

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.0150 | -61.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158504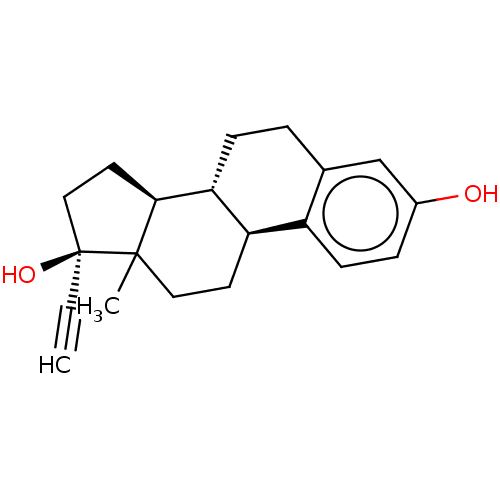 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.0250 | -60.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158504 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158504 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.0250 | -60.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158504 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158504 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158504 (US9034854, EE | US9040509, EE | US9561238, EE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248937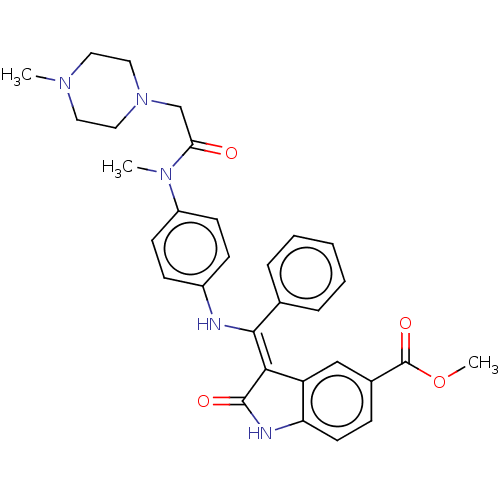 (CHEMBL4062168 | US10981896, Compound 15) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248946 (CHEMBL1908392 | US10981896, Compound 16) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248938 (CHEMBL4089284 | US10981896, Compound 21) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NUAK family SNF1-like kinase 1 (Homo sapiens (Human)) | BDBM50248946 (CHEMBL1908392 | US10981896, Compound 16) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal GST-tagged NUAK1 expressed in baculovirus infected Sf9 insect cells using CHK peptide as subst... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248936 (CHEMBL4083922 | US10981896, Compound 25) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248945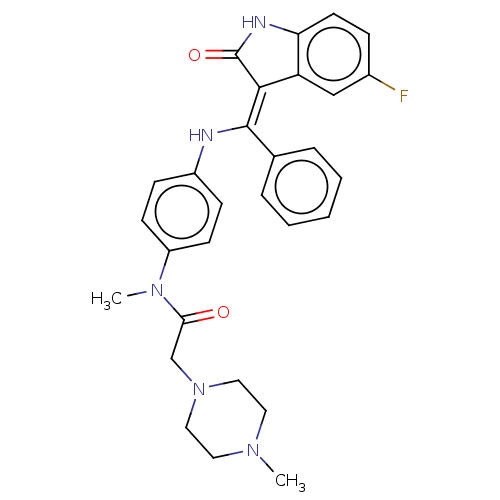 (CHEMBL4081410 | US10981896, Compound 19) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158505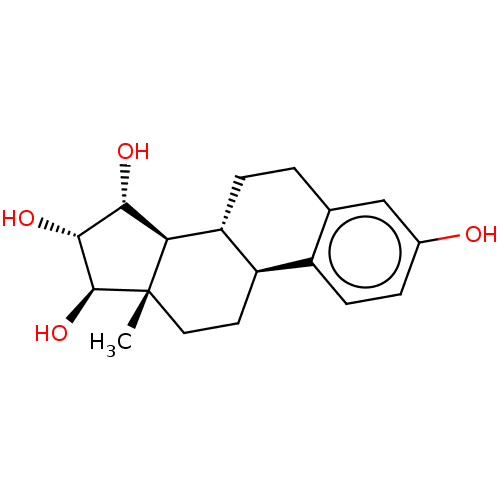 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 4.90 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158505 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 4.90 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM158505 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50248937 (CHEMBL4062168 | US10981896, Compound 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His-tagged CHK1 expressed in baculovirus infected Sf9 insect cells using CHK peptide as substr... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NUAK family SNF1-like kinase 1 (Homo sapiens (Human)) | BDBM50248938 (CHEMBL4089284 | US10981896, Compound 21) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal GST-tagged NUAK1 expressed in baculovirus infected Sf9 insect cells using CHK peptide as subst... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248950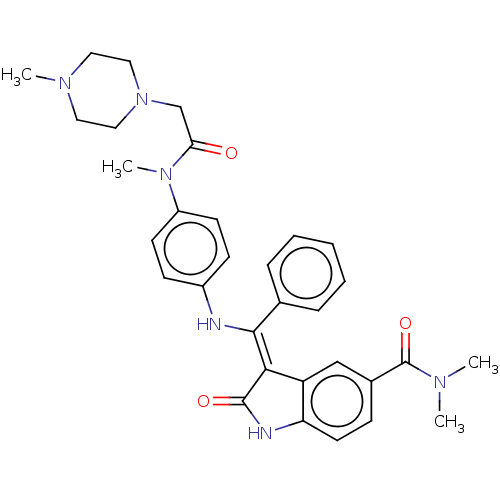 (CHEMBL4070814) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50248946 (CHEMBL1908392 | US10981896, Compound 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His-tagged CHK1 expressed in baculovirus infected Sf9 insect cells using CHK peptide as substr... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NUAK family SNF1-like kinase 1 (Homo sapiens (Human)) | BDBM50248937 (CHEMBL4062168 | US10981896, Compound 15) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal GST-tagged NUAK1 expressed in baculovirus infected Sf9 insect cells using CHK peptide as subst... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase kinase 2 (Homo sapiens (Human)) | BDBM50248946 (CHEMBL1908392 | US10981896, Compound 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of CAMKK2 (unknown origin) using NUAK2 peptide as substrate measured after 30 mins in presence of [gamma32P]ATP by liquid scintillation co... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158505 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 19 | -44.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Pantarhei Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osboum et al. (1993, Biochemistry, 32, 6229-6236). Recombin... | US Patent US9040509 (2015) BindingDB Entry DOI: 10.7270/Q2Z036WK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158505 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Donesta Bioscience B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9561238 (2017) BindingDB Entry DOI: 10.7270/Q2B56MR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM158505 (US9034854, E4 | US9040509, E4 | US9561238, E4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | 19 | -44.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
PANTARHEI BIOSCIENCE B.V. US Patent | Assay Description The method employed was adapted from the scientific literature and described in detail by Osbourn et al. (1993, Biochemistry, 32, 6229-6236). Recombi... | US Patent US9034854 (2015) BindingDB Entry DOI: 10.7270/Q2W66JJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase kinase 2 (Homo sapiens (Human)) | BDBM50248937 (CHEMBL4062168 | US10981896, Compound 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of CAMKK2 (unknown origin) using NUAK2 peptide as substrate measured after 30 mins in presence of [gamma32P]ATP by liquid scintillation co... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248947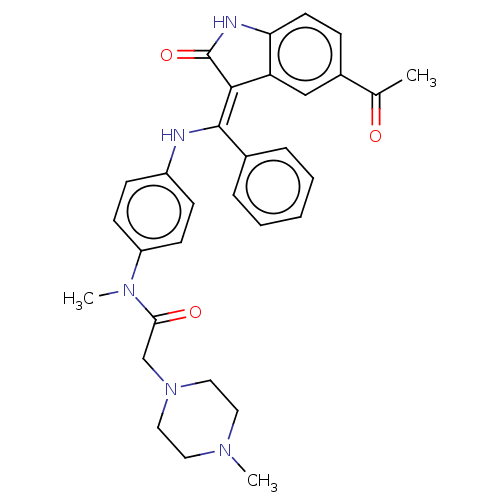 (CHEMBL4075402 | US10981896, Compound 18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM50248938 (CHEMBL4089284 | US10981896, Compound 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal His-tagged CHK1 expressed in baculovirus infected Sf9 insect cells using CHK peptide as substr... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium/calmodulin-dependent protein kinase kinase 2 (Homo sapiens (Human)) | BDBM50248938 (CHEMBL4089284 | US10981896, Compound 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of CAMKK2 (unknown origin) using NUAK2 peptide as substrate measured after 30 mins in presence of [gamma32P]ATP by liquid scintillation co... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248924 (CHEMBL4099431 | US10981896, Compound 20) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248925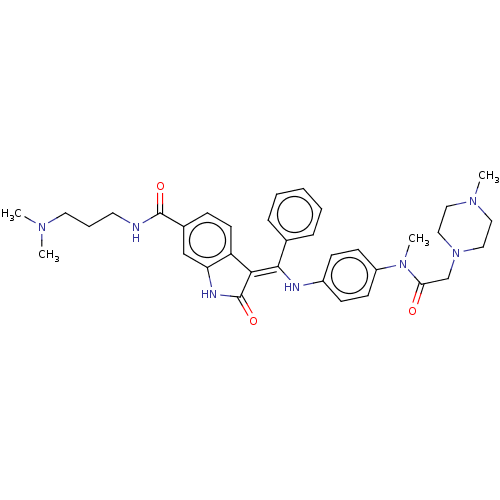 (CHEMBL4061117 | US10981896, Compound 22) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 358 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248935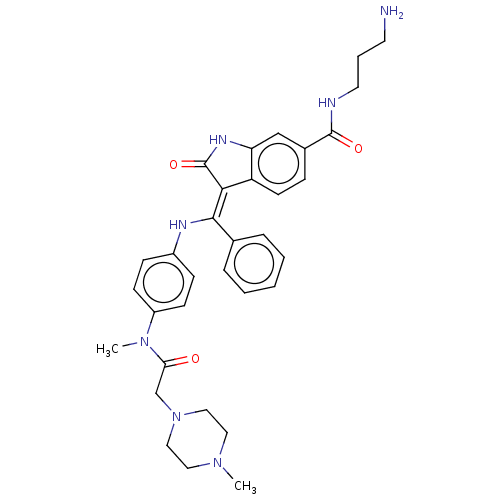 (CHEMBL4062202 | US10981896, Compound 23) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50248946 (CHEMBL1908392 | US10981896, Compound 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of ERK2 (unknown origin) using Ets-1 as substrate measured after 30 mins in presence of [gamma32P]ATP by liquid scintillation counting met... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50248937 (CHEMBL4062168 | US10981896, Compound 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of ERK2 (unknown origin) using Ets-1 as substrate measured after 30 mins in presence of [gamma32P]ATP by liquid scintillation counting met... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50248938 (CHEMBL4089284 | US10981896, Compound 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of ERK2 (unknown origin) using Ets-1 as substrate measured after 30 mins in presence of [gamma32P]ATP by liquid scintillation counting met... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248937 (CHEMBL4062168 | US10981896, Compound 15) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248946 (CHEMBL1908392 | US10981896, Compound 16) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248938 (CHEMBL4089284 | US10981896, Compound 21) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248936 (CHEMBL4083922 | US10981896, Compound 25) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248945 (CHEMBL4081410 | US10981896, Compound 19) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50026612 (BIBF-1120 | Nintedanib | US10981896, Compound Nint...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50248950 (CHEMBL4070814) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50273571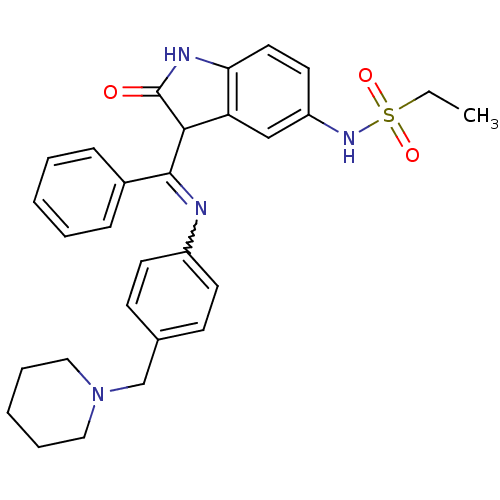 (CHEMBL514409 | Hesperadin | N-(2-oxo-3-(phenyl(4-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50109821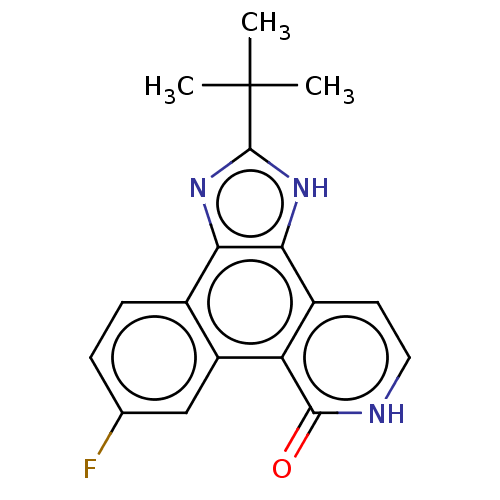 (CHEBI:87103 | CHEMBL21156 | US10981896, Compound 4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Texas at Austin Curated by ChEMBL | Assay Description Inhibition of His6-tagged MELK catalytic domain (1 to 340 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using Bcl-GL as s... | Bioorg Med Chem 25: 2609-2616 (2017) Article DOI: 10.1016/j.bmc.2017.03.018 BindingDB Entry DOI: 10.7270/Q2833VFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 664 total ) | Next | Last >> |