

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172038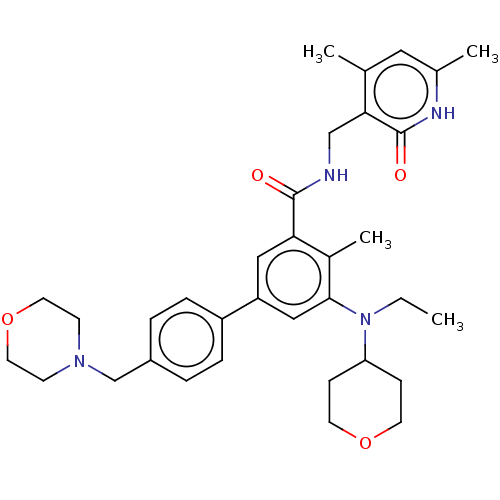 (US10155002, Compound 44 | US10647700, Compound EPZ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc.; Eisai R&D Management Co., LTD. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9394283 (2016) BindingDB Entry DOI: 10.7270/Q2WM1C8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172038 (US10155002, Compound 44 | US10647700, Compound EPZ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13.0 | n/a | n/a | n/a | n/a | n/a | 25 |
Epizyme, Inc.; Eisai R&D Management Co., LTD. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM bicine (pH=7.6), 0.5 mM DTT, 0.005% BSG and 0.002% Tween20, prepared on the day of use.... | US Patent US9394283 (2016) BindingDB Entry DOI: 10.7270/Q2WM1C8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50222222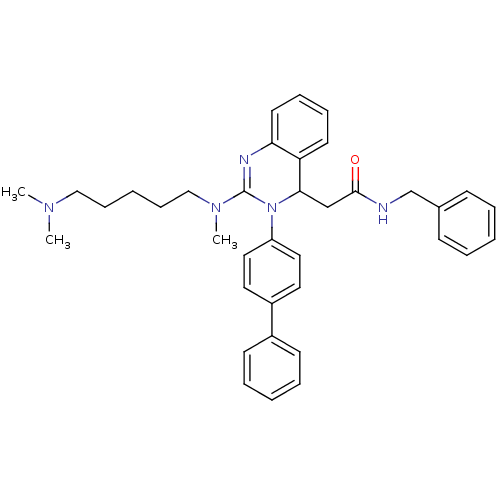 (CHEMBL394956 | KYS-05090 | N-benzyl-2-(3-(biphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of T-type calcium channel alpha 1 G | Bioorg Med Chem Lett 22: 1198-201 (2012) Article DOI: 10.1016/j.bmcl.2011.11.083 BindingDB Entry DOI: 10.7270/Q2GQ6Z59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50222222 (CHEMBL394956 | KYS-05090 | N-benzyl-2-(3-(biphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50494713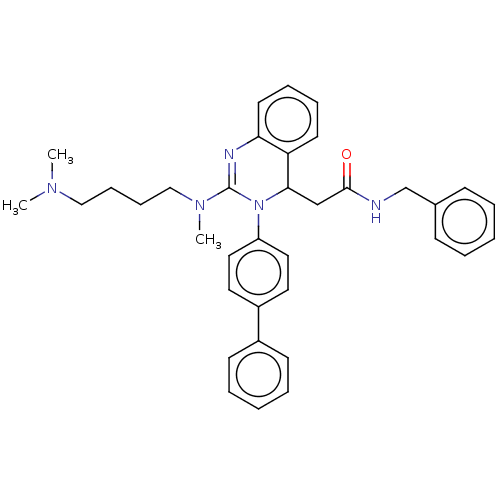 (CHEMBL3093776) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50232642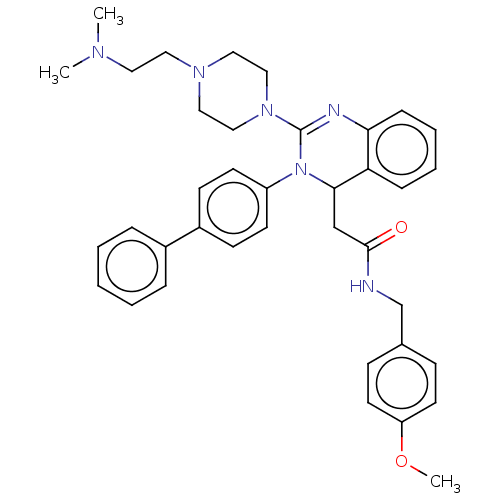 (CHEMBL3093781) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50232643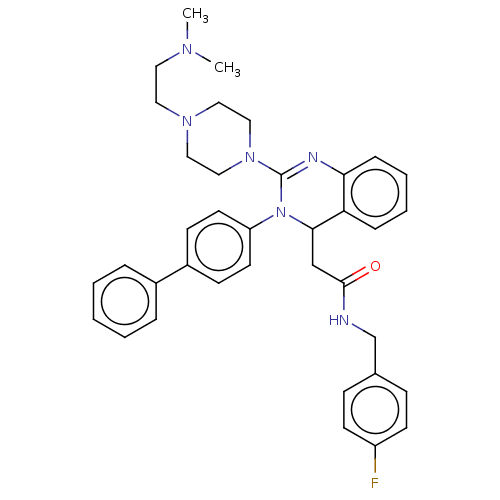 (CHEMBL3093780) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50494712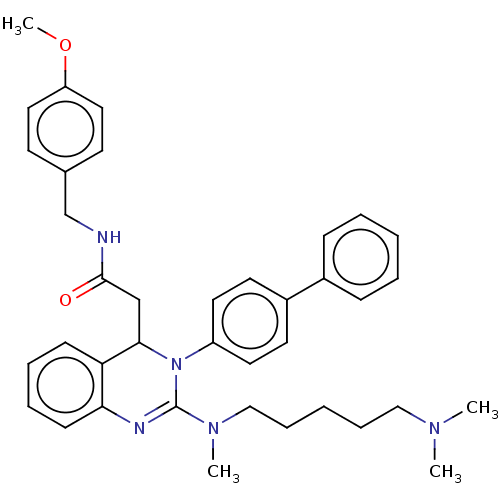 (CHEMBL3093774) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50494711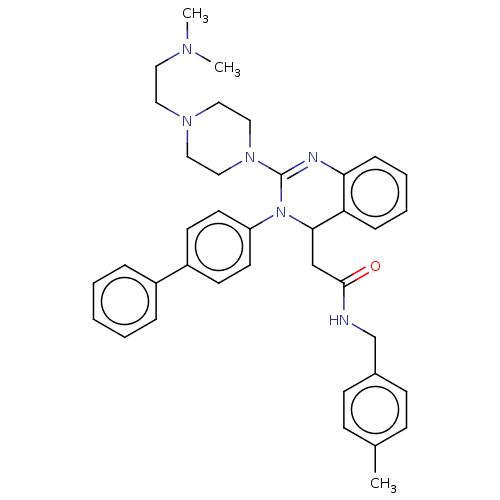 (CHEMBL3093779) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50494710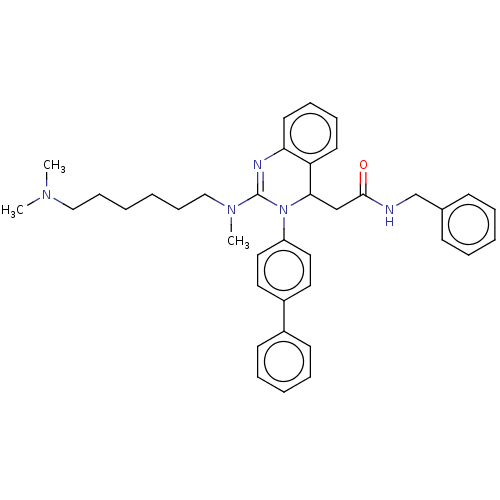 (CHEMBL3093777) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent T-type calcium channel subunit alpha-1G (Homo sapiens (Human)) | BDBM50117922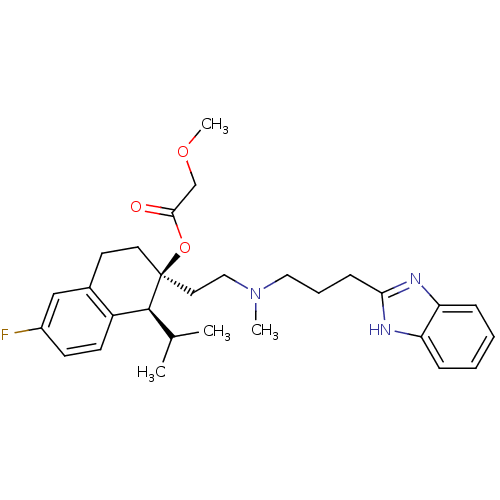 ((1S,2S)-2-(2-((3-(1H-benzo[d]imidazol-2-yl)propyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of t-type Cav3.1 channel (unknown origin) expressed in HEK293 cells assessed as inhibition of 50 ms depolarizing voltage step-induced curr... | Bioorg Med Chem Lett 23: 6656-62 (2013) Article DOI: 10.1016/j.bmcl.2013.10.049 BindingDB Entry DOI: 10.7270/Q28W3H80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117067 (US8664230, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117067 (US8664230, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117066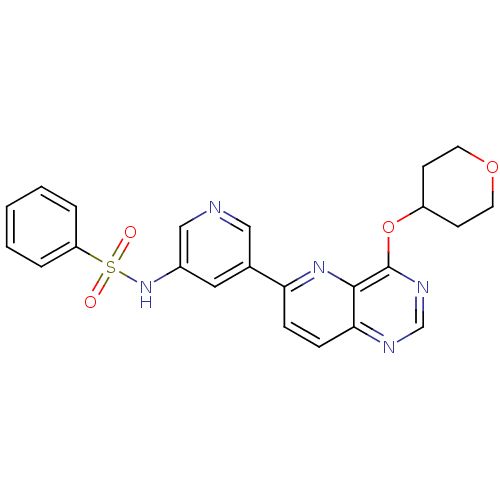 (US8664230, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117065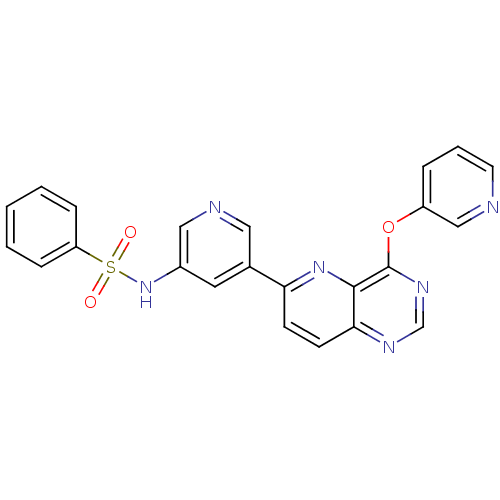 (US8664230, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117068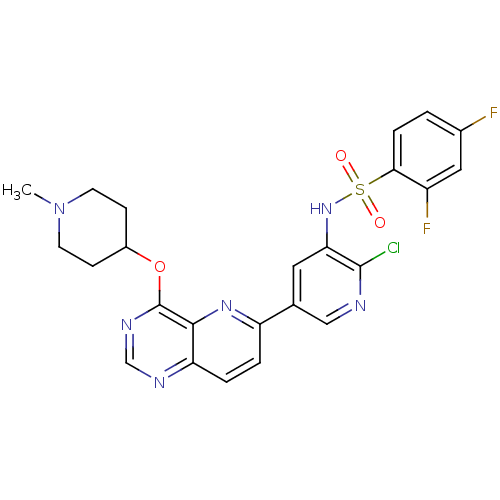 (US8664230, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117067 (US8664230, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117068 (US8664230, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50222222 (CHEMBL394956 | KYS-05090 | N-benzyl-2-(3-(biphenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of N-type calcium channel alpha 1 B | Bioorg Med Chem Lett 22: 1198-201 (2012) Article DOI: 10.1016/j.bmcl.2011.11.083 BindingDB Entry DOI: 10.7270/Q2GQ6Z59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117069 (US8664230, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117066 (US8664230, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117065 (US8664230, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117068 (US8664230, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117069 (US8664230, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117070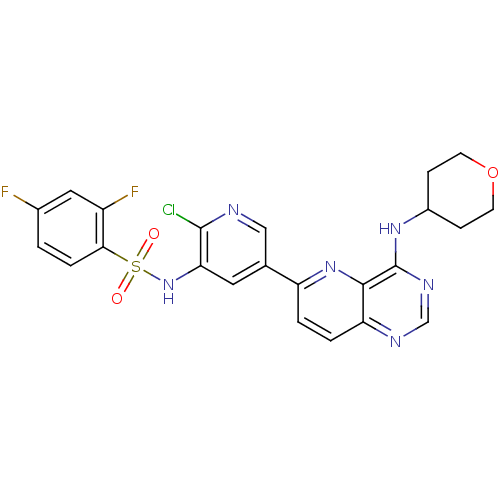 (US8664230, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117069 (US8664230, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117066 (US8664230, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase regulatory subunit alpha (Homo sapiens (Human)) | BDBM117065 (US8664230, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM117070 (US8664230, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM117070 (US8664230, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Asan Foundation US Patent | Assay Description The PI3 Kinase Activity/Inhibitor Assay is a competitive assay used for the fast and sensitive quantitation of activity of the four class I PI3 kinas... | US Patent US8664230 (2014) BindingDB Entry DOI: 10.7270/Q2N58K1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||