

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50115021 (1-(3-Cyano-phenyl)-3-{3-[4-(4-fluoro-benzyl)-piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50142464 (1-(3-Cyano-phenyl)-3-{3-[3-(4-fluoro-benzyl)-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50115023 (1-(3-Acetyl-phenyl)-3-{3-[4-(4-fluoro-benzyl)-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50115071 (1-{3-[4-(4-Fluoro-benzyl)-piperidin-1-yl]-propyl}-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50089354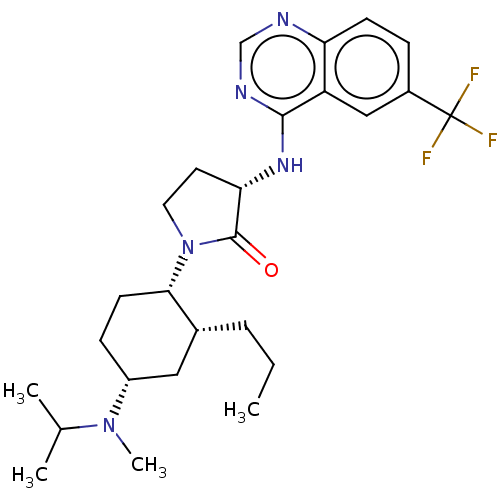 (CHEMBL3577945) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of muscarinic acetylcholine receptor M1 (unknown origin) | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50115090 (1-[3-(4-Benzyl-piperidin-1-yl)-propyl]-3-(3-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 613 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50115010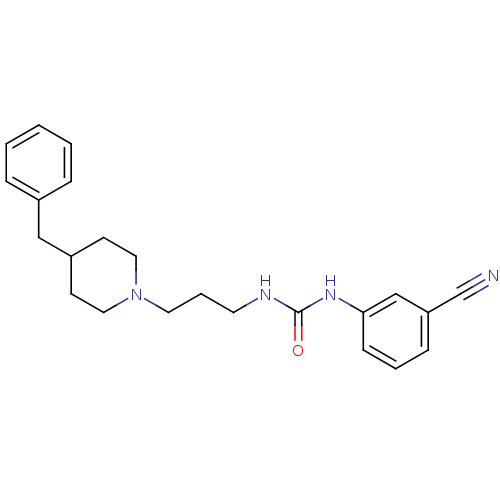 (1-[3-(4-Benzyl-piperidin-1-yl)-propyl]-3-(3-cyano-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 865 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of muscarinic acetylcholine receptor M4 (unknown origin) | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50142459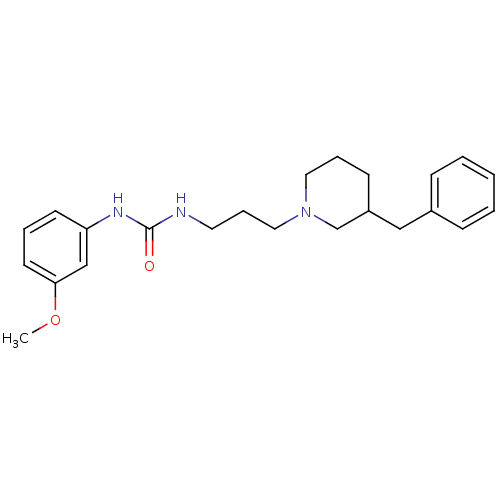 (1-[3-(3-Benzyl-piperidin-1-yl)-propyl]-3-(3-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 913 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50142448 ((S)-1-(3-(3-(4-fluorobenzyl)piperidin-1-yl)propyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50142465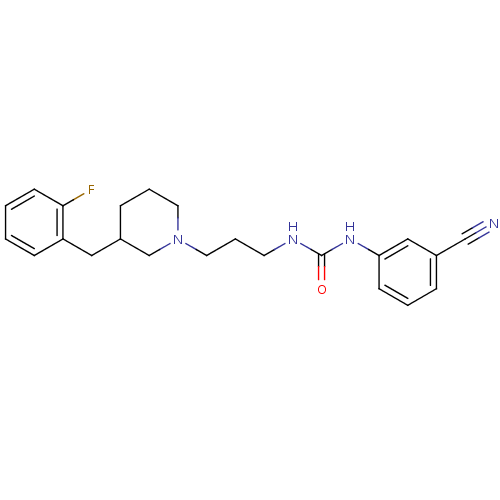 (1-(3-Cyano-phenyl)-3-{3-[3-(2-fluoro-benzyl)-piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50142466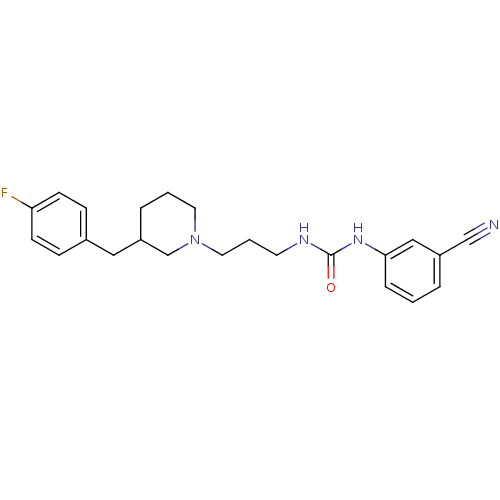 (1-(3-Cyano-phenyl)-3-{3-[3-(4-fluoro-benzyl)-piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50142455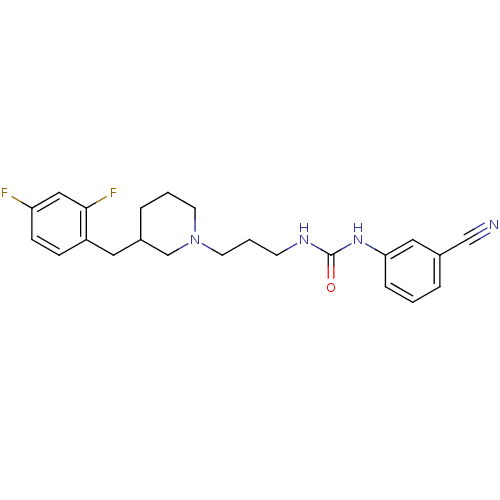 (1-(3-Cyano-phenyl)-3-{3-[3-(2,4-difluoro-benzyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50142463 (1-[3-(3-Benzyl-piperidin-1-yl)-propyl]-3-(3-cyano-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]-eotaxin binding to serotonin 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of muscarinic acetylcholine receptor M2 (unknown origin) | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089366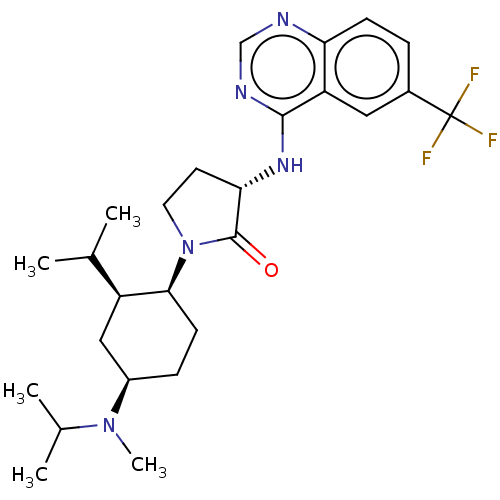 (CHEMBL3577948) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089356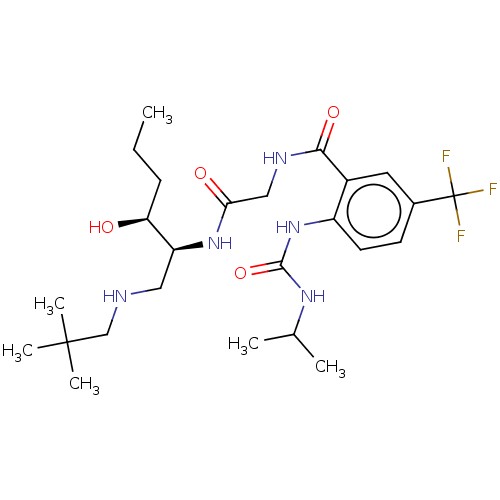 (CHEMBL3577933) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089355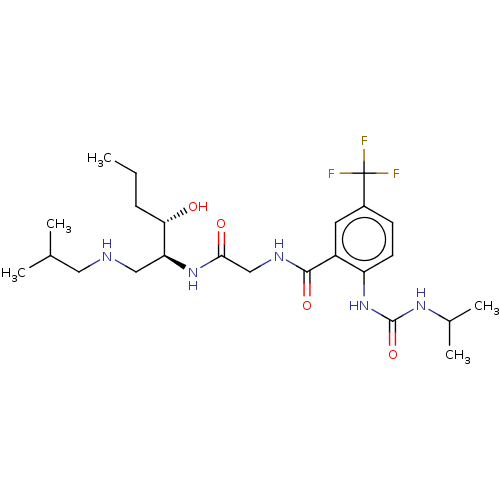 (CHEMBL3577932) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089364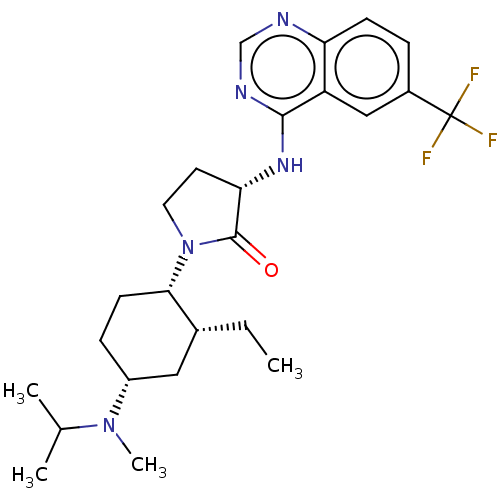 (CHEMBL3577946) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089364 (CHEMBL3577946) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142453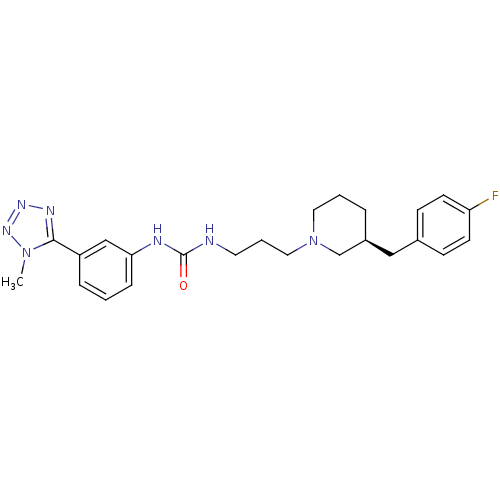 (1-(3-((S)-3-(4-fluorobenzyl)piperidin-1-yl)propyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089353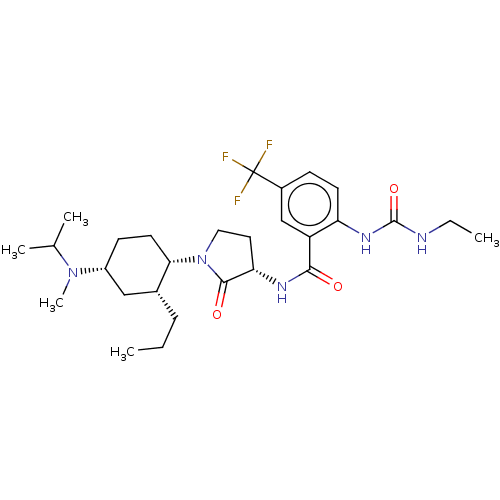 (CHEMBL3577942) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142457 (1-[3,5-Bis-(1-methyl-1H-tetrazol-5-yl)-phenyl]-3-{...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142445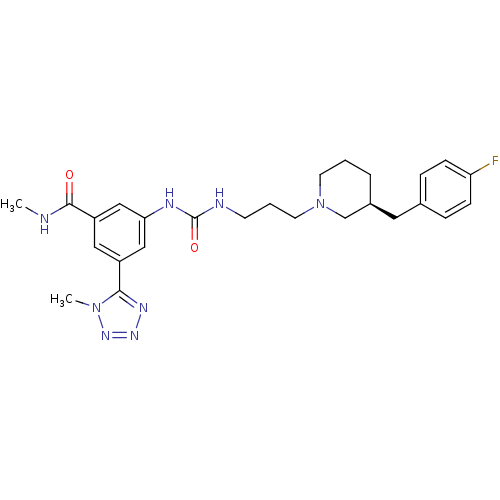 (3-(3-{3-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-yl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50315994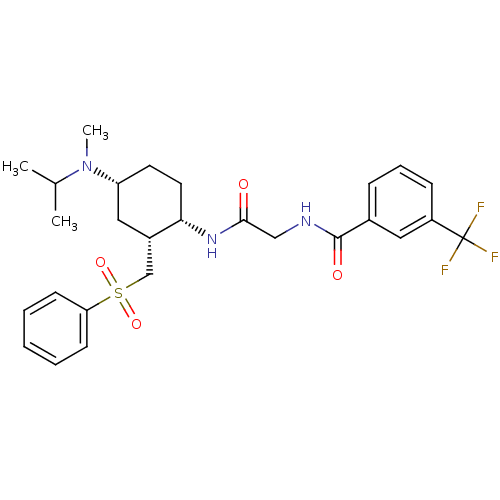 (CHEMBL1091604 | N-(2-((1S,2R,4R)-4-(isopropyl(meth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089356 (CHEMBL3577933) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142456 (1-{3-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-yl]-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089353 (CHEMBL3577942) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089355 (CHEMBL3577932) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142468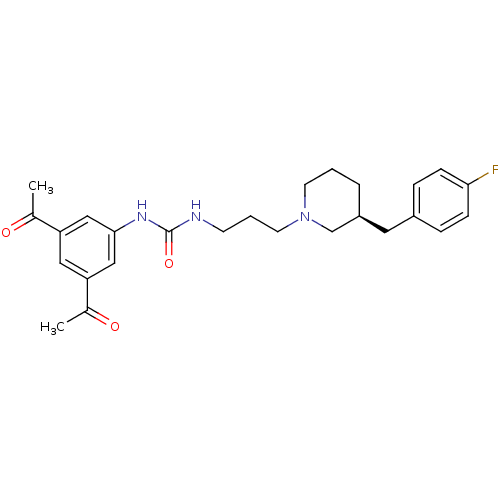 ((S)-1-(3-(3-(4-fluorobenzyl)piperidin-1-yl)propyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142458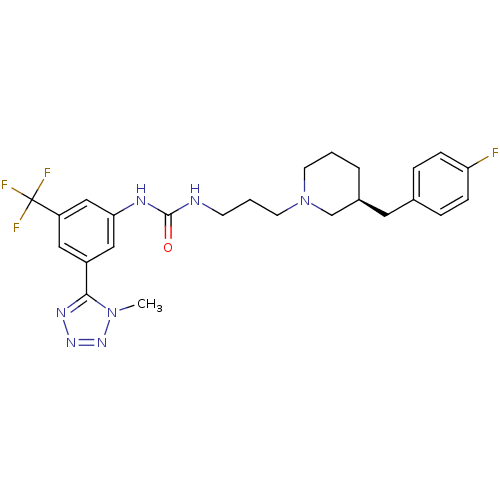 (1-{3-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-yl]-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142472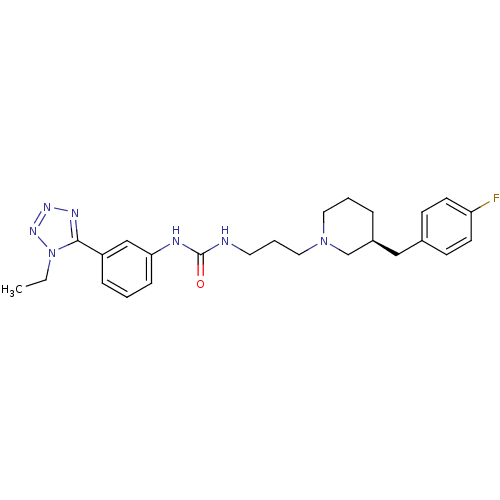 (1-[3-(1-Ethyl-1H-tetrazol-5-yl)-phenyl]-3-{3-[(S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50315994 (CHEMBL1091604 | N-(2-((1S,2R,4R)-4-(isopropyl(meth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142444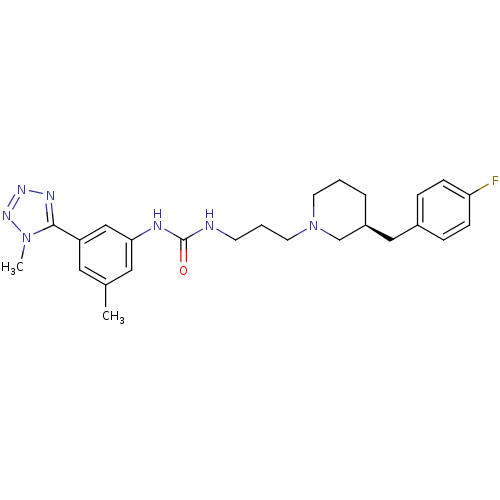 (1-(3-((S)-3-(4-fluorobenzyl)piperidin-1-yl)propyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089362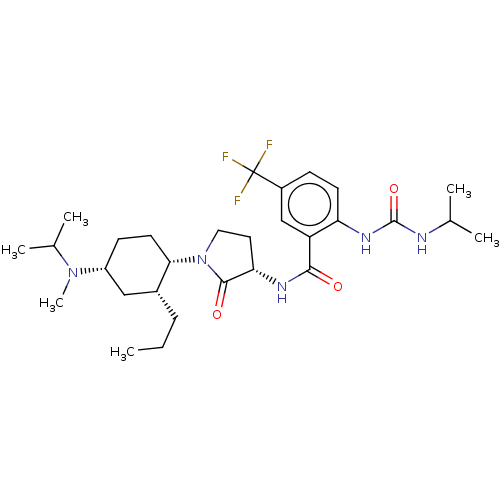 (CHEMBL3577943) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089337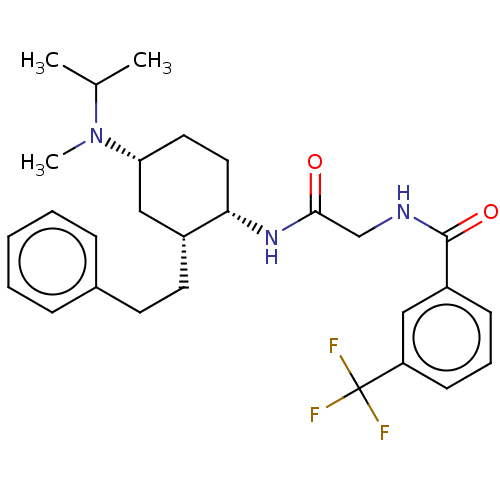 (CHEMBL3577940) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089365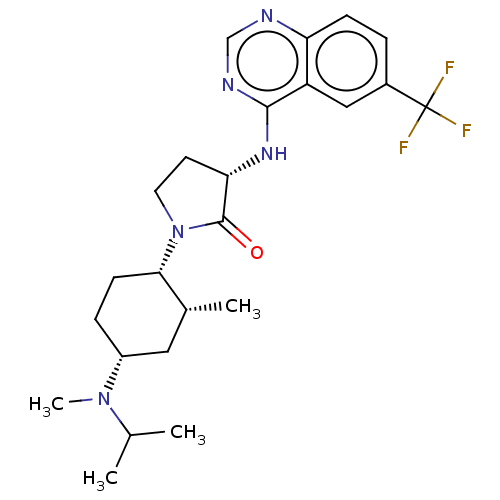 (CHEMBL3577947) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089363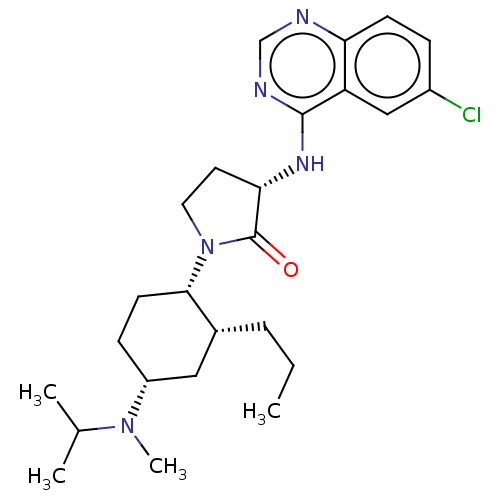 (CHEMBL3577944) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142469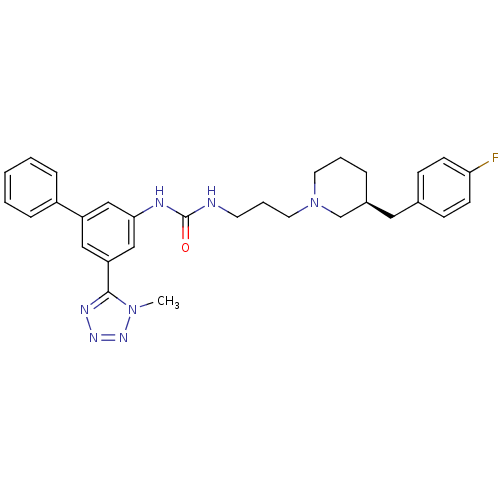 (1-{3-[(S)-3-(4-Fluoro-benzyl)-piperidin-1-yl]-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]MCP1-beta binding to CCR5 in human HT1080 cell membranes incubated for 4 to 6 hrs | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142448 ((S)-1-(3-(3-(4-fluorobenzyl)piperidin-1-yl)propyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089332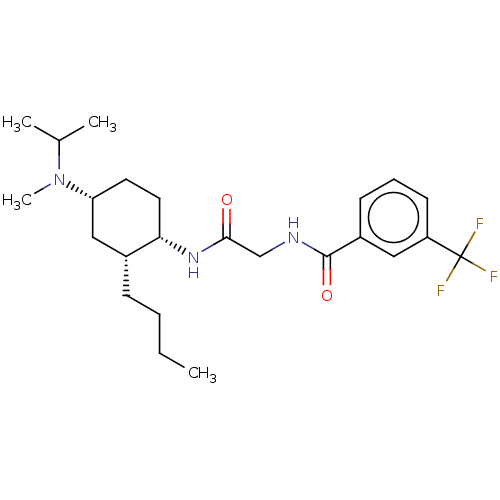 (CHEMBL3577937) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089337 (CHEMBL3577940) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50142451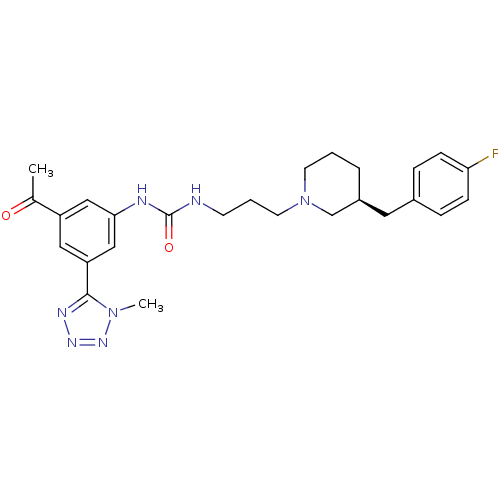 (1-(3-((S)-3-(4-fluorobenzyl)piperidin-1-yl)propyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against C-C chemokine receptor type 3 using human [125I]-eotaxin. | Bioorg Med Chem Lett 14: 1645-9 (2004) Article DOI: 10.1016/j.bmcl.2004.01.059 BindingDB Entry DOI: 10.7270/Q2RB741N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089363 (CHEMBL3577944) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089332 (CHEMBL3577937) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089367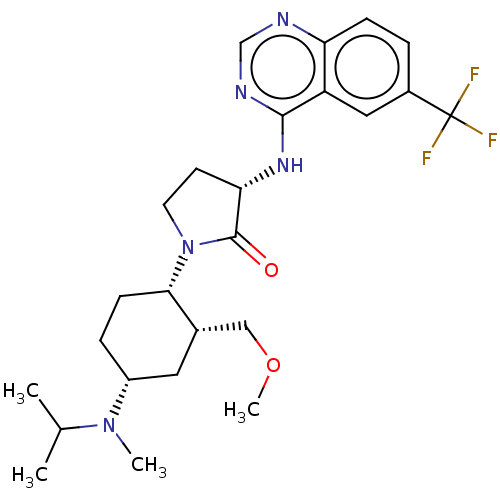 (CHEMBL3577949) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50089364 (CHEMBL3577946) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]MCP1-beta binding to CCR5 in human HT1080 cell membranes incubated for 4 to 6 hrs | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 186 total ) | Next | Last >> |