

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427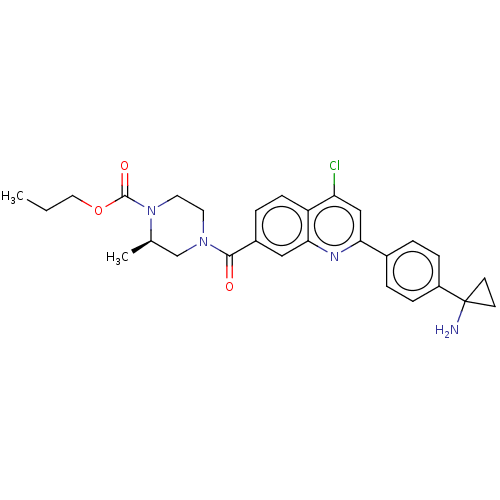 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502433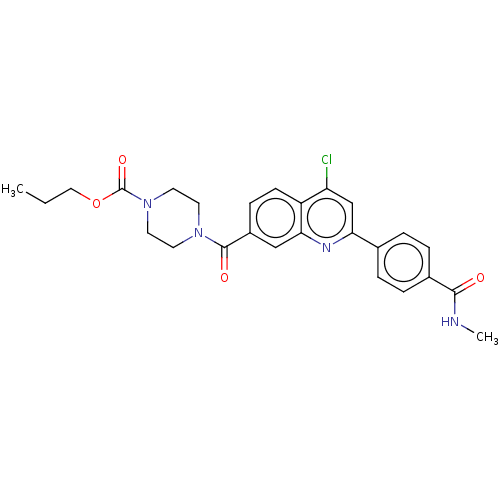 (CHEMBL4575866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502434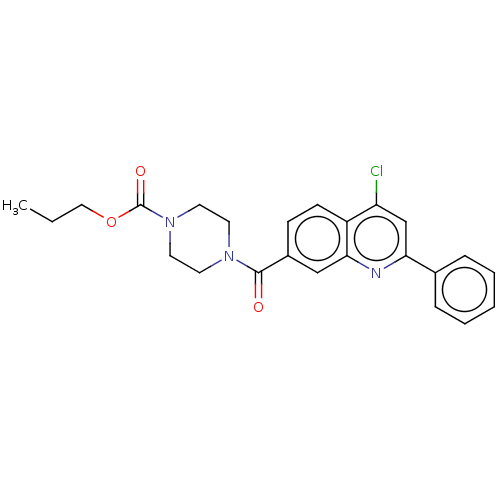 (CHEMBL4551647) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Irreversible inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 5 mins followed by substrate addition and measured... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50133870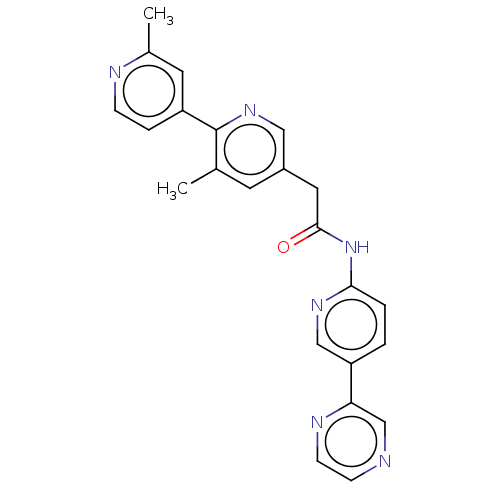 (CHEBI:78030 | CHEMBL3188386 | US10251893, Compound...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257135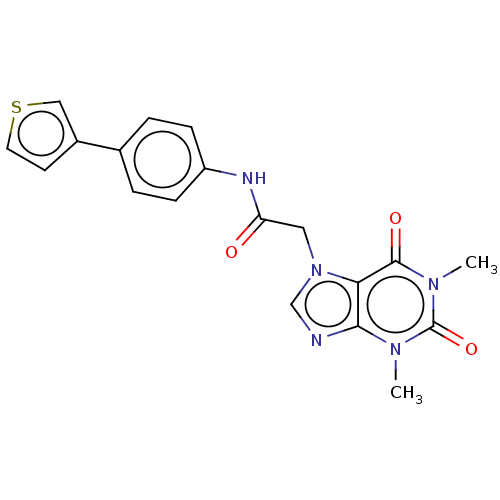 (CHEMBL4080208) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257160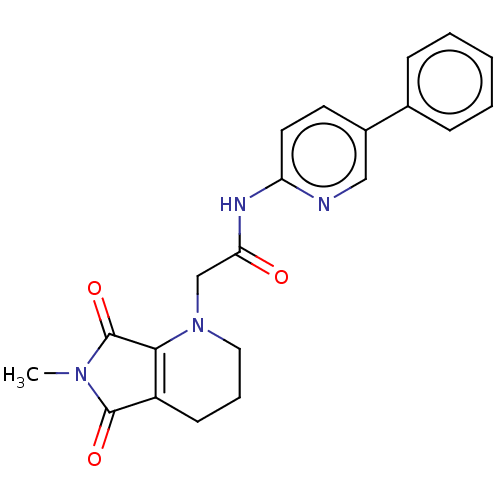 (CHEMBL4096728) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50170221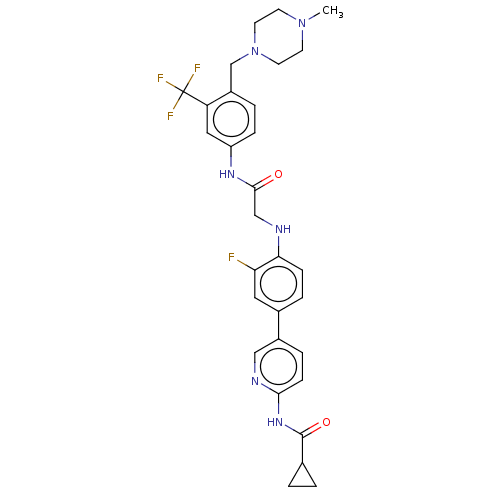 (CHEMBL3806189) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant RET expressed in sf21 cells using Poly(Glu:Tyr) as substrate in presence of [gamma32P]ATP | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233628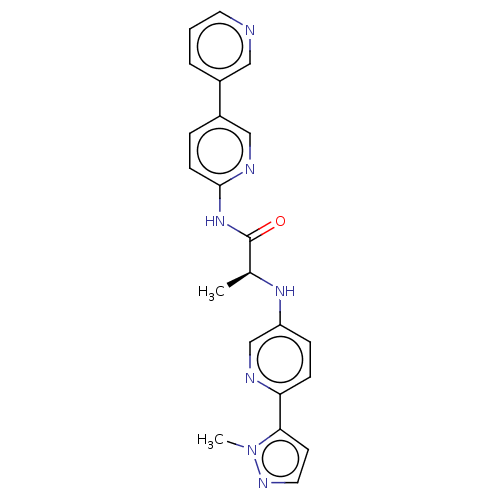 (CHEMBL4088716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233629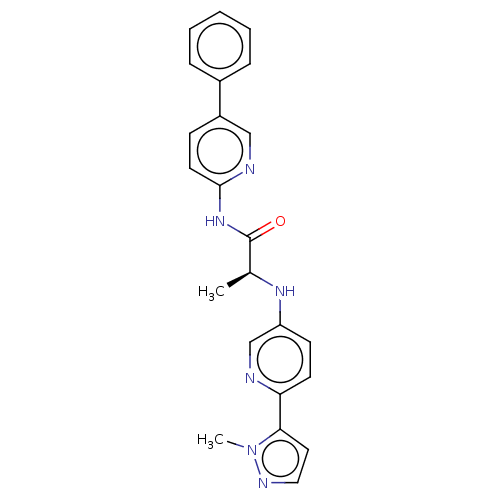 (CHEMBL4080842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257143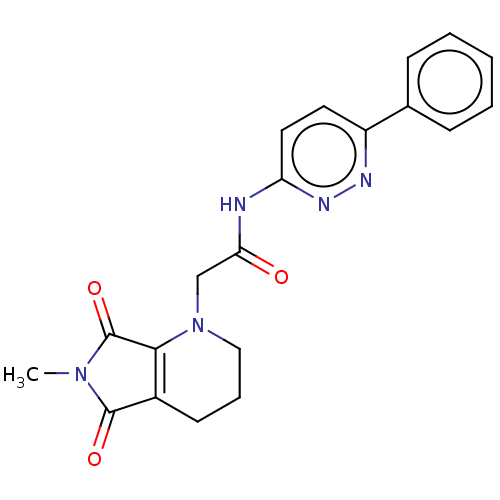 (CHEMBL4080021) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233636 (CHEMBL4070649) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50133866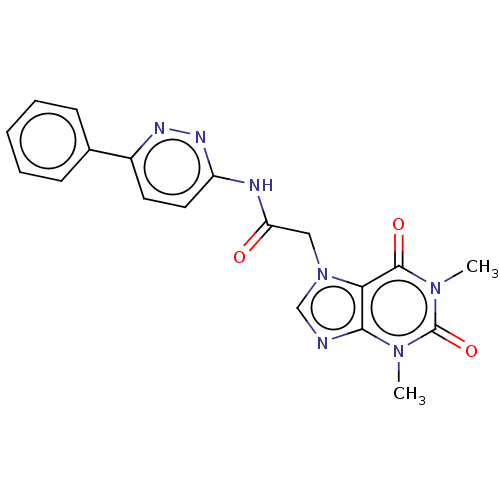 (CHEMBL3633802) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233635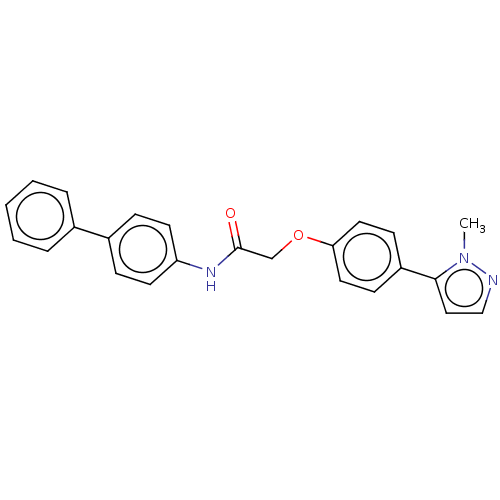 (CHEMBL4099279) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233630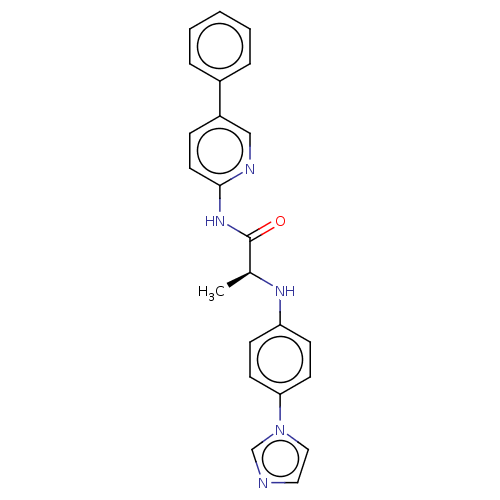 (CHEMBL4084739) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257141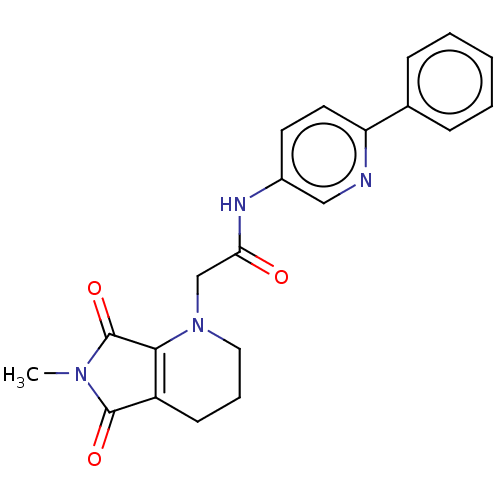 (CHEMBL4069295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233633 (CHEMBL4089128) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50170222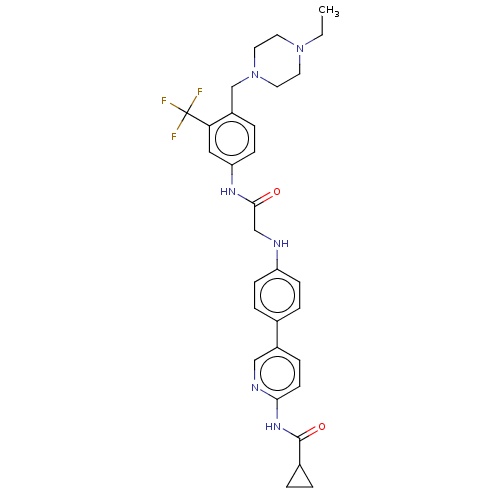 (CHEMBL3805771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant RET expressed in sf21 cells using Poly(Glu:Tyr) as substrate in presence of [gamma32P]ATP | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50170223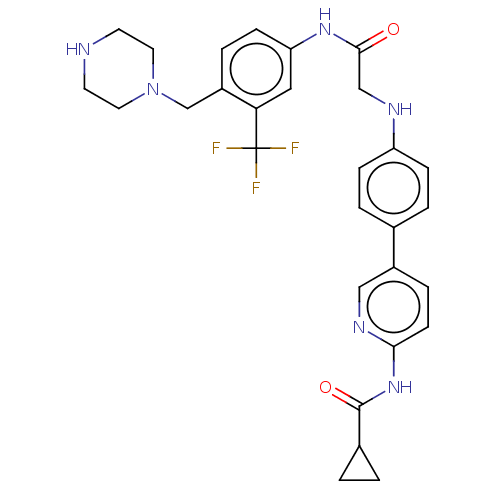 (CHEMBL3805330) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged MNK2 kinase domain (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using JH3 ... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50170218 (CHEMBL3804952) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged MNK2 kinase domain (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using JH3 ... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257139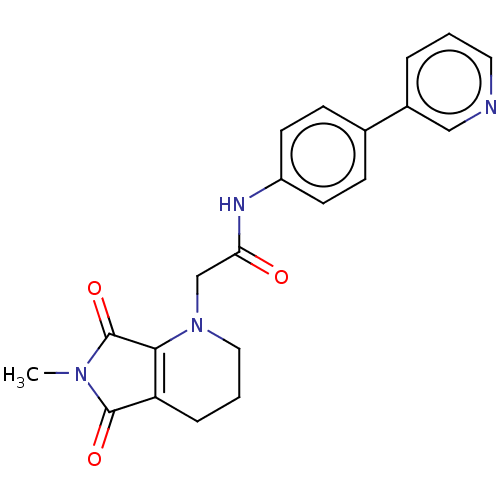 (CHEMBL4104447) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375523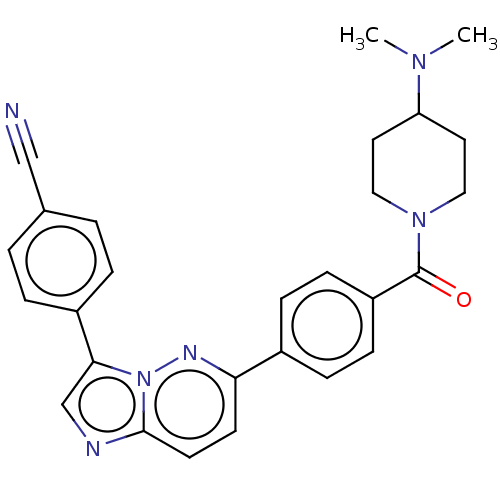 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257144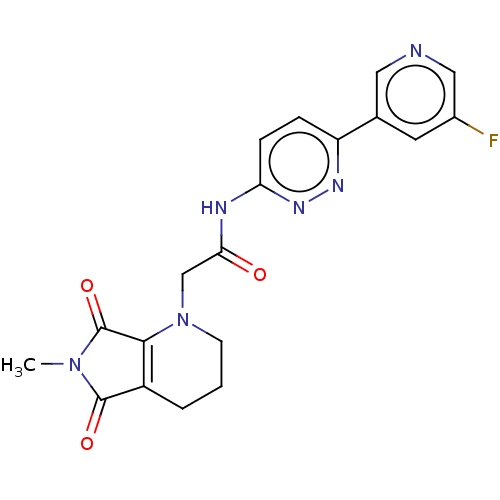 (CHEMBL4066589) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233640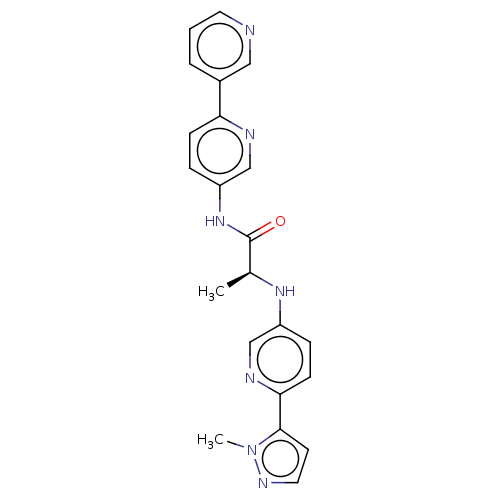 (CHEMBL4098880) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50170217 (CHEMBL3805114) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged MNK2 kinase domain (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using JH3 ... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50170221 (CHEMBL3806189) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged MNK2 kinase domain (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using JH3 ... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Breakpoint cluster region protein/Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50170221 (CHEMBL3806189) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of BCR fused full length human recombinant N-terminal His-tagged ABL (2 to 1130 residues) expressed in baculovirus expression system using... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257145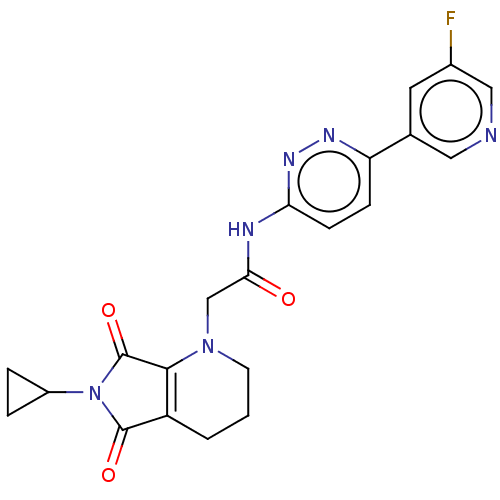 (CHEMBL4095609) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257142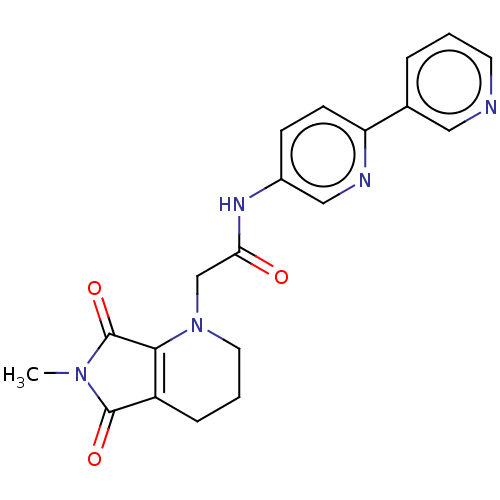 (CHEMBL4096529) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM375523 (4-(6-(4-(4-(dimethylamino)piperidine-1-carbonyl)ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK2 (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50170215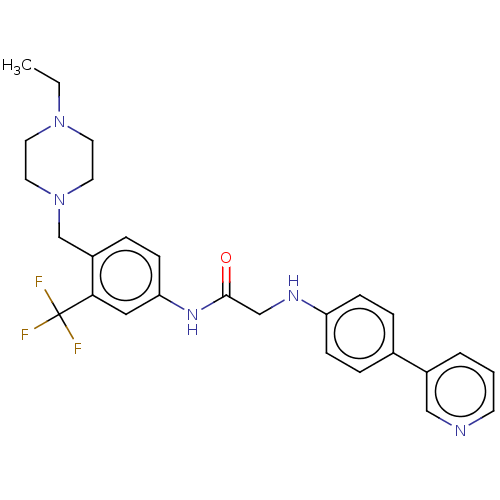 (CHEMBL3806261) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged MNK2 kinase domain (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using JH3 ... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257138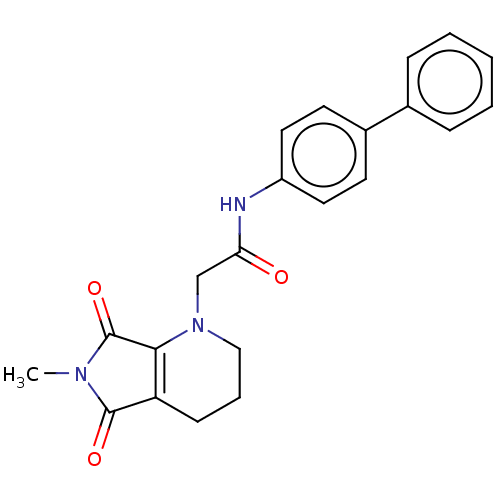 (CHEMBL4066796) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50170171 (CHEMBL3805029) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged MNK2 kinase domain (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using JH3 ... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257128 (CHEMBL4087906) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257157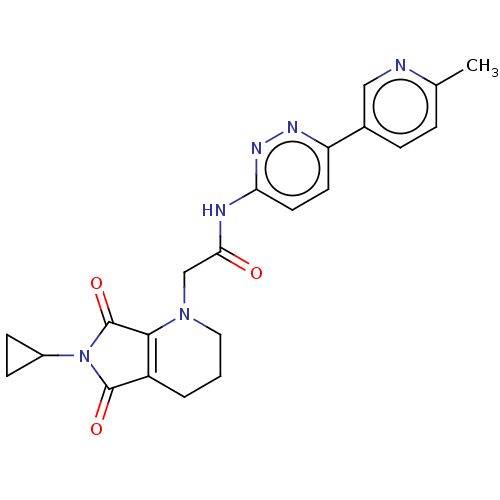 (CHEMBL4103356) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SMYD3 (Homo sapiens (Human)) | BDBM50502427 (CHEMBL4542517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Drug Development Centre Curated by ChEMBL | Assay Description Inhibition of SMYD3 (unknown origin) using MAP3K2 peptide as substrate pretreated for 30 mins followed by substrate addition and measured after 30 mi... | ACS Med Chem Lett 10: 978-984 (2019) Article DOI: 10.1021/acsmedchemlett.9b00170 BindingDB Entry DOI: 10.7270/Q23B63DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50170216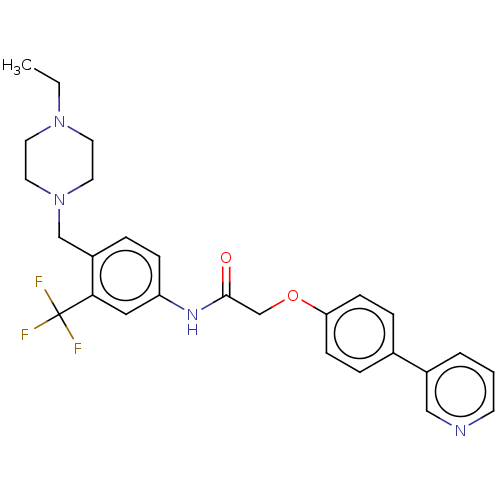 (CHEMBL3805956) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged MNK2 kinase domain (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using JH3 ... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257136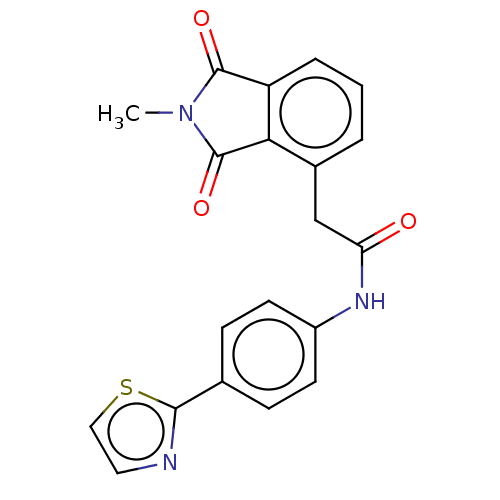 (CHEMBL4061069) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50257137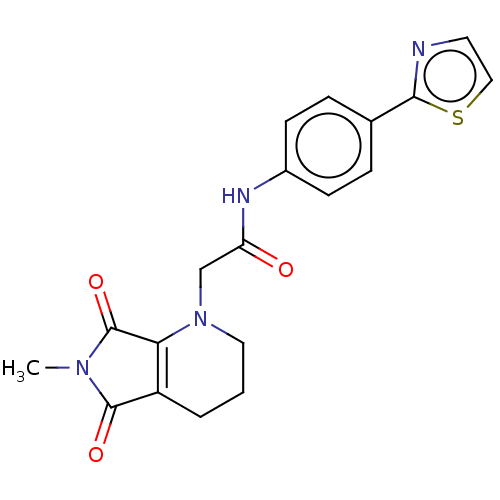 (CHEMBL4094309) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre , 31 Biopolis Way, No. 03-01 Nanos, 138669, Singapore. Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293 cells after day 1 post treatment by Super-top flash reporter gene assay | J Med Chem 60: 6678-6692 (2017) Article DOI: 10.1021/acs.jmedchem.7b00662 BindingDB Entry DOI: 10.7270/Q2571FF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-serine O-palmitoleoyltransferase porcupine (Homo sapiens (Human)) | BDBM50233639 (CHEMBL4097104) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of porcupine (unknown origin) expressed in HEK293-STF3A cells assessed as inhibition of Wnt signaling by measuring decrease in beta-cateni... | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375525 (4-(6-(4-(piperazine-1-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50170222 (CHEMBL3805771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged MNK2 kinase domain (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using JH3 ... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50170222 (CHEMBL3805771) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant FLT3 expressed in sf21 cells using Poly(Glu:Tyr) as substrate in presence of [gamma32P]ATP | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50170221 (CHEMBL3806189) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant FLT3 expressed in sf21 cells using Poly(Glu:Tyr) as substrate in presence of [gamma32P]ATP | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50233639 (CHEMBL4097104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 58: 5889-99 (2015) Article DOI: 10.1021/acs.jmedchem.5b00507 BindingDB Entry DOI: 10.7270/Q29K4DG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375513 ((4-(3-(4-chlorophenyl)imidazo[1,2-b]pyridazin-6-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM31085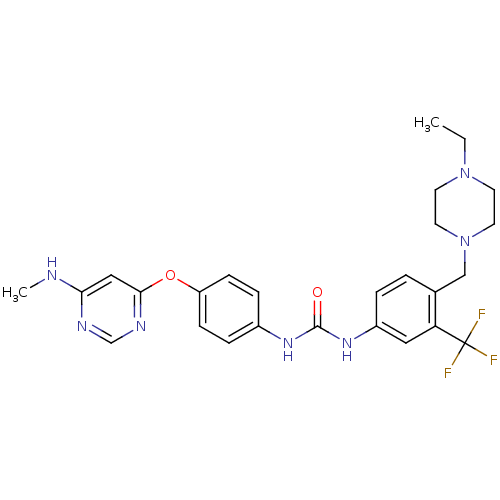 (1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged MNK2 kinase domain (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using JH3 ... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50170221 (CHEMBL3806189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of full length human recombinant N-terminal His-tagged ABL (2 to 1130 residues) T315I mutant expressed in baculovirus expression system us... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50170221 (CHEMBL3806189) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant VEGFR2 expressed in sf21 cells using Poly(Glu:Tyr) as substrate in presence of [gamma32P]ATP | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50170219 (CHEMBL3805074) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Experimental Therapeutics Centre Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged MNK2 kinase domain (72 to 385 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) cells using JH3 ... | J Med Chem 59: 3063-78 (2016) Article DOI: 10.1021/acs.jmedchem.5b01712 BindingDB Entry DOI: 10.7270/Q2028TF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MAP kinase-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM375522 (4-(6-(4-(morpholine-4-carbonyl)phenyl)imidazo[1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Agency for Science, Technology and Research (A*STAR) Curated by ChEMBL | Assay Description Inhibition of GST-tagged MNK1 (37 to 341 residues) (unknown origin) expressed in Escherichia coli BL21(DE3) using JH3 peptide as substrate preincubat... | J Med Chem 61: 4348-4369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01714 BindingDB Entry DOI: 10.7270/Q20004P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 290 total ) | Next | Last >> |