H,5-8,12H2/b16-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
The compound was tested for its binding affinity against nicotinic receptor in synaptic membrane fractions from rat cerebral cortices. |
Bioorg Med Chem Lett 9: 2795-800 (1999)
BindingDB Entry DOI: 10.7270/Q26H4GM1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334699
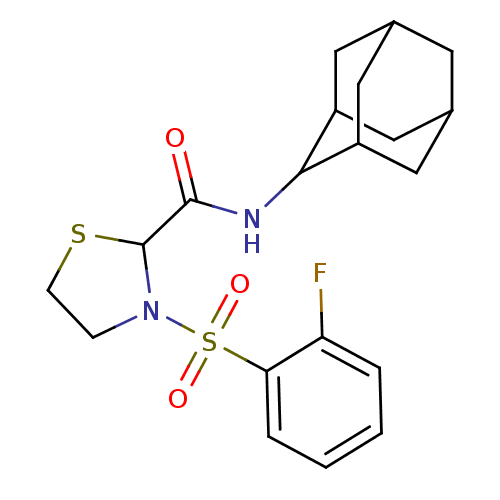
(3-(2-Fluoro-benzenesulfonyl)-thiazolidine-2-carbox...)Show SMILES Fc1ccccc1S(=O)(=O)N1CCSC1C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:17:18:20.27.21:25.23.24,27:26:24:20.21.22,THB:17:18:24:20.21.22,27:21:18.26.25:24,22:21:18:25.23.24,22:23:18:20.27.21,(8.61,-15.54,;7.27,-14.79,;5.95,-15.59,;4.6,-14.84,;4.57,-13.3,;5.89,-12.51,;7.24,-13.26,;8.56,-12.47,;7.78,-11.13,;9.32,-11.13,;9.9,-13.22,;10.38,-14.68,;11.92,-14.68,;12.39,-13.22,;11.15,-12.31,;11.14,-10.77,;9.81,-10,;12.48,-10,;13.81,-10.77,;15.01,-9.49,;16.34,-9.98,;17.73,-9.64,;17.74,-8.11,;16.35,-7.53,;15,-8.01,;15.31,-8.76,;15.31,-10.35,;16.72,-10.91,)| Show InChI InChI=1S/C20H25FN2O3S2/c21-16-3-1-2-4-17(16)28(25,26)23-5-6-27-20(23)19(24)22-18-14-8-12-7-13(10-14)11-15(18)9-12/h1-4,12-15,18,20H,5-11H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334700
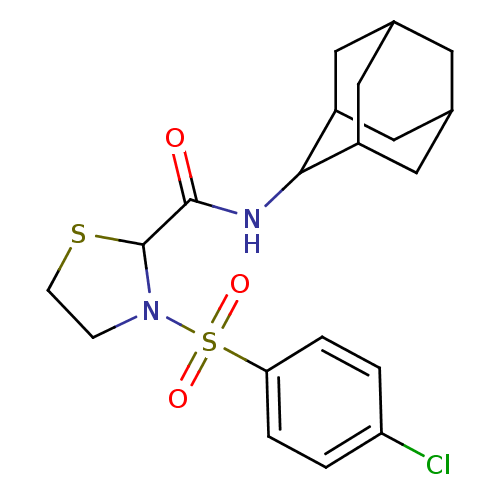
(3-(4-Chloro-benzenesulfonyl)-thiazolidine-2-carbox...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1CCSC1C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:17:18:20.27.21:25.23.24,27:26:24:20.21.22,THB:17:18:24:20.21.22,27:21:18.26.25:24,22:21:18:25.23.24,22:23:18:20.27.21,(4.15,-15.58,;5.47,-14.79,;5.44,-13.25,;6.76,-12.46,;8.11,-13.22,;8.14,-14.75,;6.82,-15.54,;9.43,-12.43,;8.65,-11.08,;10.19,-11.08,;10.77,-13.17,;11.25,-14.64,;12.79,-14.64,;13.27,-13.17,;12.02,-12.27,;12.01,-10.73,;10.68,-9.96,;13.35,-9.95,;14.68,-10.72,;15.88,-9.45,;17.21,-9.94,;18.6,-9.59,;18.61,-8.06,;17.22,-7.48,;15.87,-7.96,;16.18,-8.72,;16.18,-10.3,;17.59,-10.87,)| Show InChI InChI=1S/C20H25ClN2O3S2/c21-16-1-3-17(4-2-16)28(25,26)23-5-6-27-20(23)19(24)22-18-14-8-12-7-13(10-14)11-15(18)9-12/h1-4,12-15,18,20H,5-11H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334701
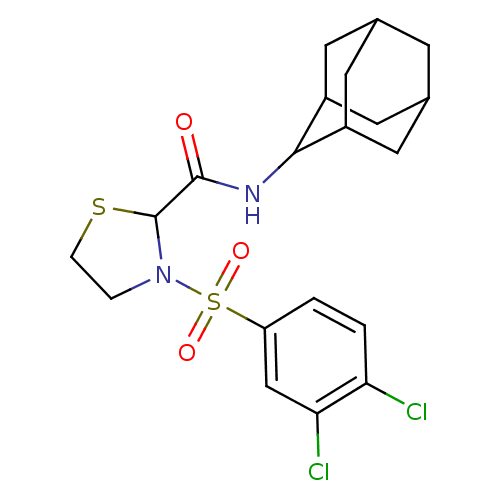
(3-(3,4-Dichloro-benzenesulfonyl)-thiazolidine-2-ca...)Show SMILES Clc1ccc(cc1Cl)S(=O)(=O)N1CCSC1C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:18:19:21.28.22:26.24.25,28:27:25:21.22.23,THB:18:19:25:21.22.23,28:22:19.27.26:25,23:22:19:26.24.25,23:24:19:21.28.22,(4.17,-15.58,;5.49,-14.79,;6.85,-15.54,;8.16,-14.75,;8.13,-13.22,;6.79,-12.46,;5.47,-13.25,;4.12,-12.5,;9.45,-12.43,;8.67,-11.08,;10.21,-11.08,;10.8,-13.17,;11.27,-14.64,;12.81,-14.64,;13.29,-13.17,;12.04,-12.27,;12.04,-10.73,;10.7,-9.96,;13.37,-9.95,;14.71,-10.72,;15.9,-9.45,;17.23,-9.94,;18.63,-9.59,;18.64,-8.06,;17.24,-7.48,;15.89,-7.96,;16.2,-8.72,;16.21,-10.3,;17.61,-10.87,)| Show InChI InChI=1S/C20H24Cl2N2O3S2/c21-16-2-1-15(10-17(16)22)29(26,27)24-3-4-28-20(24)19(25)23-18-13-6-11-5-12(8-13)9-14(18)7-11/h1-2,10-14,18,20H,3-9H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334709

(3-(3-Fluoro-benzenesulfonyl)-thiazolidine-2-carbox...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)C1SCCN1S(=O)(=O)c1cccc(F)c1)C(C3)C2 |TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(54.1,-20.21,;53.28,-18.91,;54,-17.55,;51.74,-18.97,;51.75,-17.44,;50.36,-16.86,;49.32,-18.1,;49.32,-19.68,;50.73,-20.25,;47.82,-20.1,;46.49,-19.33,;45.16,-20.11,;43.82,-19.34,;45.16,-21.65,;46.41,-22.55,;45.93,-24.02,;44.39,-24.02,;43.92,-22.55,;42.57,-21.81,;41.79,-20.46,;43.33,-20.46,;41.25,-22.6,;41.28,-24.13,;39.96,-24.92,;38.61,-24.17,;38.59,-22.63,;37.24,-21.88,;39.91,-21.84,;49.02,-18.83,;49.01,-17.34,;50.35,-19.32,)| Show InChI InChI=1S/C21H26FN3O4S2/c22-15-2-1-3-16(8-15)31(28,29)25-4-5-30-19(25)18(26)24-17-13-6-12-7-14(17)11-21(9-12,10-13)20(23)27/h1-3,8,12-14,17,19H,4-7,9-11H2,(H2,23,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334710
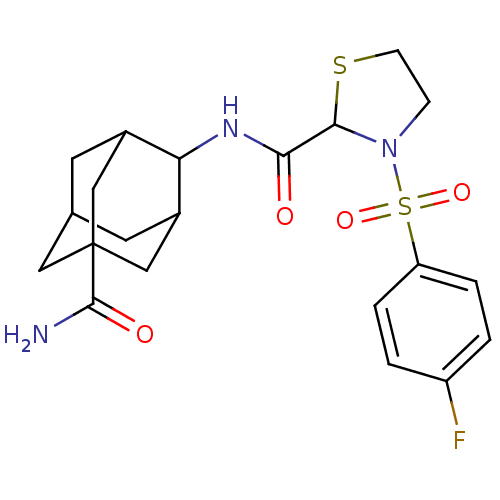
(3-(4-Fluoro-benzenesulfonyl)-thiazolidine-2-carbox...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)C1SCCN1S(=O)(=O)c1ccc(F)cc1)C(C3)C2 |TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(54.57,-13.07,;53.75,-11.77,;54.47,-10.41,;52.21,-11.83,;52.22,-10.3,;50.83,-9.72,;49.79,-10.96,;49.79,-12.54,;51.2,-13.11,;48.29,-12.96,;46.95,-12.19,;45.62,-12.97,;44.29,-12.2,;45.62,-14.51,;46.87,-15.41,;46.39,-16.88,;44.85,-16.88,;44.38,-15.41,;43.04,-14.67,;42.26,-13.32,;43.8,-13.32,;41.71,-15.46,;40.37,-14.7,;39.05,-15.49,;39.08,-17.03,;37.75,-17.82,;40.43,-17.78,;41.75,-16.99,;49.49,-11.69,;49.48,-10.2,;50.81,-12.18,)| Show InChI InChI=1S/C21H26FN3O4S2/c22-15-1-3-16(4-2-15)31(28,29)25-5-6-30-19(25)18(26)24-17-13-7-12-8-14(17)11-21(9-12,10-13)20(23)27/h1-4,12-14,17,19H,5-11H2,(H2,23,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334711
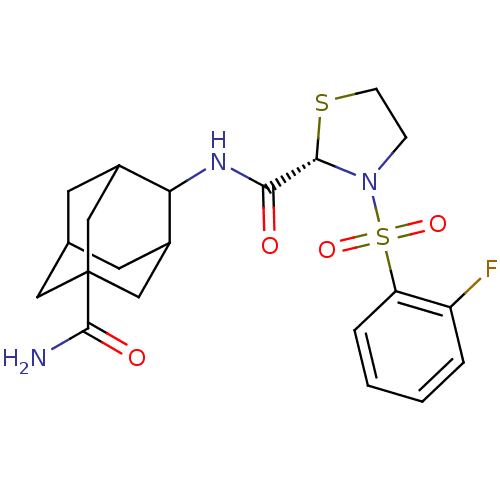
((S)-3-(2-Fluoro-benzenesulfonyl)-thiazolidine-2-ca...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)[C@@H]1SCCN1S(=O)(=O)c1ccccc1F)C(C3)C2 |r,wD:13.13,TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(17.46,-12.91,;16.64,-11.61,;17.36,-10.24,;15.1,-11.67,;15.11,-10.14,;13.72,-9.56,;12.68,-10.79,;12.68,-12.38,;14.09,-12.94,;11.18,-12.8,;9.85,-12.03,;8.51,-12.8,;7.18,-12.03,;8.52,-14.34,;9.77,-15.25,;9.29,-16.71,;7.75,-16.71,;7.27,-15.25,;5.93,-14.5,;5.15,-13.16,;6.69,-13.16,;4.61,-15.29,;4.64,-16.82,;3.32,-17.62,;1.97,-16.87,;1.94,-15.33,;3.26,-14.54,;3.25,-13,;12.38,-11.52,;12.37,-10.04,;13.71,-12.01,)| Show InChI InChI=1S/C21H26FN3O4S2/c22-15-3-1-2-4-16(15)31(28,29)25-5-6-30-19(25)18(26)24-17-13-7-12-8-14(17)11-21(9-12,10-13)20(23)27/h1-4,12-14,17,19H,5-11H2,(H2,23,27)(H,24,26)/t12?,13?,14?,17?,19-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50081733
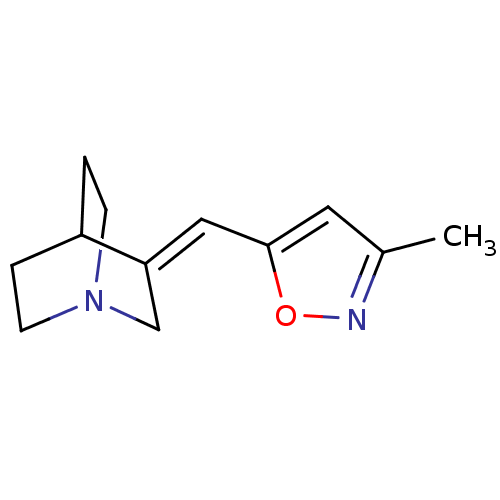
(3-(3-Methyl-isoxazol-5-ylmethylene)-1-aza-bicyclo[...)Show SMILES Cc1cc(\C=C2/CN3CCC2CC3)on1 |THB:4:5:11.12:9.8,(26.44,-10.99,;26.13,-9.47,;24.73,-8.83,;24.91,-7.3,;23.77,-6.26,;22.3,-6.71,;22.6,-8.11,;21.23,-7.48,;19.68,-8.13,;19.47,-6.75,;20.96,-6.11,;21.02,-4.47,;21.48,-5.59,;26.43,-6.99,;27.19,-8.35,)| Show InChI InChI=1S/C12H16N2O/c1-9-6-12(15-13-9)7-11-8-14-4-2-10(11)3-5-14/h6-7,10H,2-5,8H2,1H3/b11-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against nicotinic receptor in synaptic membrane fractions from rat cerebral cortices. |
Bioorg Med Chem Lett 9: 2795-800 (1999)
BindingDB Entry DOI: 10.7270/Q26H4GM1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334711

((S)-3-(2-Fluoro-benzenesulfonyl)-thiazolidine-2-ca...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)[C@@H]1SCCN1S(=O)(=O)c1ccccc1F)C(C3)C2 |r,wD:13.13,TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(17.46,-12.91,;16.64,-11.61,;17.36,-10.24,;15.1,-11.67,;15.11,-10.14,;13.72,-9.56,;12.68,-10.79,;12.68,-12.38,;14.09,-12.94,;11.18,-12.8,;9.85,-12.03,;8.51,-12.8,;7.18,-12.03,;8.52,-14.34,;9.77,-15.25,;9.29,-16.71,;7.75,-16.71,;7.27,-15.25,;5.93,-14.5,;5.15,-13.16,;6.69,-13.16,;4.61,-15.29,;4.64,-16.82,;3.32,-17.62,;1.97,-16.87,;1.94,-15.33,;3.26,-14.54,;3.25,-13,;12.38,-11.52,;12.37,-10.04,;13.71,-12.01,)| Show InChI InChI=1S/C21H26FN3O4S2/c22-15-3-1-2-4-16(15)31(28,29)25-5-6-30-19(25)18(26)24-17-13-7-12-8-14(17)11-21(9-12,10-13)20(23)27/h1-4,12-14,17,19H,5-11H2,(H2,23,27)(H,24,26)/t12?,13?,14?,17?,19-,21?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against nicotinic receptor in synaptic membrane fractions from rat cerebral cortices. |
Bioorg Med Chem Lett 9: 2795-800 (1999)
BindingDB Entry DOI: 10.7270/Q26H4GM1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334707
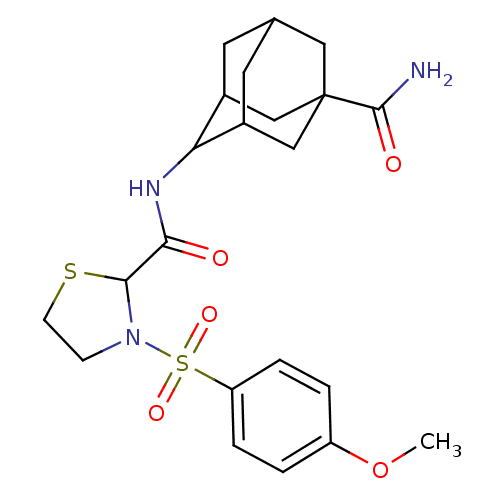
(3-(4-Methoxy-benzenesulfonyl)-thiazolidine-2-carbo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCSC1C(=O)NC1C2CC3CC1CC(C3)(C2)C(N)=O |TLB:18:19:25.28.26:21.22.23,18:19:23:25.26.27,28:26:19.20.21:23,THB:28:20:23:25.26.27,27:26:19:21.22.23,27:22:19:25.28.26,29:26:19:21.22.23,29:26:19.20.21:23,(37.63,-44.84,;38.98,-45.59,;40.3,-44.8,;40.27,-43.26,;41.59,-42.47,;42.94,-43.22,;42.97,-44.75,;41.65,-45.55,;44.26,-42.43,;43.48,-41.09,;45.02,-41.09,;45.6,-43.18,;46.08,-44.64,;47.62,-44.64,;48.09,-43.18,;46.85,-42.27,;46.84,-40.73,;45.51,-39.96,;48.18,-39.96,;49.51,-40.73,;51.01,-40.31,;51.01,-38.72,;52.05,-37.49,;50.7,-37.97,;50.71,-39.45,;52.04,-39.94,;53.43,-39.6,;53.44,-38.07,;52.42,-40.87,;54.97,-39.54,;55.79,-40.84,;55.69,-38.17,)| Show InChI InChI=1S/C22H29N3O5S2/c1-30-16-2-4-17(5-3-16)32(28,29)25-6-7-31-20(25)19(26)24-18-14-8-13-9-15(18)12-22(10-13,11-14)21(23)27/h2-5,13-15,18,20H,6-12H2,1H3,(H2,23,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334698
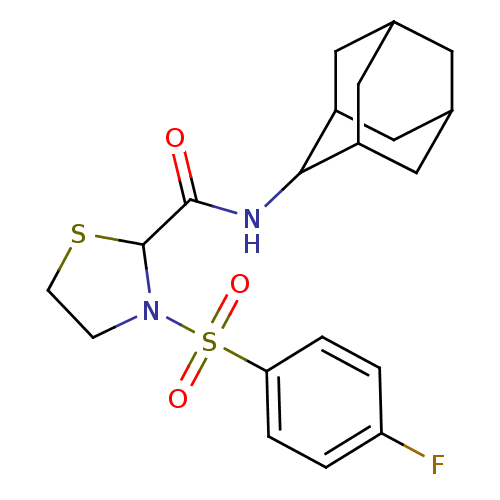
(3-(4-Fluoro-benzenesulfonyl)-thiazolidine-2-carbox...)Show SMILES Fc1ccc(cc1)S(=O)(=O)N1CCSC1C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:17:18:20.27.21:25.23.24,27:26:24:20.21.22,THB:17:18:24:20.21.22,27:21:18.26.25:24,22:21:18:25.23.24,22:23:18:20.27.21,(3.92,-15.58,;5.24,-14.79,;5.22,-13.25,;6.54,-12.46,;7.88,-13.22,;7.91,-14.75,;6.6,-15.54,;9.2,-12.43,;8.42,-11.08,;9.96,-11.08,;10.55,-13.17,;11.02,-14.64,;12.56,-14.64,;13.04,-13.17,;11.79,-12.27,;11.79,-10.73,;10.45,-9.96,;13.12,-9.95,;14.46,-10.72,;15.65,-9.45,;16.98,-9.94,;18.38,-9.59,;18.39,-8.06,;16.99,-7.48,;15.64,-7.96,;15.95,-8.72,;15.96,-10.3,;17.36,-10.87,)| Show InChI InChI=1S/C20H25FN2O3S2/c21-16-1-3-17(4-2-16)28(25,26)23-5-6-27-20(23)19(24)22-18-14-8-12-7-13(10-14)11-15(18)9-12/h1-4,12-15,18,20H,5-11H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334695
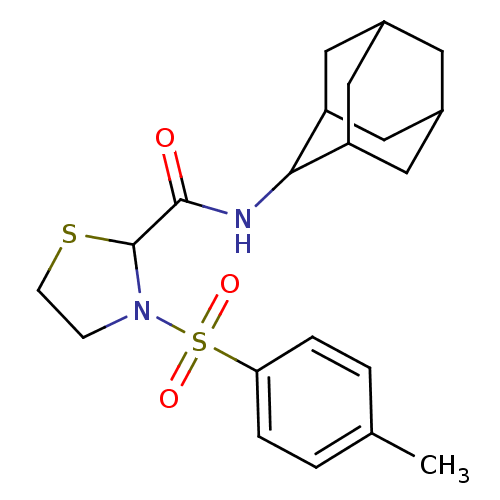
(3-(Toluene-4-sulfonyl)-thiazolidine-2-carboxylic a...)Show SMILES Cc1ccc(cc1)S(=O)(=O)N1CCSC1C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:17:18:20.27.21:25.23.24,27:26:24:20.21.22,THB:17:18:24:20.21.22,27:21:18.26.25:24,22:21:18:25.23.24,22:23:18:20.27.21,(-10.48,-18.33,;-9.15,-17.54,;-9.18,-15.99,;-7.86,-15.21,;-6.52,-15.96,;-6.48,-17.49,;-7.8,-18.29,;-5.19,-15.17,;-5.97,-13.83,;-4.43,-13.83,;-3.85,-15.92,;-3.38,-17.38,;-1.84,-17.38,;-1.36,-15.92,;-2.61,-15.01,;-2.61,-13.47,;-3.94,-12.7,;-1.28,-12.7,;.06,-13.47,;1.26,-12.19,;2.58,-12.68,;3.98,-12.33,;3.99,-10.81,;2.6,-10.23,;1.25,-10.71,;1.56,-11.46,;1.56,-13.05,;2.97,-13.61,)| Show InChI InChI=1S/C21H28N2O3S2/c1-13-2-4-18(5-3-13)28(25,26)23-6-7-27-21(23)20(24)22-19-16-9-14-8-15(11-16)12-17(19)10-14/h2-5,14-17,19,21H,6-12H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334689
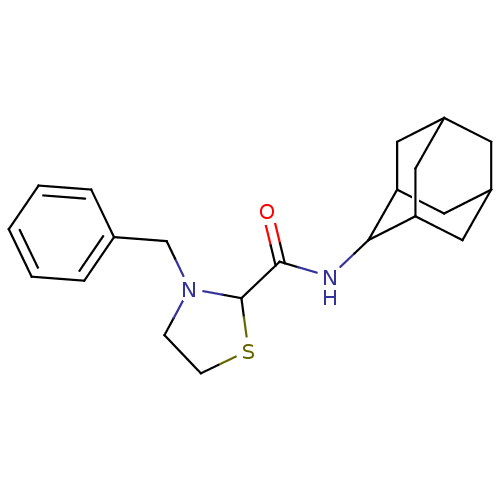
(3-Benzyl-thiazolidine-2-carboxylic acid adamantan-...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)C1SCCN1Cc1ccccc1 |TLB:2:3:5.12.6:10.8.9,12:11:9:5.6.7,THB:2:3:9:5.6.7,12:6:3.11.10:9,7:6:3:10.8.9,7:8:3:5.12.6,(8.22,-4.07,;9.56,-4.84,;10.89,-4.07,;12.22,-4.83,;13.42,-3.56,;14.75,-4.05,;16.14,-3.7,;16.16,-2.17,;14.76,-1.59,;13.41,-2.07,;13.72,-2.83,;13.73,-4.41,;15.13,-4.98,;9.56,-6.38,;10.81,-7.29,;10.33,-8.75,;8.79,-8.75,;8.32,-7.29,;6.97,-6.54,;5.65,-7.33,;4.31,-6.57,;2.99,-7.36,;3.01,-8.9,;4.36,-9.65,;5.68,-8.86,)| Show InChI InChI=1S/C21H28N2OS/c24-20(21-23(6-7-25-21)13-14-4-2-1-3-5-14)22-19-17-9-15-8-16(11-17)12-18(19)10-15/h1-5,15-19,21H,6-13H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM91658
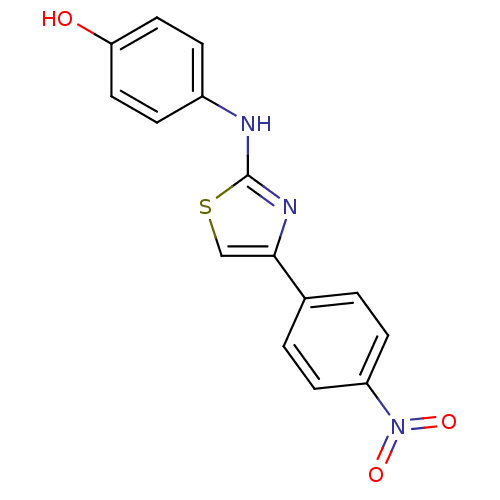
(N-aryl-4-aryl-1,3-thiazole-2-amine, 6)Show InChI InChI=1S/C15H11N3O3S/c19-13-7-3-11(4-8-13)16-15-17-14(9-22-15)10-1-5-12(6-2-10)18(20)21/h1-9,19H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Korea Research Institute of Chemical Technology
| Assay Description
5-LOX enzyme assay was carried out with some modifications of ferric oxidation of xylenol orange (FOX) assay, which is based on the complex formation... |
Chem Biol Drug Des 80: 89-98 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01371.x
BindingDB Entry DOI: 10.7270/Q21G0JW1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50309943
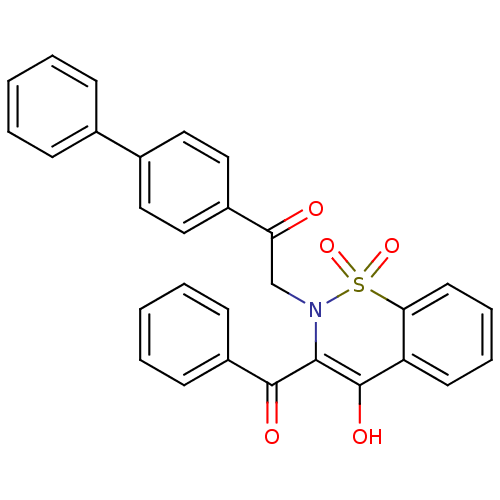
(2-(3-Benzoyl-4-hydroxy-1,1-dioxo-1H-1lambda*6*-ben...)Show SMILES OC1=C(N(CC(=O)c2ccc(cc2)-c2ccccc2)S(=O)(=O)c2ccccc12)C(=O)c1ccccc1 |t:1| Show InChI InChI=1S/C29H21NO5S/c31-25(22-17-15-21(16-18-22)20-9-3-1-4-10-20)19-30-27(28(32)23-11-5-2-6-12-23)29(33)24-13-7-8-14-26(24)36(30,34)35/h1-18,33H,19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes by HTRF cortisol assay |
Bioorg Med Chem Lett 20: 1065-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.035
BindingDB Entry DOI: 10.7270/Q26M36XD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50309944
(2-(3-Benzoyl-4-hydroxy-1,1-dioxo-1H-1lambda*6*-ben...)Show SMILES OC1=C(N(CC(=O)c2ccc(cc2)[N+]([O-])=O)S(=O)(=O)c2ccccc12)C(=O)c1ccccc1 |t:1| Show InChI InChI=1S/C23H16N2O7S/c26-19(15-10-12-17(13-11-15)25(29)30)14-24-21(22(27)16-6-2-1-3-7-16)23(28)18-8-4-5-9-20(18)33(24,31)32/h1-13,28H,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes by HTRF cortisol assay |
Bioorg Med Chem Lett 20: 1065-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.035
BindingDB Entry DOI: 10.7270/Q26M36XD |
More data for this
Ligand-Target Pair | |
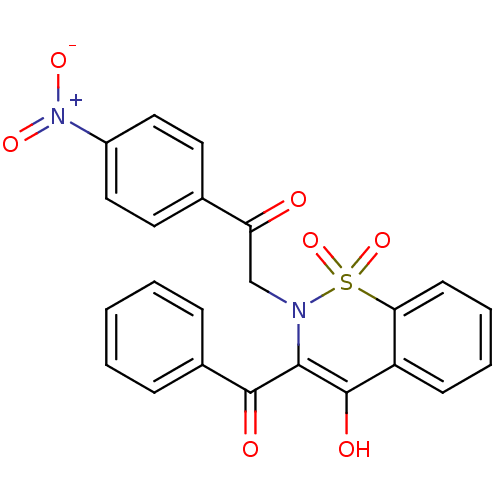
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50309945
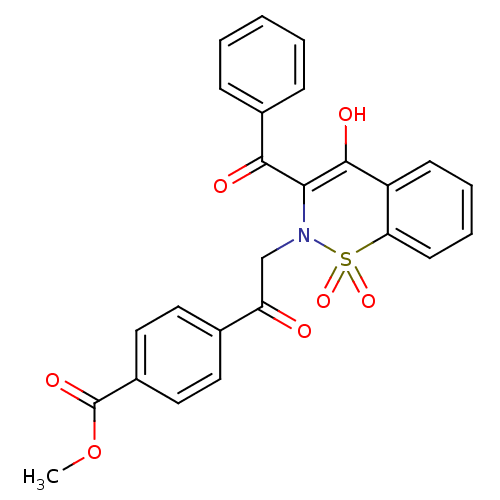
(4-[2-(3-Benzoyl-4-hydroxy-1,1-dioxo-1H-1lambda*6*-...)Show SMILES COC(=O)c1ccc(cc1)C(=O)CN1C(C(=O)c2ccccc2)=C(O)c2ccccc2S1(=O)=O |t:24| Show InChI InChI=1S/C25H19NO7S/c1-33-25(30)18-13-11-16(12-14-18)20(27)15-26-22(23(28)17-7-3-2-4-8-17)24(29)19-9-5-6-10-21(19)34(26,31)32/h2-14,29H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes by HTRF cortisol assay |
Bioorg Med Chem Lett 20: 1065-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.035
BindingDB Entry DOI: 10.7270/Q26M36XD |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50309934
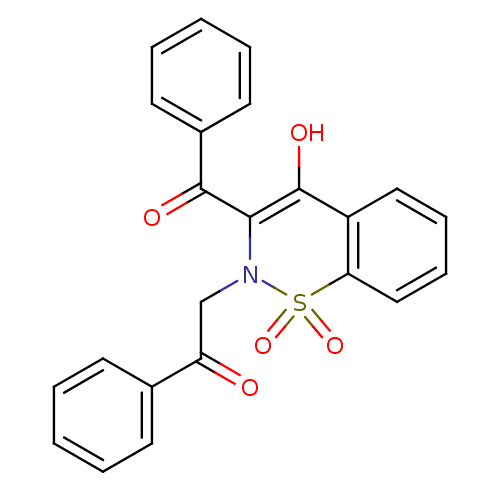
(2-(3-Benzoyl-4-hydroxy-1,1-dioxo-1H-1lambda*6*-ben...)Show SMILES OC1=C(N(CC(=O)c2ccccc2)S(=O)(=O)c2ccccc12)C(=O)c1ccccc1 |t:1| Show InChI InChI=1S/C23H17NO5S/c25-19(16-9-3-1-4-10-16)15-24-21(22(26)17-11-5-2-6-12-17)23(27)18-13-7-8-14-20(18)30(24,28)29/h1-14,27H,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes by HTRF cortisol assay |
Bioorg Med Chem Lett 20: 1065-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.035
BindingDB Entry DOI: 10.7270/Q26M36XD |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50312869

(4-(4-(4-chlorophenyl)thiazol-2-ylamino)phenol | CH...)Show InChI InChI=1S/C15H11ClN2OS/c16-11-3-1-10(2-4-11)14-9-20-15(18-14)17-12-5-7-13(19)8-6-12/h1-9,19H,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Korea Research Institute of Chemical Technology
| Assay Description
5-LOX enzyme assay was carried out with some modifications of ferric oxidation of xylenol orange (FOX) assay, which is based on the complex formation... |
Chem Biol Drug Des 80: 89-98 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01371.x
BindingDB Entry DOI: 10.7270/Q21G0JW1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334713
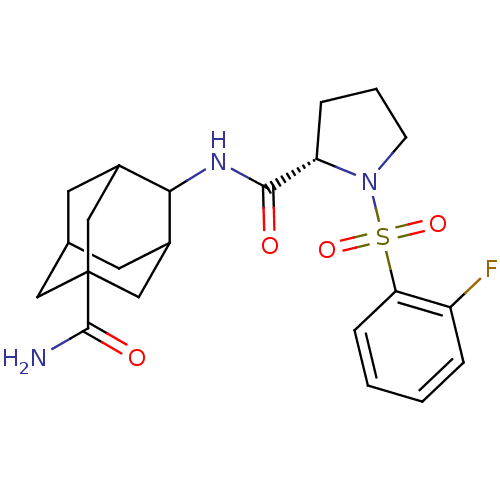
((S)-1-(2-Fluoro-benzenesulfonyl)-pyrrolidine-2-car...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)[C@@H]1CCCN1S(=O)(=O)c1ccccc1F)C(C3)C2 |r,wD:13.13,TLB:10:9:30.8.3:6.5.29,10:9:29:30.3.4,8:3:9.7.6:29,THB:8:7:29:30.3.4,4:3:9:6.5.29,4:5:9:30.8.3,1:3:9:6.5.29,1:3:9.7.6:29,(29.23,.08,;28.41,1.38,;29.13,2.75,;26.87,1.32,;26.88,2.85,;25.49,3.43,;24.45,2.19,;24.45,.61,;25.86,.05,;22.95,.19,;21.61,.96,;20.28,.19,;18.95,.95,;20.28,-1.35,;21.53,-2.26,;21.05,-3.73,;19.51,-3.73,;19.04,-2.26,;17.7,-1.51,;16.92,-.17,;18.46,-.17,;16.37,-2.3,;16.41,-3.83,;15.09,-4.63,;13.74,-3.88,;13.71,-2.34,;15.03,-1.55,;15.01,-.01,;24.15,1.47,;24.14,2.95,;25.47,.98,)| Show InChI InChI=1S/C22H28FN3O4S/c23-16-4-1-2-6-18(16)31(29,30)26-7-3-5-17(26)20(27)25-19-14-8-13-9-15(19)12-22(10-13,11-14)21(24)28/h1-2,4,6,13-15,17,19H,3,5,7-12H2,(H2,24,28)(H,25,27)/t13?,14?,15?,17-,19?,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50309946
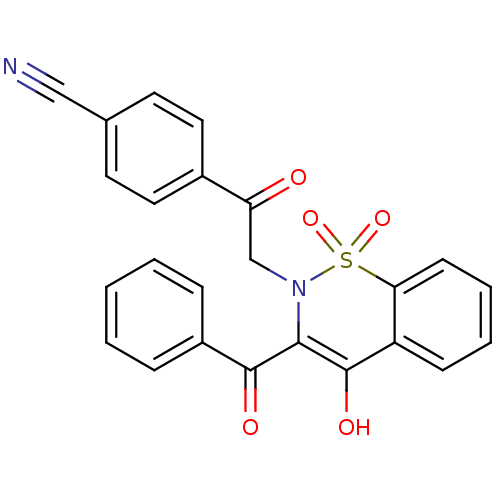
(4-[2-(3-Benzoyl-4-hydroxy-1,1-dioxo-1H-1lambda*6*-...)Show SMILES OC1=C(N(CC(=O)c2ccc(cc2)C#N)S(=O)(=O)c2ccccc12)C(=O)c1ccccc1 |t:1| Show InChI InChI=1S/C24H16N2O5S/c25-14-16-10-12-17(13-11-16)20(27)15-26-22(23(28)18-6-2-1-3-7-18)24(29)19-8-4-5-9-21(19)32(26,30)31/h1-13,29H,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of 11beta-HSD1 in human microsomes by HTRF cortisol assay |
Bioorg Med Chem Lett 20: 1065-9 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.035
BindingDB Entry DOI: 10.7270/Q26M36XD |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 2
(Homo sapiens (Human)) | BDBM50256618
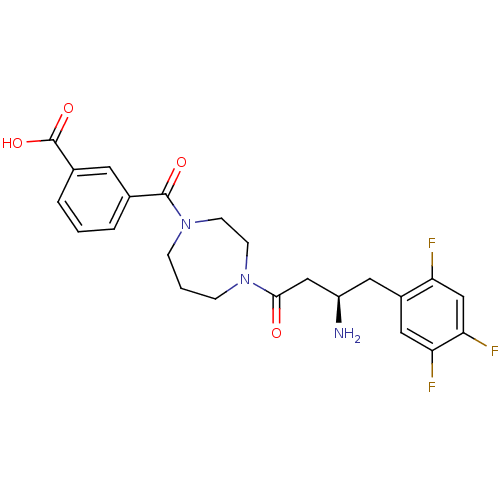
((R)-3-(4-(3-amino-4-(2,4,5-trifluorophenyl)butanoy...)Show SMILES N[C@@H](CC(=O)N1CCCN(CC1)C(=O)c1cccc(c1)C(O)=O)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C23H24F3N3O4/c24-18-13-20(26)19(25)11-16(18)10-17(27)12-21(30)28-5-2-6-29(8-7-28)22(31)14-3-1-4-15(9-14)23(32)33/h1,3-4,9,11,13,17H,2,5-8,10,12,27H2,(H,32,33)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of DPP2 (unknown origin) |
Bioorg Med Chem Lett 18: 6525-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.076
BindingDB Entry DOI: 10.7270/Q25D8RQV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50256618

((R)-3-(4-(3-amino-4-(2,4,5-trifluorophenyl)butanoy...)Show SMILES N[C@@H](CC(=O)N1CCCN(CC1)C(=O)c1cccc(c1)C(O)=O)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C23H24F3N3O4/c24-18-13-20(26)19(25)11-16(18)10-17(27)12-21(30)28-5-2-6-29(8-7-28)22(31)14-3-1-4-15(9-14)23(32)33/h1,3-4,9,11,13,17H,2,5-8,10,12,27H2,(H,32,33)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 18: 6525-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.076
BindingDB Entry DOI: 10.7270/Q25D8RQV |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 2622-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.111
BindingDB Entry DOI: 10.7270/Q21V5FS4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50206821

((R)-3-amino-1-(2-benzoylpiperazin-1-yl)-4-(2,4,5-t...)Show SMILES N[C@@H](CC(=O)N1CCCCN1C(=O)c1ccccc1)Cc1cc(F)c(F)cc1F Show InChI InChI=1S/C21H22F3N3O2/c22-17-13-19(24)18(23)11-15(17)10-16(25)12-20(28)26-8-4-5-9-27(26)21(29)14-6-2-1-3-7-14/h1-3,6-7,11,13,16H,4-5,8-10,12,25H2/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 2622-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.111
BindingDB Entry DOI: 10.7270/Q21V5FS4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334696
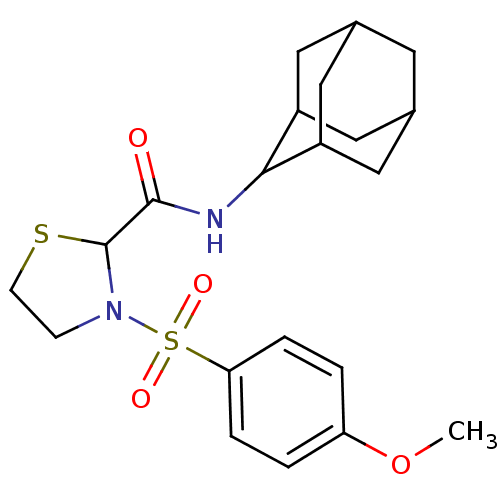
(3-(4-Methoxy-benzenesulfonyl)-thiazolidine-2-carbo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCSC1C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:18:19:21.28.22:26.24.25,28:27:25:21.22.23,THB:18:19:25:21.22.23,28:22:19.27.26:25,23:22:19:26.24.25,23:24:19:21.28.22,(2.68,-17,;4.02,-17.75,;5.34,-16.95,;5.32,-15.41,;6.64,-14.62,;7.98,-15.38,;8.01,-16.91,;6.7,-17.7,;9.3,-14.59,;8.52,-13.25,;10.06,-13.25,;10.65,-15.34,;11.12,-16.8,;12.66,-16.8,;13.14,-15.34,;11.89,-14.43,;11.89,-12.89,;10.55,-12.12,;13.22,-12.12,;14.56,-12.88,;15.75,-11.61,;17.08,-12.1,;18.48,-11.75,;18.49,-10.22,;17.09,-9.64,;15.75,-10.12,;16.05,-10.88,;16.06,-12.46,;17.46,-13.03,)| Show InChI InChI=1S/C21H28N2O4S2/c1-27-17-2-4-18(5-3-17)29(25,26)23-6-7-28-21(23)20(24)22-19-15-9-13-8-14(11-15)12-16(19)10-13/h2-5,13-16,19,21H,6-12H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50334707

(3-(4-Methoxy-benzenesulfonyl)-thiazolidine-2-carbo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCSC1C(=O)NC1C2CC3CC1CC(C3)(C2)C(N)=O |TLB:18:19:25.28.26:21.22.23,18:19:23:25.26.27,28:26:19.20.21:23,THB:28:20:23:25.26.27,27:26:19:21.22.23,27:22:19:25.28.26,29:26:19:21.22.23,29:26:19.20.21:23,(37.63,-44.84,;38.98,-45.59,;40.3,-44.8,;40.27,-43.26,;41.59,-42.47,;42.94,-43.22,;42.97,-44.75,;41.65,-45.55,;44.26,-42.43,;43.48,-41.09,;45.02,-41.09,;45.6,-43.18,;46.08,-44.64,;47.62,-44.64,;48.09,-43.18,;46.85,-42.27,;46.84,-40.73,;45.51,-39.96,;48.18,-39.96,;49.51,-40.73,;51.01,-40.31,;51.01,-38.72,;52.05,-37.49,;50.7,-37.97,;50.71,-39.45,;52.04,-39.94,;53.43,-39.6,;53.44,-38.07,;52.42,-40.87,;54.97,-39.54,;55.79,-40.84,;55.69,-38.17,)| Show InChI InChI=1S/C22H29N3O5S2/c1-30-16-2-4-17(5-3-16)32(28,29)25-6-7-31-20(25)19(26)24-18-14-8-13-9-15(18)12-22(10-13,11-14)21(23)27/h2-5,13-15,18,20H,6-12H2,1H3,(H2,23,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM91673
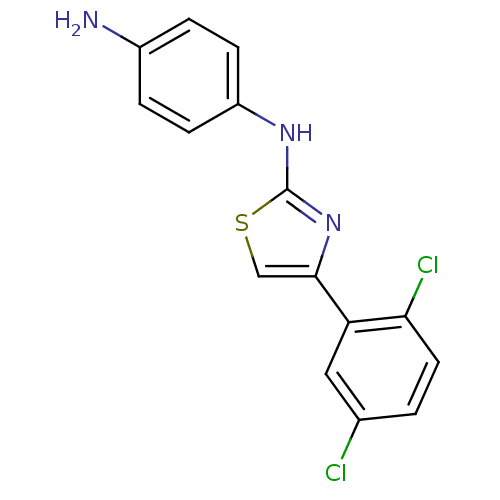
(N-aryl-4-aryl-1,3-thiazole-2-amine, 31)Show InChI InChI=1S/C15H11Cl2N3S/c16-9-1-6-13(17)12(7-9)14-8-21-15(20-14)19-11-4-2-10(18)3-5-11/h1-8H,18H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Korea Research Institute of Chemical Technology
| Assay Description
5-LOX enzyme assay was carried out with some modifications of ferric oxidation of xylenol orange (FOX) assay, which is based on the complex formation... |
Chem Biol Drug Des 80: 89-98 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01371.x
BindingDB Entry DOI: 10.7270/Q21G0JW1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Rattus norvegicus (rat)) | BDBM50150870
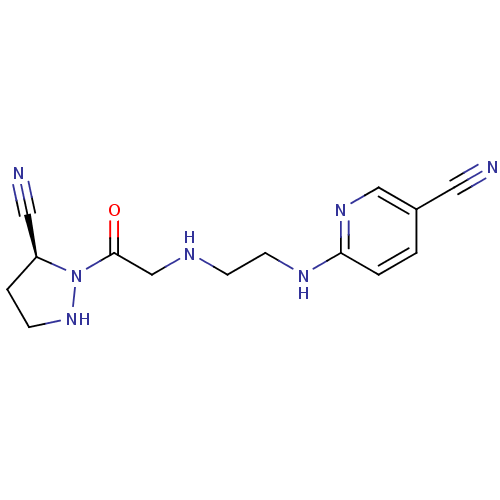
(6-{2-[2-((S)-5-Cyano-pyrazolidin-1-yl)-2-oxo-ethyl...)Show InChI InChI=1S/C14H17N7O/c15-7-11-1-2-13(19-9-11)18-6-5-17-10-14(22)21-12(8-16)3-4-20-21/h1-2,9,12,17,20H,3-6,10H2,(H,18,19)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rat dipeptidyl peptidase IV |
Bioorg Med Chem Lett 14: 4461-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.046
BindingDB Entry DOI: 10.7270/Q2J9674N |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM91665
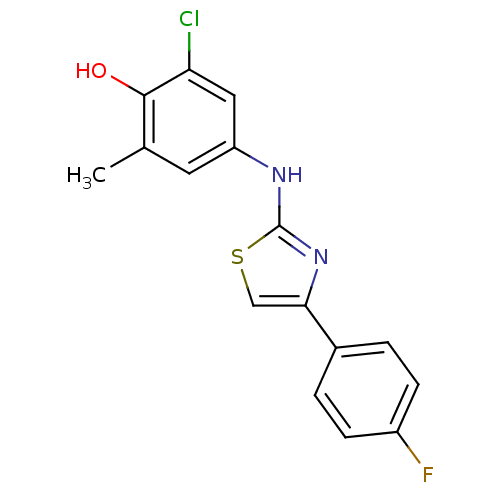
(N-aryl-4-aryl-1,3-thiazole-2-amine, 15)Show InChI InChI=1S/C16H12ClFN2OS/c1-9-6-12(7-13(17)15(9)21)19-16-20-14(8-22-16)10-2-4-11(18)5-3-10/h2-8,21H,1H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Korea Research Institute of Chemical Technology
| Assay Description
5-LOX enzyme assay was carried out with some modifications of ferric oxidation of xylenol orange (FOX) assay, which is based on the complex formation... |
Chem Biol Drug Des 80: 89-98 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01371.x
BindingDB Entry DOI: 10.7270/Q21G0JW1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334692
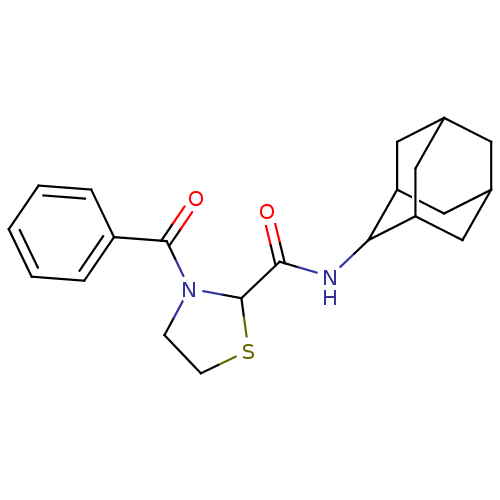
(3-Benzoyl-thiazolidine-2-carboxylic acid adamantan...)Show SMILES O=C(NC1C2CC3CC(C2)CC1C3)C1SCCN1C(=O)c1ccccc1 |TLB:2:3:5.12.6:10.8.9,12:11:9:5.6.7,THB:2:3:9:5.6.7,12:6:3.11.10:9,7:6:3:10.8.9,7:8:3:5.12.6,(21.33,4.44,;22.66,3.67,;23.99,4.44,;25.33,3.68,;26.53,4.95,;27.85,4.46,;29.25,4.81,;29.26,6.34,;27.87,6.91,;26.52,6.44,;26.83,5.68,;26.83,4.1,;28.24,3.53,;22.66,2.13,;23.91,1.22,;23.43,-.24,;21.89,-.24,;21.42,1.22,;20.08,1.98,;20.07,3.52,;18.75,1.2,;17.42,1.97,;16.09,1.19,;16.1,-.35,;17.45,-1.11,;18.77,-.33,)| Show InChI InChI=1S/C21H26N2O2S/c24-19(22-18-16-9-13-8-14(11-16)12-17(18)10-13)21-23(6-7-26-21)20(25)15-4-2-1-3-5-15/h1-5,13-14,16-18,21H,6-12H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM91659
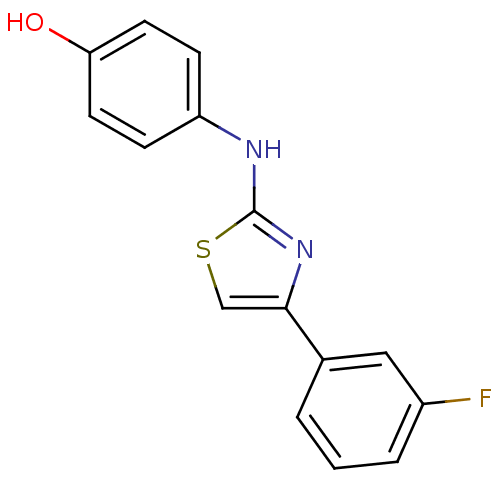
(N-aryl-4-aryl-1,3-thiazole-2-amine, 8)Show InChI InChI=1S/C15H11FN2OS/c16-11-3-1-2-10(8-11)14-9-20-15(18-14)17-12-4-6-13(19)7-5-12/h1-9,19H,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Korea Research Institute of Chemical Technology
| Assay Description
5-LOX enzyme assay was carried out with some modifications of ferric oxidation of xylenol orange (FOX) assay, which is based on the complex formation... |
Chem Biol Drug Des 80: 89-98 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01371.x
BindingDB Entry DOI: 10.7270/Q21G0JW1 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50334705

(3-Benzenesulfonyl-thiazolidine-2-carboxylic acid(5...)Show SMILES NC(=O)C12CC3CC(C1)C(NC(=O)C1SCCN1S(=O)(=O)c1ccccc1)C(C3)C2 |TLB:10:9:29.8.3:6.5.28,10:9:28:29.3.4,8:3:9.7.6:28,THB:8:7:28:29.3.4,4:3:9:6.5.28,4:5:9:29.8.3,1:3:9:6.5.28,1:3:9.7.6:28,(22.28,-41.5,;21.46,-40.2,;22.17,-38.83,;19.92,-40.26,;19.93,-38.73,;18.53,-38.15,;17.49,-39.39,;17.5,-40.97,;18.9,-41.53,;16,-41.39,;14.66,-40.62,;13.33,-41.39,;11.99,-40.63,;13.33,-42.93,;14.58,-43.84,;14.1,-45.31,;12.56,-45.31,;12.09,-43.84,;10.74,-43.09,;9.96,-41.75,;11.5,-41.75,;9.42,-43.88,;8.08,-43.13,;6.76,-43.92,;6.78,-45.46,;8.14,-46.21,;9.45,-45.41,;17.19,-40.11,;17.18,-38.63,;18.52,-40.6,)| Show InChI InChI=1S/C21H27N3O4S2/c22-20(26)21-10-13-8-14(11-21)17(15(9-13)12-21)23-18(25)19-24(6-7-29-19)30(27,28)16-4-2-1-3-5-16/h1-5,13-15,17,19H,6-12H2,(H2,22,26)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 expressed in CHO cells after 3 hrs by HTRF cortisol assay |
Bioorg Med Chem Lett 21: 435-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.123
BindingDB Entry DOI: 10.7270/Q2FJ2H1K |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50256619
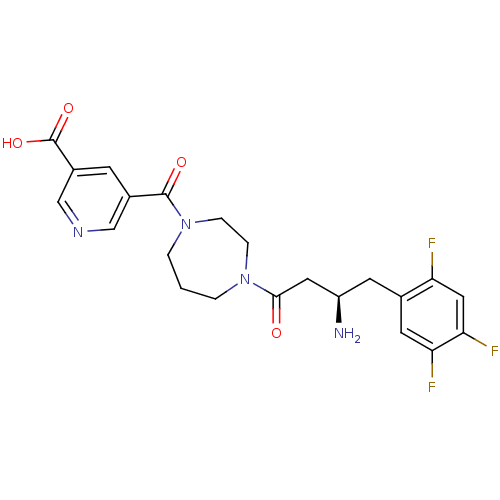
((R)-5-(4-(3-amino-4-(2,4,5-trifluorophenyl)butanoy...)Show SMILES N[C@@H](CC(=O)N1CCCN(CC1)C(=O)c1cncc(c1)C(O)=O)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C22H23F3N4O4/c23-17-10-19(25)18(24)8-13(17)7-16(26)9-20(30)28-2-1-3-29(5-4-28)21(31)14-6-15(22(32)33)12-27-11-14/h6,8,10-12,16H,1-5,7,9,26H2,(H,32,33)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 (unknown origin) |
Bioorg Med Chem Lett 18: 6525-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.076
BindingDB Entry DOI: 10.7270/Q25D8RQV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data