

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50241203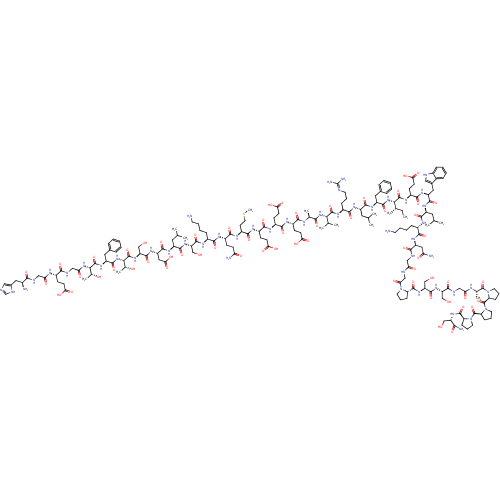 (CHEMBL414357 | HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGS...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of GLP-1-red from human GLP-1R expressed in CHO-K1 cells by fluorescent competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50557258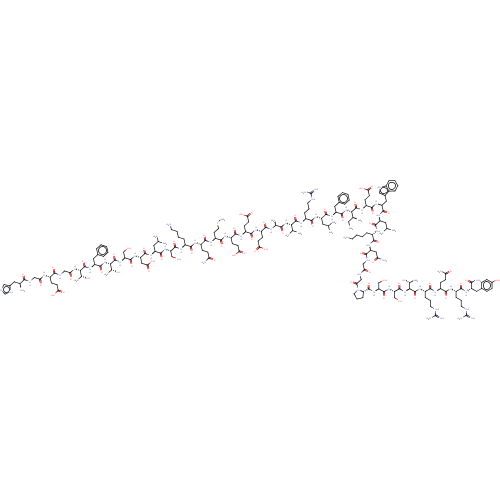 (CHEMBL4796216) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of GLP-1-red from human GLP-1R expressed in CHO-K1 cells by fluorescent competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50557264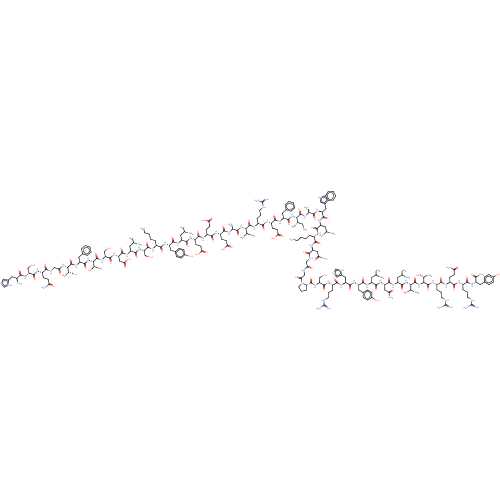 (CHEMBL4751466) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY2R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50466532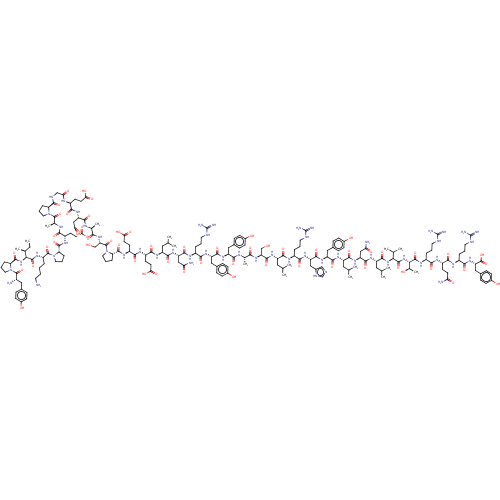 (CHEMBL4281479) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY1R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50197025 (CHEMBL439904 | PYY(3-36)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY2R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50557262 (CHEMBL4759334) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY2R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50557263 (CHEMBL4752195) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY2R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50557264 (CHEMBL4751466) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY1R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50557261 (CHEMBL4749279) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of GLP-1-red from human GLP-1R expressed in CHO-K1 cells by fluorescent competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50557261 (CHEMBL4749279) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY2R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50557261 (CHEMBL4749279) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY1R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50557259 (CHEMBL4748874) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY2R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50557260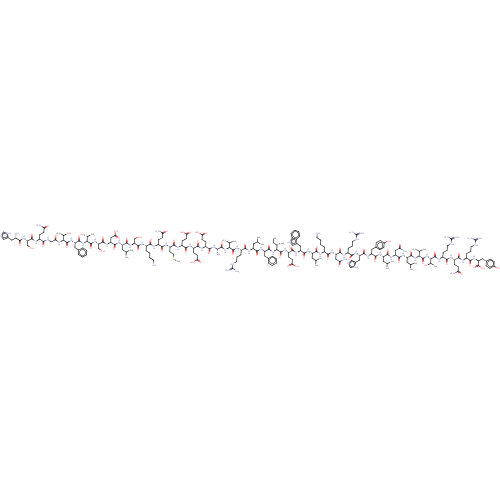 (CHEMBL4741000) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY2R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50557262 (CHEMBL4759334) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY1R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50557264 (CHEMBL4751466) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of GLP-1-red from human GLP-1R expressed in CHO-K1 cells by fluorescent competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50557258 (CHEMBL4796216) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human NPY2R expressed in HEK293 cells assessed as inhibition of adenosine-induced stimulation of cAMP accumulation by FRET assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425531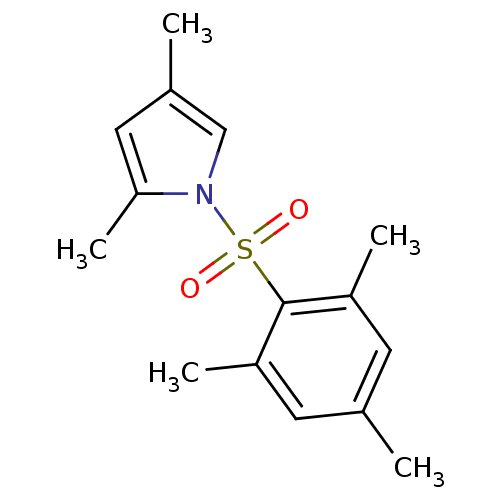 (CHEMBL2313646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50557260 (CHEMBL4741000) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 321 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of GLP-1-red from human GLP-1R expressed in CHO-K1 cells by fluorescent competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425541 (CHEMBL2313637) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425539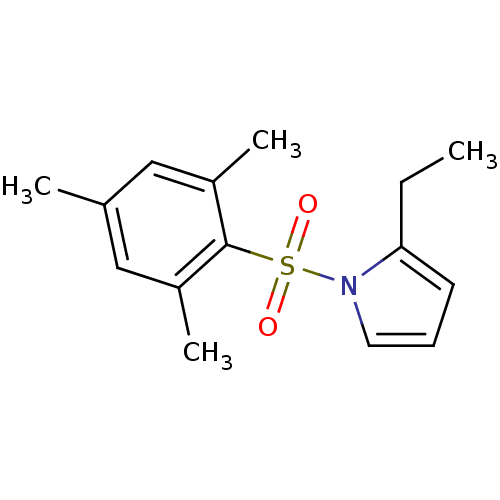 (CHEMBL2313639) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425555 (CHEMBL2313653) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425554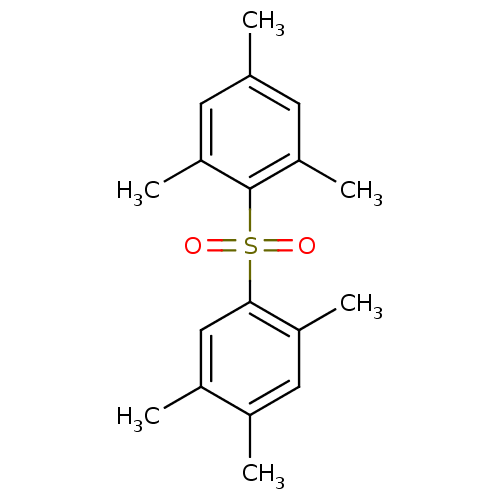 (CHEMBL2313654) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425542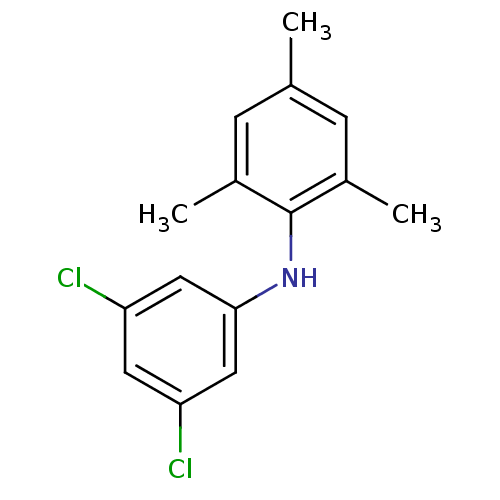 (CHEMBL2313636) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50557263 (CHEMBL4752195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of GLP-1-red from human GLP-1R expressed in CHO-K1 cells by fluorescent competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50557262 (CHEMBL4759334) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of GLP-1-red from human GLP-1R expressed in CHO-K1 cells by fluorescent competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01783 BindingDB Entry DOI: 10.7270/Q2H41W34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM89901 (1-(2,4,6-Trimethyl-benzenesulfonyl)-1H-indole | 1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425532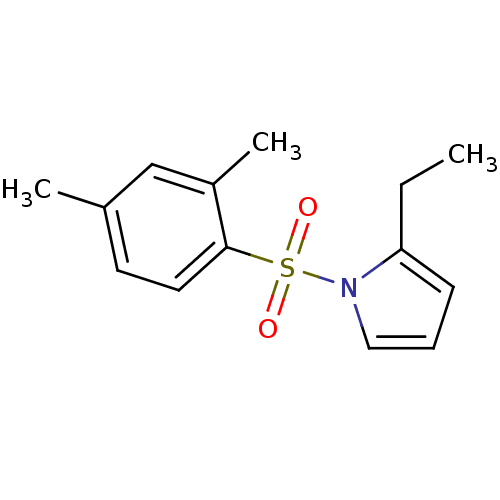 (CHEMBL2313645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425550 (CHEMBL2313658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425525 (CHEMBL2313652) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425543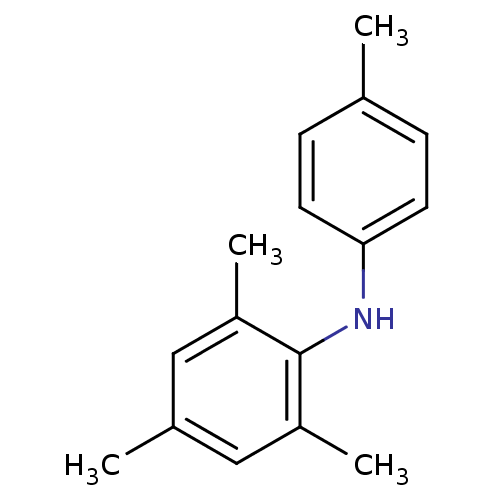 (CHEMBL2313635) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425528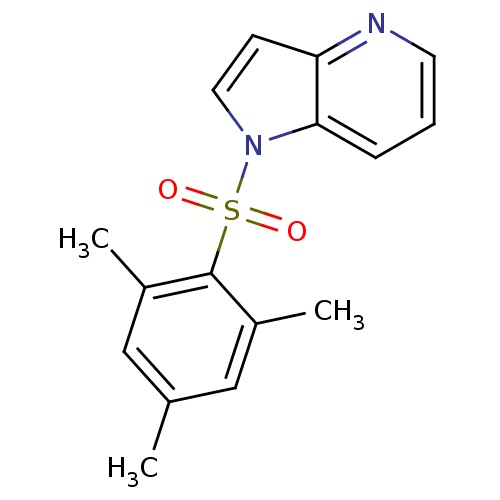 (CHEMBL2313649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425538 (CHEMBL2313640) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425551 (CHEMBL2313657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425533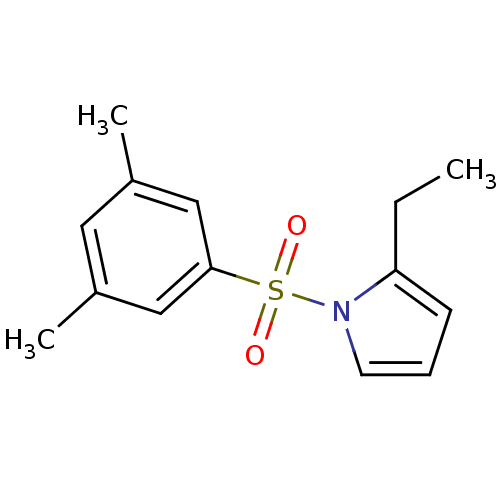 (CHEMBL2313644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425534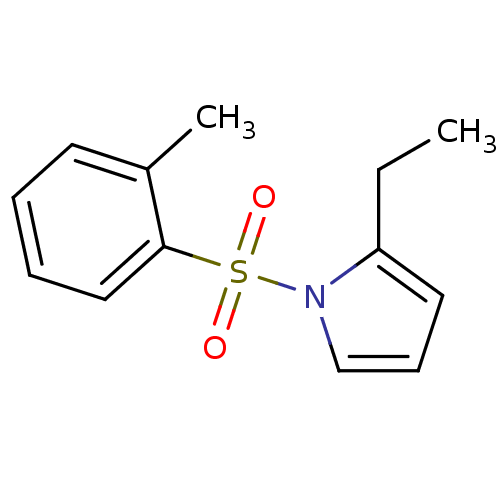 (CHEMBL2313643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425536 (CHEMBL2311560) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425537 (CHEMBL2313641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425527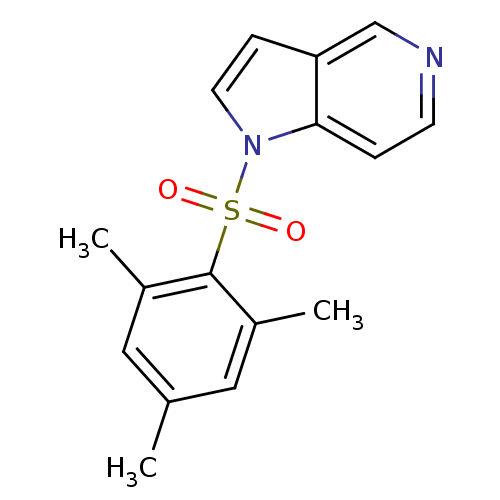 (CHEMBL2313650) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425529 (CHEMBL2313648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425526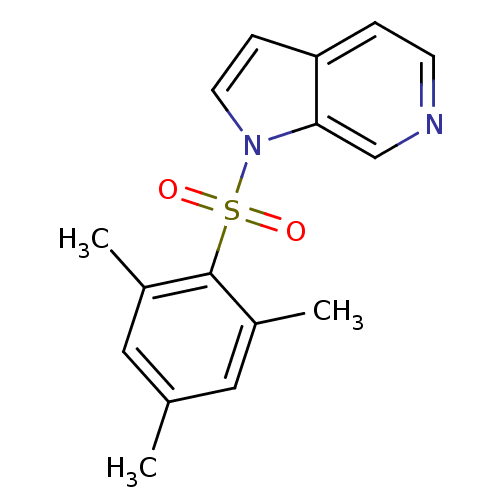 (CHEMBL2313651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425548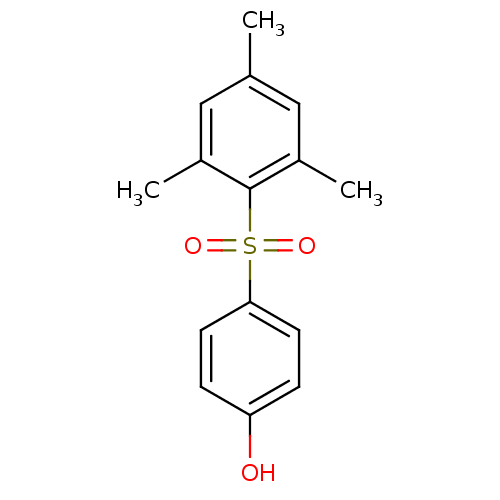 (4-(2,4,6-Trimethylbenzenesulfonyl)Phenol | CHEMBL2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50294336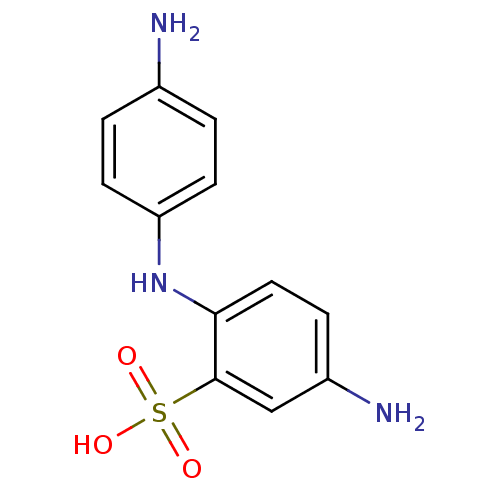 (5-amino-2-(4-aminophenylamino)benzenesulfonic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50384264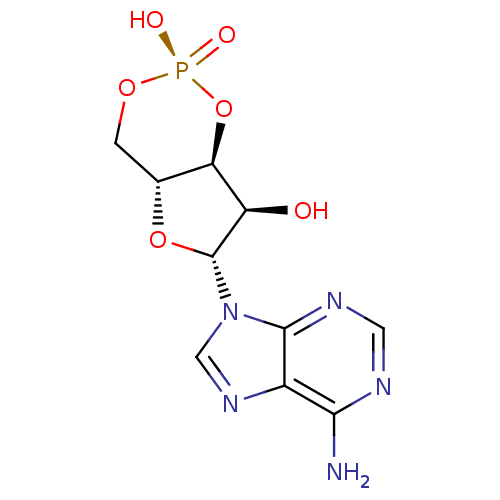 (CHEMBL316966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425544 (CHEMBL2313634) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425540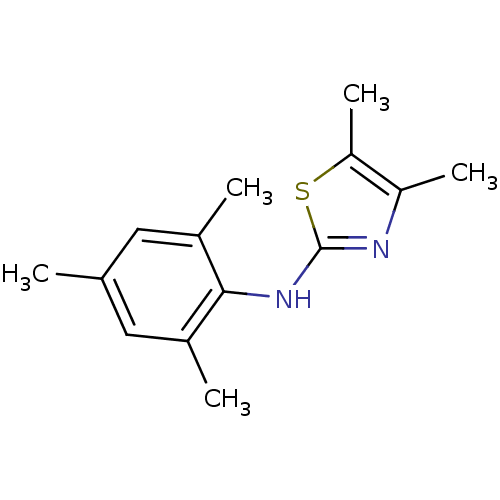 (CHEMBL2313638) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425535 (CHEMBL2313642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425530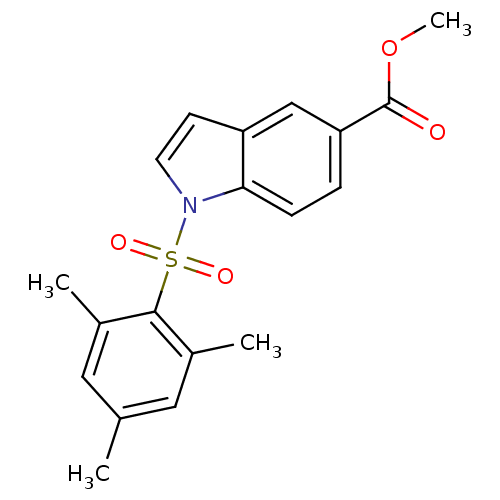 (CHEMBL2313647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425545 (CHEMBL2313633) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425552 (CHEMBL2313656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rap guanine nucleotide exchange factor 4 (Homo sapiens (Human)) | BDBM50425549 (CHEMBL2313659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Antagonist activity at recombinant EPAC2 (unknown origin) assessed 8-NBD-cAMP substrate fluorescence intensity by substrate competing assay | J Med Chem 56: 952-62 (2013) Article DOI: 10.1021/jm3014162 BindingDB Entry DOI: 10.7270/Q2D21ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 68 total ) | Next | Last >> |