

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase 17B (Homo sapiens (Human)) | BDBM50166121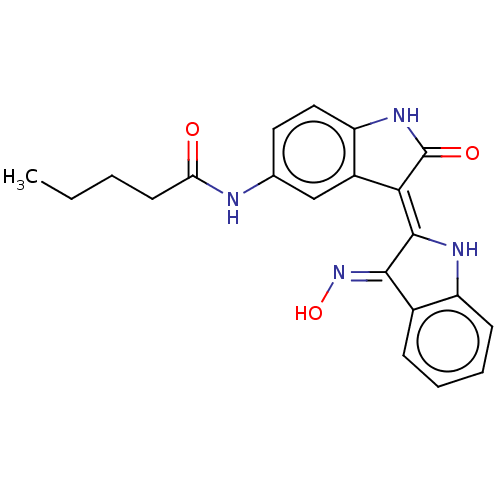 (CHEMBL3797480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Competitive inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by Lineweaver-Burk plot analysis in presence of... | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50384024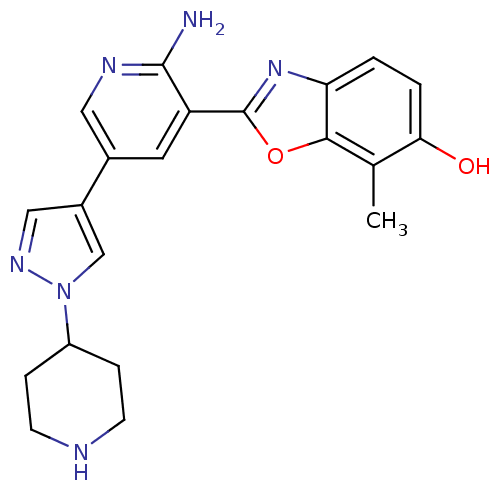 (CHEMBL2032280 | CHEMBL2079349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50384038 (CHEMBL2032155) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant FLT3 by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238943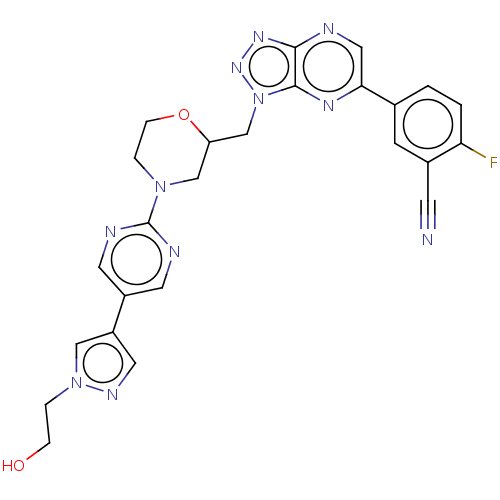 (US9403831, 32) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50384038 (CHEMBL2032155) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50205347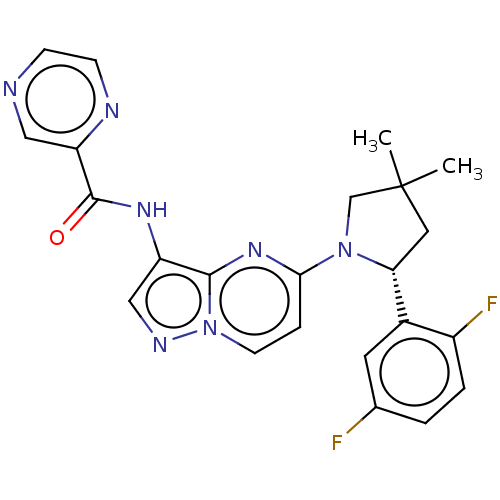 (CHEMBL3947950) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University Curated by ChEMBL | Assay Description Inhibition of TrkA (unknown origin) by ELISA | Bioorg Med Chem 25: 389-396 (2017) Article DOI: 10.1016/j.bmc.2016.11.005 BindingDB Entry DOI: 10.7270/Q2JM2CNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM193503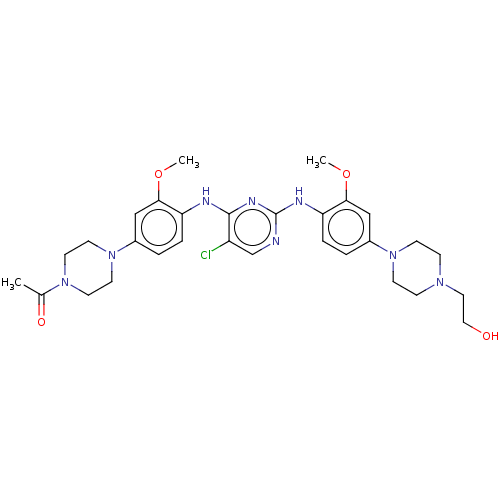 (US9199944, 85) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM193475 (US9199944, 57) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM193476 (US9199944, 58) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238925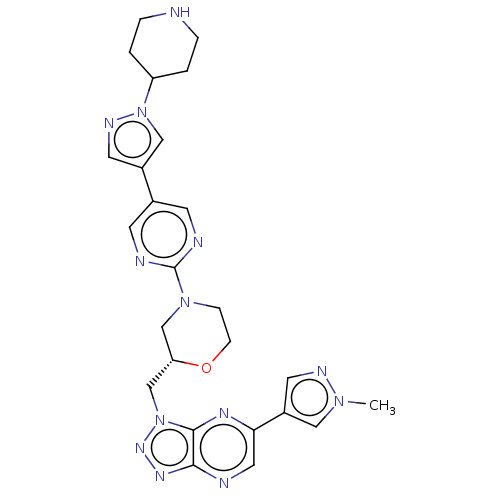 (US9403831, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238935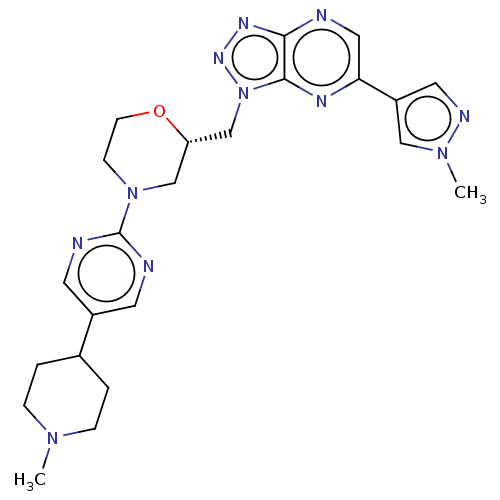 (US9403831, 20) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238928 (US9403831, 24) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238964 (US9403831, 29) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238944 (US9403831, 30) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238942 (US9403831, 31) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238951 (US9403831, 36) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238952 (US9403831, 37) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238946 (US9403831, 39) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50384004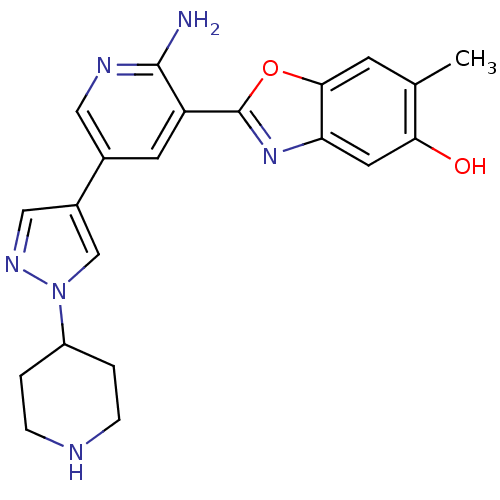 (CHEMBL2032276) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant FLT3 by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50358291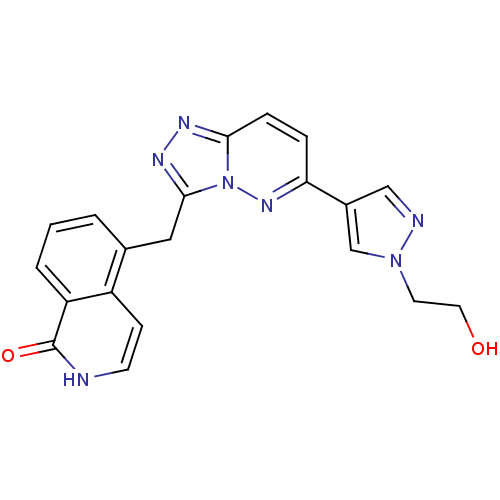 (CHEMBL1922406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-met by HTRF assay | Bioorg Med Chem Lett 21: 7185-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.066 BindingDB Entry DOI: 10.7270/Q24Q7VDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238947 (US9403831, 40) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50030308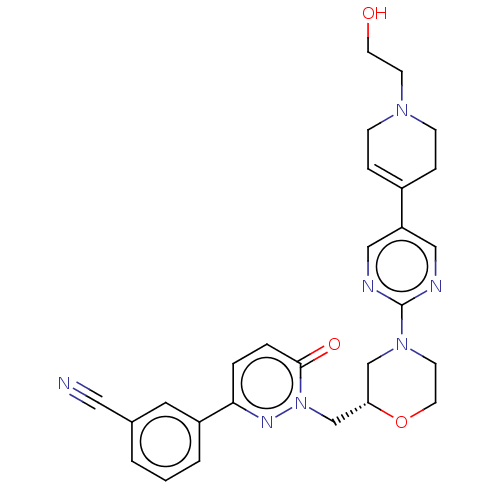 (CHEMBL3335781) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) by HTRF assay | Bioorg Med Chem Lett 24: 5093-7 (2014) Article DOI: 10.1016/j.bmcl.2014.08.067 BindingDB Entry DOI: 10.7270/Q2T43VQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50358288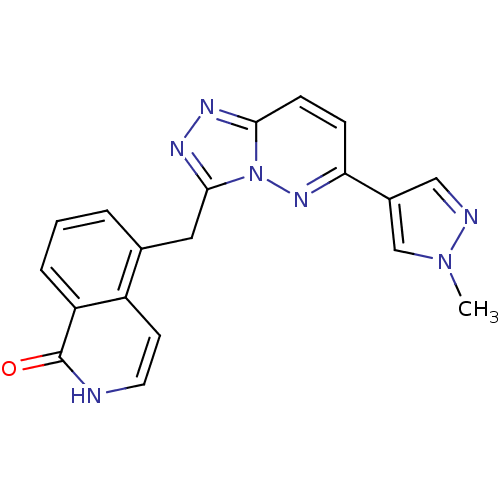 (CHEMBL1922404) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-met by HTRF assay | Bioorg Med Chem Lett 21: 7185-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.066 BindingDB Entry DOI: 10.7270/Q24Q7VDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50030291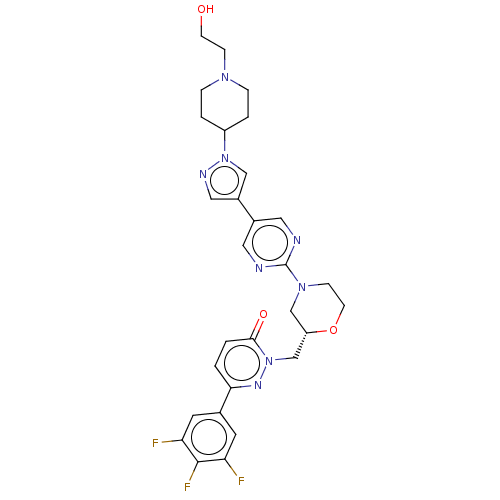 (CHEMBL3335768) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) by HTRF assay | Bioorg Med Chem Lett 24: 5093-7 (2014) Article DOI: 10.1016/j.bmcl.2014.08.067 BindingDB Entry DOI: 10.7270/Q2T43VQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17B (Homo sapiens (Human)) | BDBM50166121 (CHEMBL3797480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by ADP-Glo kinase assay | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50358289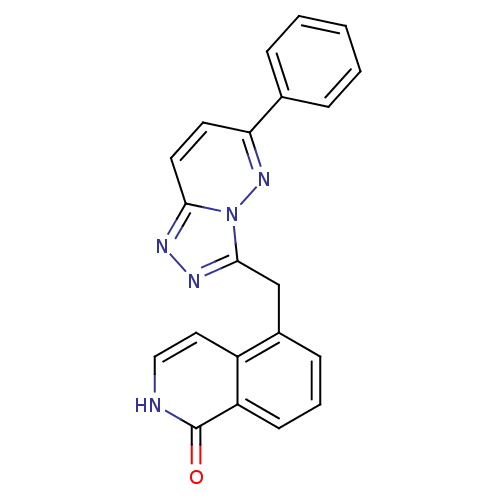 (CHEMBL1922405) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-met by HTRF assay | Bioorg Med Chem Lett 21: 7185-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.066 BindingDB Entry DOI: 10.7270/Q24Q7VDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50358301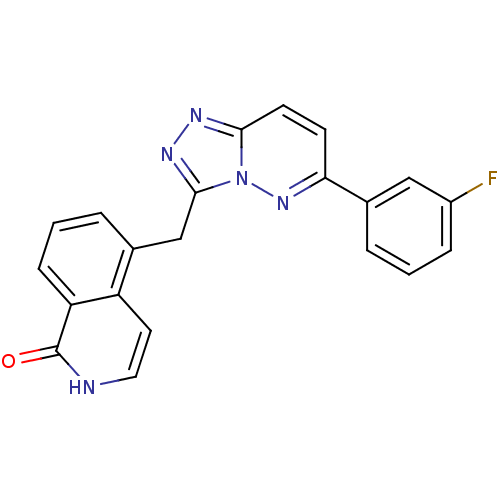 (CHEMBL1922416) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-met by HTRF assay | Bioorg Med Chem Lett 21: 7185-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.066 BindingDB Entry DOI: 10.7270/Q24Q7VDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50358303 (CHEMBL1922418) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-met by HTRF assay | Bioorg Med Chem Lett 21: 7185-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.066 BindingDB Entry DOI: 10.7270/Q24Q7VDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50358304 (CHEMBL1922419) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-met by HTRF assay | Bioorg Med Chem Lett 21: 7185-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.066 BindingDB Entry DOI: 10.7270/Q24Q7VDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50384027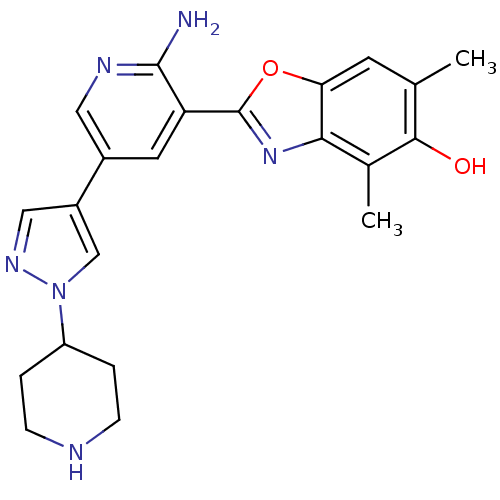 (CHEMBL2032277) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant c-Met by time resolved-fluorescence resonance energy transfer analysis | Bioorg Med Chem Lett 22: 4044-8 (2012) Article DOI: 10.1016/j.bmcl.2012.04.083 BindingDB Entry DOI: 10.7270/Q2FF3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50142026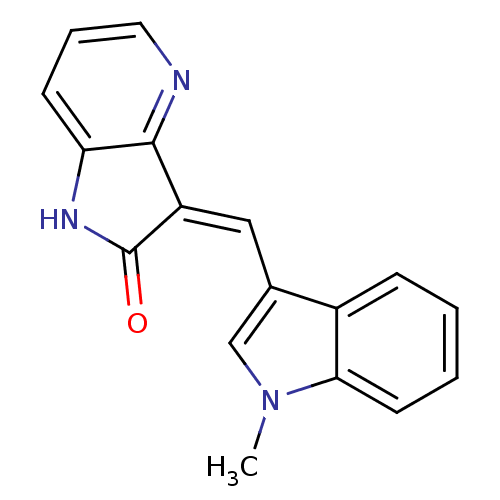 (3-((1-methyl-1H-indol-3-yl)methylene)-1H-pyrrolo[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University Curated by ChEMBL | Assay Description Inhibition of human N- terminal GST-tagged TrkA cytoplasmic domain (436 to 790 residues) expressed in baculovirus infected sf21 cells using biotinyla... | Bioorg Med Chem 25: 389-396 (2017) Article DOI: 10.1016/j.bmc.2016.11.005 BindingDB Entry DOI: 10.7270/Q2JM2CNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM193475 (US9199944, 57) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM193481 (US9199944, 63) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM193486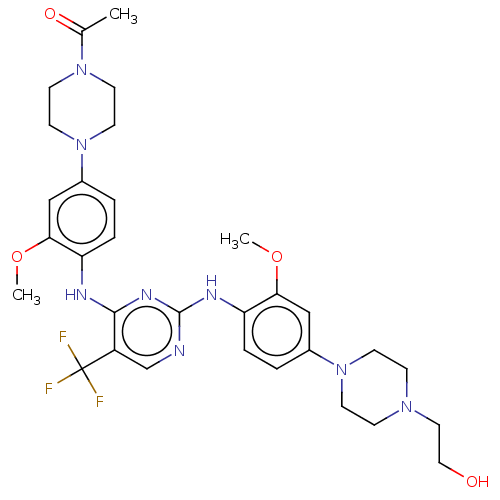 (US9199944, 68) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM193487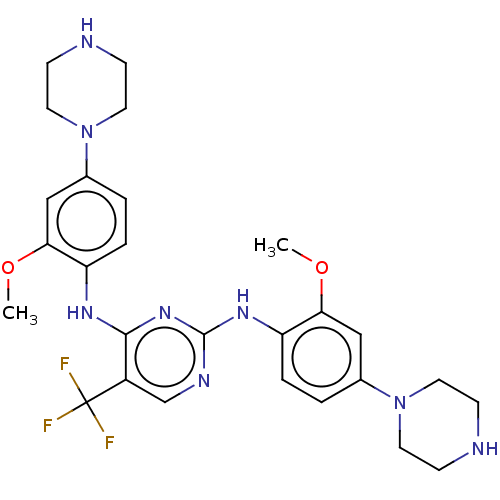 (US9199944, 69) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM193490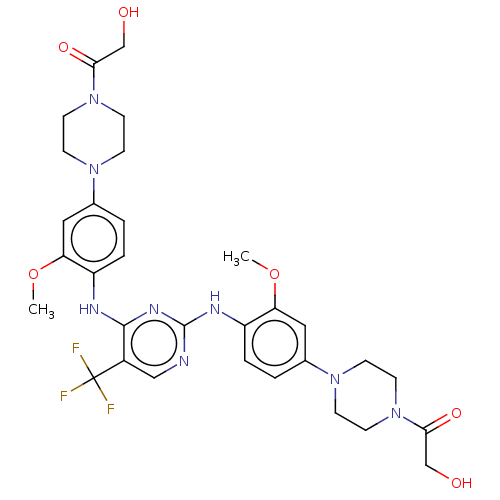 (US9199944, 72) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM193495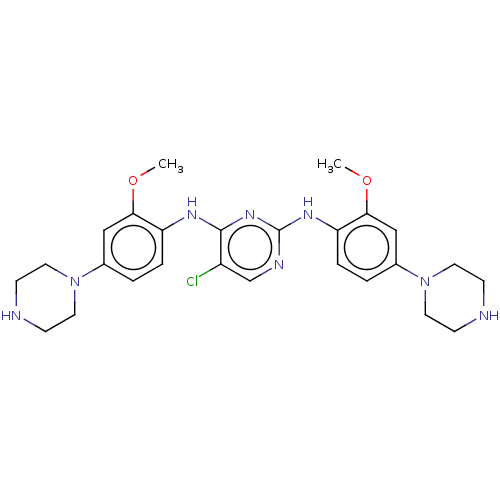 (US9199944, 77) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM193477 (US9199944, 59) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM193478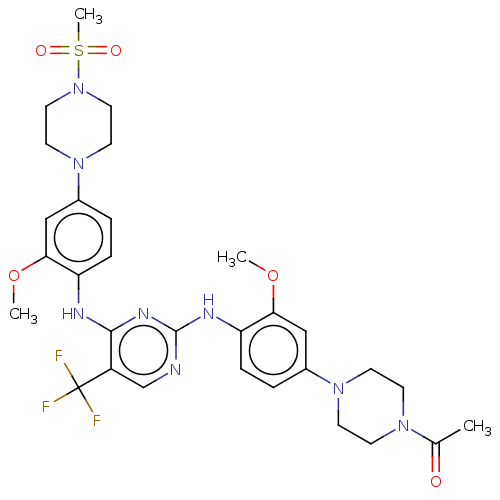 (US9199944, 60) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM193481 (US9199944, 63) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM193483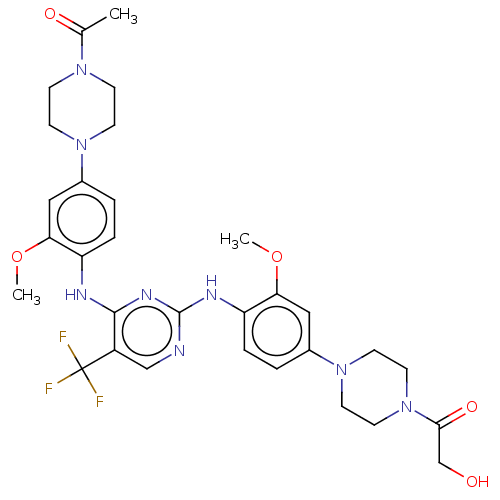 (US9199944, 65) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor [L1196M] (Homo sapiens (Human)) | BDBM193484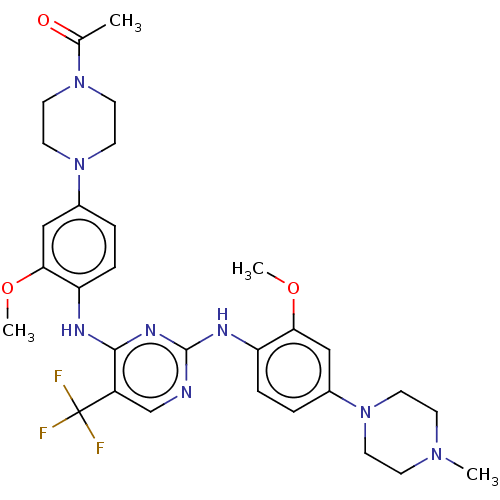 (US9199944, 66) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY US Patent | Assay Description In order to measure the inhibitory activity against ALK, a Grainer 96-well round type bottom plate was added with the compounds (2 μl) prepared ... | US Patent US9199944 (2015) BindingDB Entry DOI: 10.7270/Q2J96558 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238937 (US9403831, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238939 (US9403831, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238953 (US9403831, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238959 (US9403831, 23 | US9403831, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238949 (US9403831, 33) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238948 (US9403831, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238950 (US9403831, 35) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM238965 (US9403831, 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; HANDOK INC. US Patent | Assay Description The c-Met kinase inhibitory activity was analyzed using dissociation enhanced lanthanide fluoro-immunoassay (DELFIA, Perkin Elmer), which is a kind o... | US Patent US9403831 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 459 total ) | Next | Last >> |