 Found 347 hits with Last Name = 'colletti' and Initial = 'ae'
Found 347 hits with Last Name = 'colletti' and Initial = 'ae' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003168
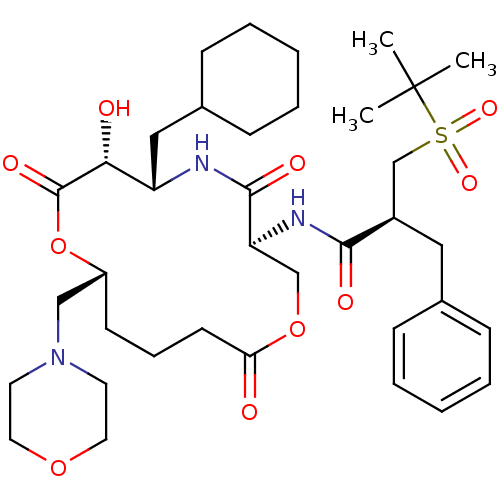
(2-Benzyl-N-(6-cyclohexylmethyl-7-hydroxy-10-morpho...)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@H]1COC(=O)CCC[C@H](CN2CCOCC2)OC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O Show InChI InChI=1S/C37H57N3O10S/c1-37(2,3)51(46,47)25-28(21-26-11-6-4-7-12-26)34(43)39-31-24-49-32(41)16-10-15-29(23-40-17-19-48-20-18-40)50-36(45)33(42)30(38-35(31)44)22-27-13-8-5-9-14-27/h4,6-7,11-12,27-31,33,42H,5,8-10,13-25H2,1-3H3,(H,38,44)(H,39,43)/t28-,29-,30+,31+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003195
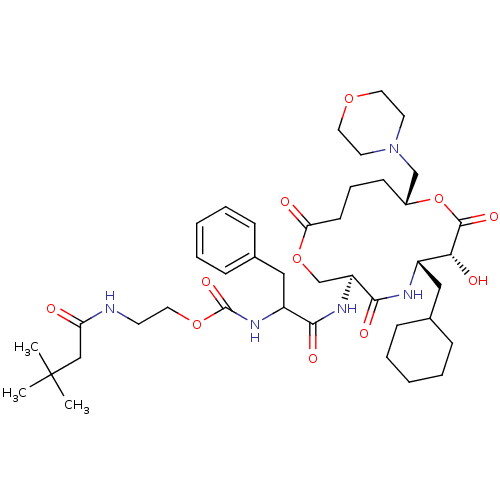
(CHEMBL126115 | [1-(6-Cyclohexylmethyl-7-hydroxy-10...)Show SMILES CC(C)(C)CC(=O)NCCOC(=O)NC(Cc1ccccc1)C(=O)N[C@H]1COC(=O)CCC[C@H](CN2CCOCC2)OC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O Show InChI InChI=1S/C41H63N5O11/c1-41(2,3)25-34(47)42-17-20-55-40(53)45-32(24-29-13-8-5-9-14-29)37(50)44-33-27-56-35(48)16-10-15-30(26-46-18-21-54-22-19-46)57-39(52)36(49)31(43-38(33)51)23-28-11-6-4-7-12-28/h5,8-9,13-14,28,30-33,36,49H,4,6-7,10-12,15-27H2,1-3H3,(H,42,47)(H,43,51)(H,44,50)(H,45,53)/t30-,31+,32?,33+,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003191
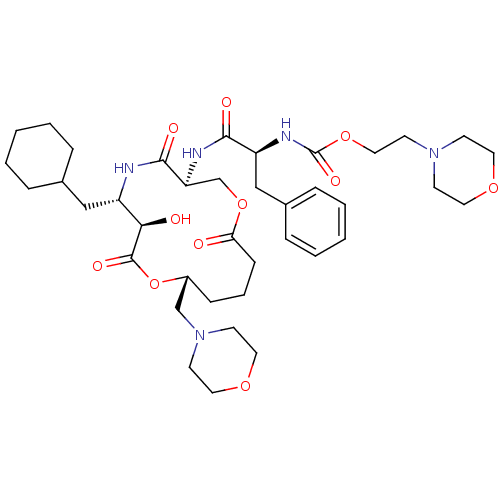
(CHEMBL338008 | [1-(6-Cyclohexylmethyl-7-hydroxy-10...)Show SMILES O[C@@H]1[C@H](CC2CCCCC2)NC(=O)[C@H](COC(=O)CCC[C@H](CN2CCOCC2)OC1=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCCN1CCOCC1 Show InChI InChI=1S/C39H59N5O11/c45-34-13-7-12-30(26-44-16-21-52-22-17-44)55-38(49)35(46)31(24-28-8-3-1-4-9-28)40-37(48)33(27-54-34)41-36(47)32(25-29-10-5-2-6-11-29)42-39(50)53-23-18-43-14-19-51-20-15-43/h2,5-6,10-11,28,30-33,35,46H,1,3-4,7-9,12-27H2,(H,40,48)(H,41,47)(H,42,50)/t30-,31+,32+,33+,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003194

(CHEMBL124616 | [1-(6-Cyclohexylmethyl-7-hydroxy-10...)Show SMILES COCCOCCOCCOC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1COC(=O)CCC[C@H](CN2CCOCC2)OC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O Show InChI InChI=1S/C40H62N4O13/c1-51-19-20-53-21-22-54-23-24-55-40(50)43-33(26-30-11-6-3-7-12-30)37(47)42-34-28-56-35(45)14-8-13-31(27-44-15-17-52-18-16-44)57-39(49)36(46)32(41-38(34)48)25-29-9-4-2-5-10-29/h3,6-7,11-12,29,31-34,36,46H,2,4-5,8-10,13-28H2,1H3,(H,41,48)(H,42,47)(H,43,50)/t31-,32+,33+,34+,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003174

(CHEMBL339289 | {2-[1-(6-Cyclohexylmethyl-7-hydroxy...)Show SMILES CC(C)(C)OC(=O)NC(C)(C)CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1COC(=O)CCC[C@H](CN2CCOCC2)OC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O Show InChI InChI=1S/C42H65N5O11/c1-41(2,3)58-40(54)46-42(4,5)25-34(48)43-32(24-29-15-10-7-11-16-29)37(51)45-33-27-56-35(49)18-12-17-30(26-47-19-21-55-22-20-47)57-39(53)36(50)31(44-38(33)52)23-28-13-8-6-9-14-28/h7,10-11,15-16,28,30-33,36,50H,6,8-9,12-14,17-27H2,1-5H3,(H,43,48)(H,44,52)(H,45,51)(H,46,54)/t30-,31+,32+,33+,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003176

(2-[1-(6-Cyclohexylmethyl-7-hydroxy-10-morpholin-4-...)Show SMILES CC(C)(C)OC(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1COC(=O)CCC[C@H](CN2CCOCC2)OC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O Show InChI InChI=1S/C42H63N5O11/c1-42(2,3)58-41(54)47-19-11-17-34(47)39(52)44-32(25-29-14-8-5-9-15-29)37(50)45-33-27-56-35(48)18-10-16-30(26-46-20-22-55-23-21-46)57-40(53)36(49)31(43-38(33)51)24-28-12-6-4-7-13-28/h5,8-9,14-15,28,30-34,36,49H,4,6-7,10-13,16-27H2,1-3H3,(H,43,51)(H,44,52)(H,45,50)/t30-,31+,32?,33+,34?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003165

(1N-[1-[12-cyclohexylmethyl-11-hydroxy-8-(1,4-oxazi...)Show SMILES CC(C)(N)CC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H]1COC(=O)CCC[C@H](CN2CCOCC2)OC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O Show InChI InChI=1S/C37H57N5O9/c1-37(2,38)22-31(43)39-29(21-26-12-7-4-8-13-26)34(46)41-30-24-50-32(44)15-9-14-27(23-42-16-18-49-19-17-42)51-36(48)33(45)28(40-35(30)47)20-25-10-5-3-6-11-25/h4,7-8,12-13,25,27-30,33,45H,3,5-6,9-11,14-24,38H2,1-2H3,(H,39,43)(H,40,47)(H,41,46)/t27-,28+,29+,30+,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003181

((diastereomer-1) (1-{6-Cyclohexylmethyl-7-hydroxy-...)Show SMILES CC(C)(C)OC(=O)NC(Cc1ccccc1)C(=O)N[C@H]1COC(=O)CCCC(CNC(=O)OCC(Cl)(Cl)Cl)OC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O Show InChI InChI=1S/C36H51Cl3N4O11/c1-35(2,3)54-34(50)43-26(18-23-13-8-5-9-14-23)30(46)42-27-20-51-28(44)16-10-15-24(19-40-33(49)52-21-36(37,38)39)53-32(48)29(45)25(41-31(27)47)17-22-11-6-4-7-12-22/h5,8-9,13-14,22,24-27,29,45H,4,6-7,10-12,15-21H2,1-3H3,(H,40,49)(H,41,47)(H,42,46)(H,43,50)/t24?,25-,26?,27-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003181

((diastereomer-1) (1-{6-Cyclohexylmethyl-7-hydroxy-...)Show SMILES CC(C)(C)OC(=O)NC(Cc1ccccc1)C(=O)N[C@H]1COC(=O)CCCC(CNC(=O)OCC(Cl)(Cl)Cl)OC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O Show InChI InChI=1S/C36H51Cl3N4O11/c1-35(2,3)54-34(50)43-26(18-23-13-8-5-9-14-23)30(46)42-27-20-51-28(44)16-10-15-24(19-40-33(49)52-21-36(37,38)39)53-32(48)29(45)25(41-31(27)47)17-22-11-6-4-7-12-22/h5,8-9,13-14,22,24-27,29,45H,4,6-7,10-12,15-21H2,1-3H3,(H,40,49)(H,41,47)(H,42,46)(H,43,50)/t24?,25-,26?,27-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003192

(2-(1-Aza-bicyclo[2.2.2]oct-3-ylamino)-N-(6-cyclohe...)Show SMILES O[C@@H]1[C@H](CC2CCCCC2)NC(=O)[C@H](COC(=O)CCC[C@H](CN2CCOCC2)OC1=O)NC(=O)[C@H](Cc1ccccc1)NC1CN2CCC1CC2 |wU:13.34,wD:35.46,2.2,21.22,1.0,(15.07,-8.78,;13.73,-8.02,;12.4,-8.78,;12.4,-10.34,;13.51,-11.43,;14.96,-11.01,;16.02,-12.12,;15.65,-13.56,;14.17,-13.96,;13.1,-12.89,;11.06,-8.06,;9.73,-8.83,;9.75,-10.38,;8.39,-8.08,;8.36,-6.53,;7.02,-5.78,;6.99,-4.23,;5.65,-3.47,;8.34,-3.44,;9.69,-4.2,;11.03,-3.4,;12.38,-4.16,;13.72,-3.4,;15.26,-3.4,;16.05,-4.74,;17.58,-4.74,;18.34,-3.4,;17.57,-2.05,;16.02,-2.07,;12.37,-5.71,;13.72,-6.48,;15.05,-5.69,;7.04,-8.87,;5.69,-8.11,;5.67,-6.55,;4.36,-8.9,;4.37,-10.43,;3.61,-11.77,;4.35,-13.1,;3.58,-14.42,;2.03,-14.41,;1.28,-13.07,;2.05,-11.75,;3.03,-8.13,;1.68,-8.9,;1.67,-10.43,;.33,-11.2,;-.99,-10.41,;-.97,-8.87,;.36,-8.12,;-.35,-9.41,;.88,-10.05,)| Show InChI InChI=1S/C39H59N5O8/c45-35-13-7-12-30(24-44-18-20-50-21-19-44)52-39(49)36(46)31(22-27-8-3-1-4-9-27)41-38(48)34(26-51-35)42-37(47)32(23-28-10-5-2-6-11-28)40-33-25-43-16-14-29(33)15-17-43/h2,5-6,10-11,27,29-34,36,40,46H,1,3-4,7-9,12-26H2,(H,41,48)(H,42,47)/t30-,31+,32+,33?,34+,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50230157

(CHEMBL3144232)Show SMILES OC(=O)C(F)(F)F.O[C@@H]1[C@H](CC2CCCCC2)NC(=O)[C@H](COC(=O)CCC[C@H](CN2CCOCC2)OC1=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1 |r| Show InChI InChI=1S/C37H55N5O9.C2HF3O2/c43-32-15-7-13-27(23-42-17-19-49-20-18-42)51-37(48)33(44)29(21-25-9-3-1-4-10-25)39-36(47)31(24-50-32)41-35(46)30(22-26-11-5-2-6-12-26)40-34(45)28-14-8-16-38-28;3-2(4,5)1(6)7/h2,5-6,11-12,25,27-31,33,38,44H,1,3-4,7-10,13-24H2,(H,39,47)(H,40,45)(H,41,46);(H,6,7)/t27-,28+,29+,30+,31+,33-;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341035

(1-(4-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-b...)Show SMILES CNC(=O)Nc1ccc(Nc2nc3ccccc3n2-c2nc(C)nc(N)n2)cc1 Show InChI InChI=1S/C19H19N9O/c1-11-22-16(20)27-17(23-11)28-15-6-4-3-5-14(15)26-18(28)24-12-7-9-13(10-8-12)25-19(29)21-2/h3-10H,1-2H3,(H,24,26)(H2,21,25,29)(H2,20,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396298
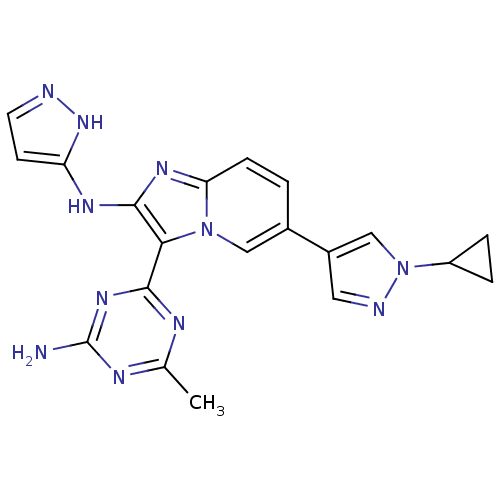
(CHEMBL2172489)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cnn(c1)C1CC1 Show InChI InChI=1S/C20H19N11/c1-11-24-18(28-20(21)25-11)17-19(26-15-6-7-22-29-15)27-16-5-2-12(9-30(16)17)13-8-23-31(10-13)14-3-4-14/h2,5-10,14H,3-4H2,1H3,(H2,22,26,29)(H2,21,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396305
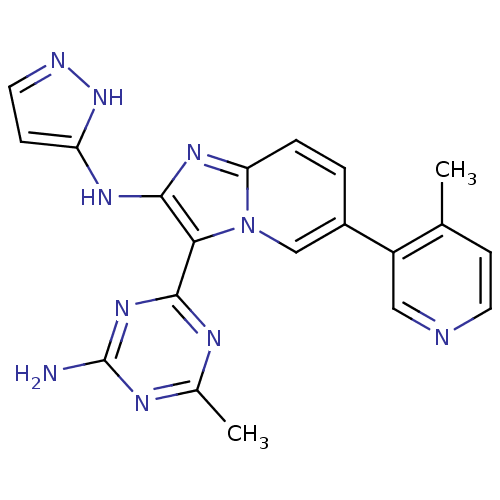
(CHEMBL2172482)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cnccc1C Show InChI InChI=1S/C20H18N10/c1-11-5-7-22-9-14(11)13-3-4-16-27-19(26-15-6-8-23-29-15)17(30(16)10-13)18-24-12(2)25-20(21)28-18/h3-10H,1-2H3,(H2,23,26,29)(H2,21,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396302
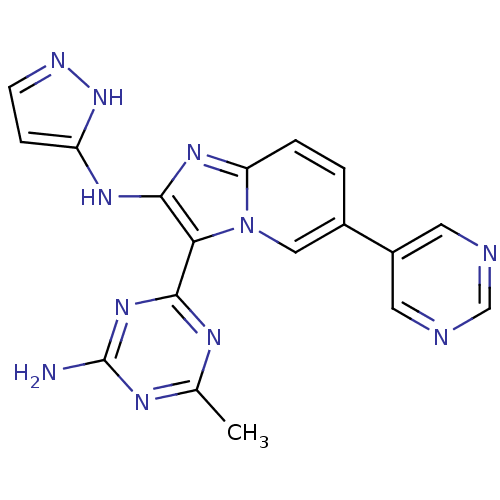
(CHEMBL2172485)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cncnc1 Show InChI InChI=1S/C18H15N11/c1-10-23-16(27-18(19)24-10)15-17(25-13-4-5-22-28-13)26-14-3-2-11(8-29(14)15)12-6-20-9-21-7-12/h2-9H,1H3,(H2,22,25,28)(H2,19,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396301
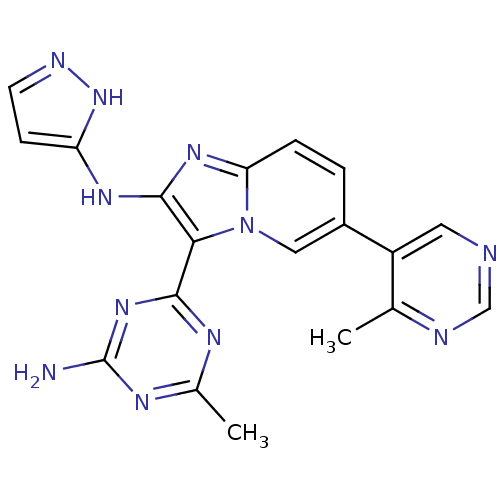
(CHEMBL2172486)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cncnc1C Show InChI InChI=1S/C19H17N11/c1-10-13(7-21-9-22-10)12-3-4-15-27-18(26-14-5-6-23-29-14)16(30(15)8-12)17-24-11(2)25-19(20)28-17/h3-9H,1-2H3,(H2,23,26,29)(H2,20,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396316
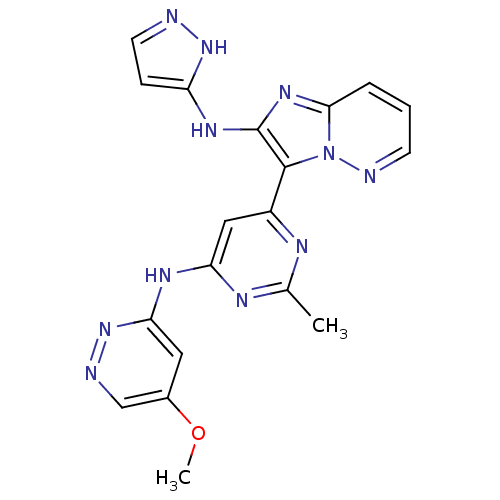
(CHEMBL2172635)Show SMILES COc1cnnc(Nc2cc(nc(C)n2)-c2c(Nc3ccn[nH]3)nc3cccnn23)c1 Show InChI InChI=1S/C19H17N11O/c1-11-23-13(9-15(24-11)25-16-8-12(31-2)10-21-29-16)18-19(26-14-5-7-20-28-14)27-17-4-3-6-22-30(17)18/h3-10H,1-2H3,(H2,20,26,28)(H,23,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396297
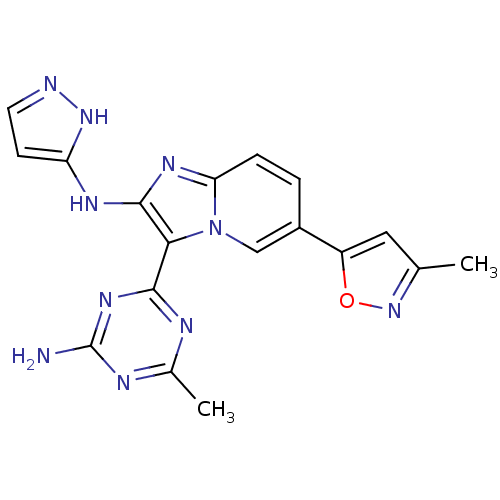
(CHEMBL2172490)Show SMILES Cc1cc(on1)-c1ccc2nc(Nc3ccn[nH]3)c(-c3nc(C)nc(N)n3)n2c1 Show InChI InChI=1S/C18H16N10O/c1-9-7-12(29-27-9)11-3-4-14-24-17(23-13-5-6-20-26-13)15(28(14)8-11)16-21-10(2)22-18(19)25-16/h3-8H,1-2H3,(H2,20,23,26)(H2,19,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50341054
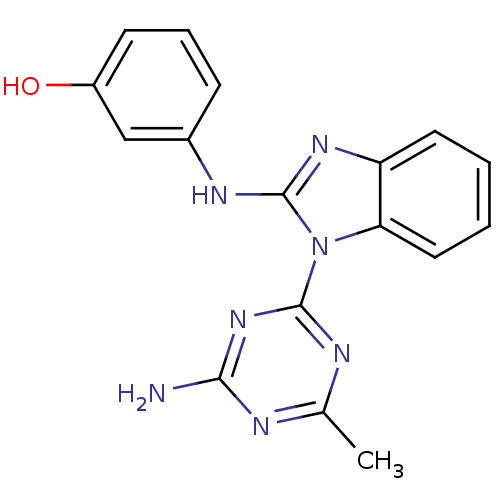
(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396322
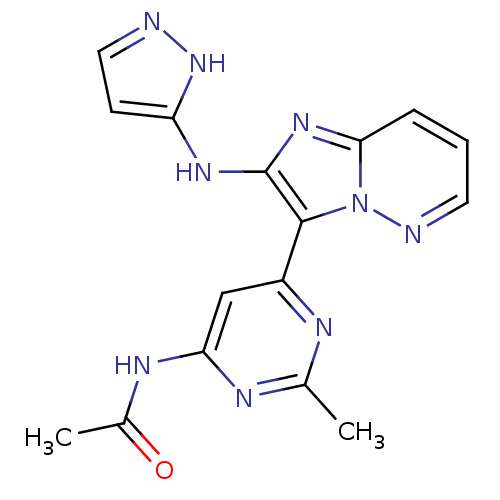
(CHEMBL2172629)Show SMILES CC(=O)Nc1cc(nc(C)n1)-c1c(Nc2ccn[nH]2)nc2cccnn12 Show InChI InChI=1S/C16H15N9O/c1-9-19-11(8-13(20-9)21-10(2)26)15-16(22-12-5-7-17-24-12)23-14-4-3-6-18-25(14)15/h3-8H,1-2H3,(H2,17,22,24)(H,19,20,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396307
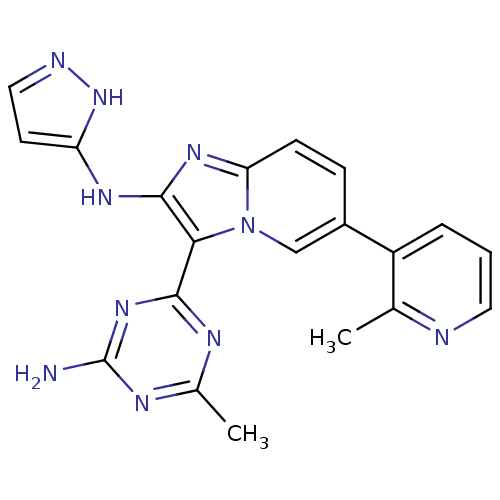
(CHEMBL2172480)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cccnc1C Show InChI InChI=1S/C20H18N10/c1-11-14(4-3-8-22-11)13-5-6-16-27-19(26-15-7-9-23-29-15)17(30(16)10-13)18-24-12(2)25-20(21)28-18/h3-10H,1-2H3,(H2,23,26,29)(H2,21,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396308
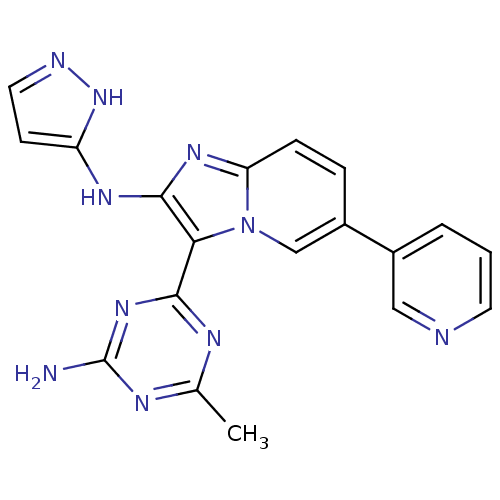
(CHEMBL2172636)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cccnc1 Show InChI InChI=1S/C19H16N10/c1-11-23-17(27-19(20)24-11)16-18(25-14-6-8-22-28-14)26-15-5-4-13(10-29(15)16)12-3-2-7-21-9-12/h2-10H,1H3,(H2,22,25,28)(H2,20,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003190

((diastereomer-1)[1-(6-Cyclohexylmethyl-7-hydroxy-4...)Show SMILES CC(C)(C)OC(=O)NC(Cc1ccccc1)C(=O)N[C@H]1COC(=O)CCCCOC(=O)[C@H](O)[C@H](CC2CCCCC2)NC1=O Show InChI InChI=1S/C32H47N3O9/c1-32(2,3)44-31(41)35-24(19-22-14-8-5-9-15-22)28(38)34-25-20-43-26(36)16-10-11-17-42-30(40)27(37)23(33-29(25)39)18-21-12-6-4-7-13-21/h5,8-9,14-15,21,23-25,27,37H,4,6-7,10-13,16-20H2,1-3H3,(H,33,39)(H,34,38)(H,35,41)/t23-,24?,25-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003188

((diastereomer-1) [1-(6-Cyclohexylmethyl-10-dimethy...)Show SMILES CN(C)CC1CCCC(=O)OC[C@H](NC(=O)C(Cc2ccccc2)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)C(=O)O1 Show InChI InChI=1S/C35H54N4O9/c1-35(2,3)48-34(45)38-27(20-24-15-10-7-11-16-24)31(42)37-28-22-46-29(40)18-12-17-25(21-39(4)5)47-33(44)30(41)26(36-32(28)43)19-23-13-8-6-9-14-23/h7,10-11,15-16,23,25-28,30,41H,6,8-9,12-14,17-22H2,1-5H3,(H,36,43)(H,37,42)(H,38,45)/t25?,26-,27?,28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003177
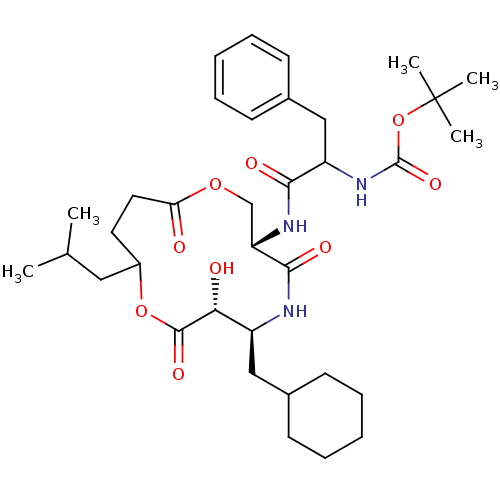
((diastereomer-1) [1-(6-Cyclohexylmethyl-7-hydroxy-...)Show SMILES CC(C)CC1CCC(=O)OC[C@H](NC(=O)C(Cc2ccccc2)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)C(=O)O1 Show InChI InChI=1S/C35H53N3O9/c1-22(2)18-25-16-17-29(39)45-21-28(32(42)36-26(30(40)33(43)46-25)19-23-12-8-6-9-13-23)37-31(41)27(20-24-14-10-7-11-15-24)38-34(44)47-35(3,4)5/h7,10-11,14-15,22-23,25-28,30,40H,6,8-9,12-13,16-21H2,1-5H3,(H,36,42)(H,37,41)(H,38,44)/t25?,26-,27?,28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003177

((diastereomer-1) [1-(6-Cyclohexylmethyl-7-hydroxy-...)Show SMILES CC(C)CC1CCC(=O)OC[C@H](NC(=O)C(Cc2ccccc2)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)C(=O)O1 Show InChI InChI=1S/C35H53N3O9/c1-22(2)18-25-16-17-29(39)45-21-28(32(42)36-26(30(40)33(43)46-25)19-23-12-8-6-9-13-23)37-31(41)27(20-24-14-10-7-11-15-24)38-34(44)47-35(3,4)5/h7,10-11,14-15,22-23,25-28,30,40H,6,8-9,12-13,16-21H2,1-5H3,(H,36,42)(H,37,41)(H,38,44)/t25?,26-,27?,28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003188

((diastereomer-1) [1-(6-Cyclohexylmethyl-10-dimethy...)Show SMILES CN(C)CC1CCCC(=O)OC[C@H](NC(=O)C(Cc2ccccc2)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)C(=O)O1 Show InChI InChI=1S/C35H54N4O9/c1-35(2,3)48-34(45)38-27(20-24-15-10-7-11-16-24)31(42)37-28-22-46-29(40)18-12-17-25(21-39(4)5)47-33(44)30(41)26(36-32(28)43)19-23-13-8-6-9-14-23/h7,10-11,15-16,23,25-28,30,41H,6,8-9,12-14,17-22H2,1-5H3,(H,36,43)(H,37,42)(H,38,45)/t25?,26-,27?,28-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396303
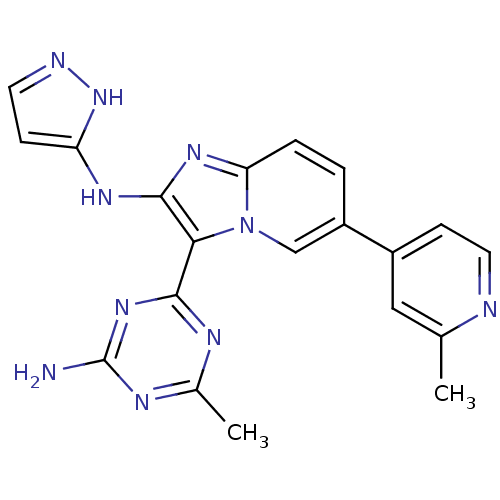
(CHEMBL2172484)Show SMILES Cc1cc(ccn1)-c1ccc2nc(Nc3ccn[nH]3)c(-c3nc(C)nc(N)n3)n2c1 Show InChI InChI=1S/C20H18N10/c1-11-9-13(5-7-22-11)14-3-4-16-27-19(26-15-6-8-23-29-15)17(30(16)10-14)18-24-12(2)25-20(21)28-18/h3-10H,1-2H3,(H2,23,26,29)(H2,21,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396299
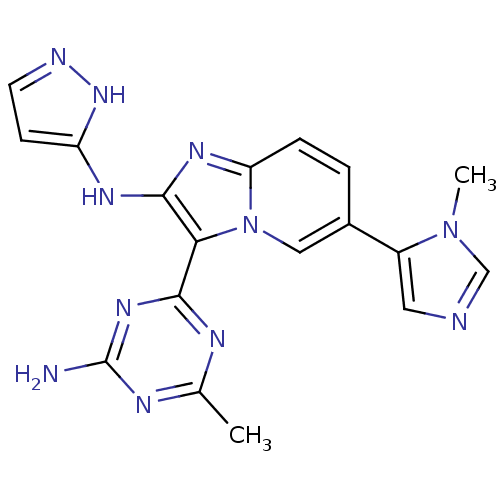
(CHEMBL2172488)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1cncn1C Show InChI InChI=1S/C18H17N11/c1-10-22-16(26-18(19)23-10)15-17(24-13-5-6-21-27-13)25-14-4-3-11(8-29(14)15)12-7-20-9-28(12)2/h3-9H,1-2H3,(H2,21,24,27)(H2,19,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341056

(5-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show SMILES Cc1nc(N)nc(n1)-n1c(Nc2cc(O)cc(O)c2)nc2ccccc12 Show InChI InChI=1S/C17H15N7O2/c1-9-19-15(18)23-16(20-9)24-14-5-3-2-4-13(14)22-17(24)21-10-6-11(25)8-12(26)7-10/h2-8,25-26H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human U-87 cells assessed as inhibition of phosphorylation of AKT at S473 |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341035

(1-(4-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-b...)Show SMILES CNC(=O)Nc1ccc(Nc2nc3ccccc3n2-c2nc(C)nc(N)n2)cc1 Show InChI InChI=1S/C19H19N9O/c1-11-22-16(20)27-17(23-11)28-15-6-4-3-5-14(15)26-18(28)24-12-7-9-13(10-8-12)25-19(29)21-2/h3-10H,1-2H3,(H,24,26)(H2,21,25,29)(H2,20,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396321
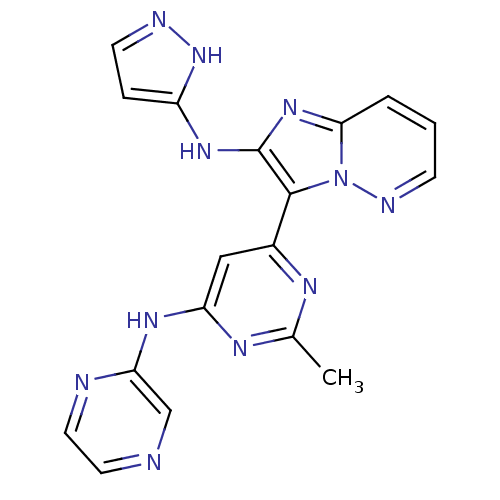
(CHEMBL2172630)Show SMILES Cc1nc(Nc2cnccn2)cc(n1)-c1c(Nc2ccn[nH]2)nc2cccnn12 Show InChI InChI=1S/C18H15N11/c1-11-23-12(9-14(24-11)25-15-10-19-7-8-20-15)17-18(26-13-4-6-21-28-13)27-16-3-2-5-22-29(16)17/h2-10H,1H3,(H2,21,26,28)(H,20,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396309
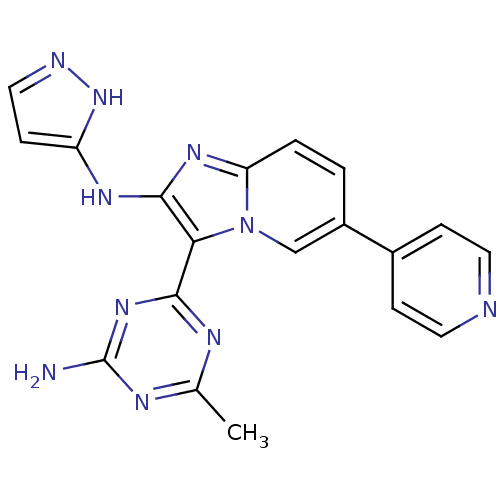
(CHEMBL2172640)Show SMILES Cc1nc(N)nc(n1)-c1c(Nc2ccn[nH]2)nc2ccc(cn12)-c1ccncc1 Show InChI InChI=1S/C19H16N10/c1-11-23-17(27-19(20)24-11)16-18(25-14-6-9-22-28-14)26-15-3-2-13(10-29(15)16)12-4-7-21-8-5-12/h2-10H,1H3,(H2,22,25,28)(H2,20,23,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003171

((diastereomer-1) [1-(6-Cyclohexylmethyl-10-diethyl...)Show SMILES CCN(CC)CC1CCCC(=O)OC[C@H](NC(=O)C(Cc2ccccc2)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)C(=O)O1 Show InChI InChI=1S/C37H58N4O9/c1-6-41(7-2)23-27-19-14-20-31(42)48-24-30(34(45)38-28(32(43)35(46)49-27)21-25-15-10-8-11-16-25)39-33(44)29(22-26-17-12-9-13-18-26)40-36(47)50-37(3,4)5/h9,12-13,17-18,25,27-30,32,43H,6-8,10-11,14-16,19-24H2,1-5H3,(H,38,45)(H,39,44)(H,40,47)/t27?,28-,29?,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003171

((diastereomer-1) [1-(6-Cyclohexylmethyl-10-diethyl...)Show SMILES CCN(CC)CC1CCCC(=O)OC[C@H](NC(=O)C(Cc2ccccc2)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)C(=O)O1 Show InChI InChI=1S/C37H58N4O9/c1-6-41(7-2)23-27-19-14-20-31(42)48-24-30(34(45)38-28(32(43)35(46)49-27)21-25-15-10-8-11-16-25)39-33(44)29(22-26-17-12-9-13-18-26)40-36(47)50-37(3,4)5/h9,12-13,17-18,25,27-30,32,43H,6-8,10-11,14-16,19-24H2,1-5H3,(H,38,45)(H,39,44)(H,40,47)/t27?,28-,29?,30-,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341063
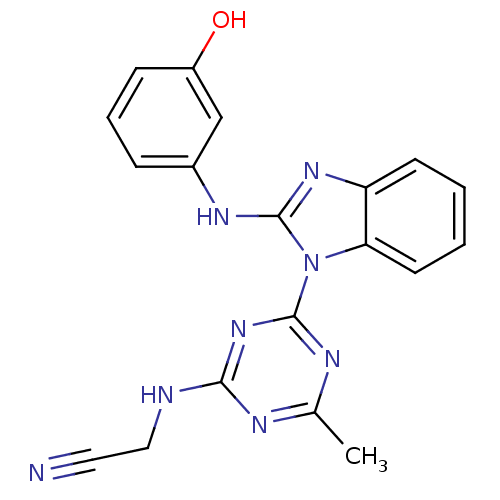
(2-(4-(2-(3-hydroxyphenylamino)-1H-benzo[d]imidazol...)Show SMILES Cc1nc(NCC#N)nc(n1)-n1c(Nc2cccc(O)c2)nc2ccccc12 Show InChI InChI=1S/C19H16N8O/c1-12-22-17(21-10-9-20)26-18(23-12)27-16-8-3-2-7-15(16)25-19(27)24-13-5-4-6-14(28)11-13/h2-8,11,28H,10H2,1H3,(H,24,25)(H,21,22,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR assessed as inhibition of phosphorylation of 4EBP1 by Lantha-Screen enzyme assay |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human U-87 cells assessed as inhibition of phosphorylation of AKT at S473 |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341054

(3-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-benz...)Show InChI InChI=1S/C17H15N7O/c1-10-19-15(18)23-16(20-10)24-14-8-3-2-7-13(14)22-17(24)21-11-5-4-6-12(25)9-11/h2-9,25H,1H3,(H,21,22)(H2,18,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50396314
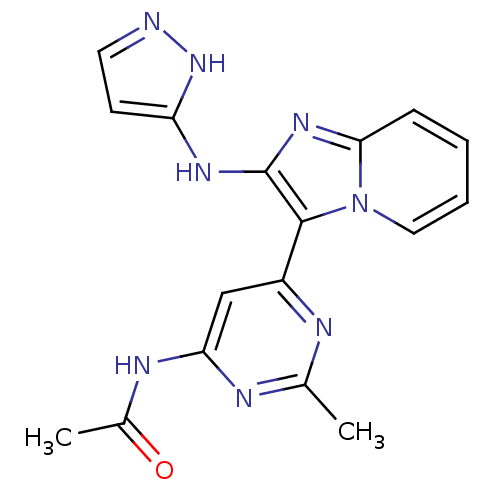
(CHEMBL2172475)Show SMILES CC(=O)Nc1cc(nc(C)n1)-c1c(Nc2ccn[nH]2)nc2ccccn12 Show InChI InChI=1S/C17H16N8O/c1-10-19-12(9-14(20-10)21-11(2)26)16-17(22-13-6-7-18-24-13)23-15-5-3-4-8-25(15)16/h3-9H,1-2H3,(H2,18,22,24)(H,19,20,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
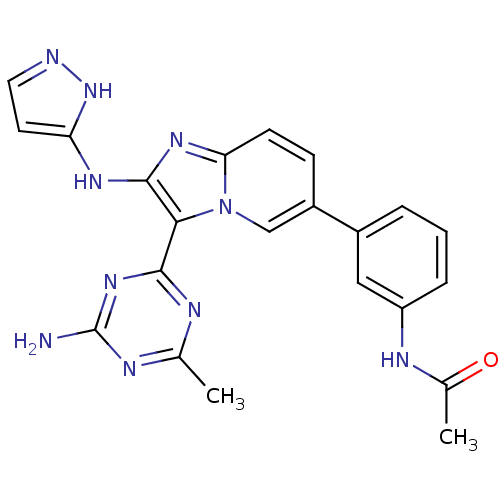
(Homo sapiens (Human)) | BDBM50396300
(CHEMBL2172487)Show SMILES CC(=O)Nc1cccc(c1)-c1ccc2nc(Nc3ccn[nH]3)c(-c3nc(C)nc(N)n3)n2c1 Show InChI InChI=1S/C22H20N10O/c1-12-25-20(30-22(23)26-12)19-21(28-17-8-9-24-31-17)29-18-7-6-15(11-32(18)19)14-4-3-5-16(10-14)27-13(2)33/h3-11H,1-2H3,(H,27,33)(H2,24,28,31)(H2,23,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by LanthaScreen assay |
Bioorg Med Chem Lett 22: 4967-74 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.033
BindingDB Entry DOI: 10.7270/Q2BK1DHN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341035

(1-(4-(1-(4-amino-6-methyl-1,3,5-triazin-2-yl)-1H-b...)Show SMILES CNC(=O)Nc1ccc(Nc2nc3ccccc3n2-c2nc(C)nc(N)n2)cc1 Show InChI InChI=1S/C19H19N9O/c1-11-22-16(20)27-17(23-11)28-15-6-4-3-5-14(15)26-18(28)24-12-7-9-13(10-8-12)25-19(29)21-2/h3-10H,1-2H3,(H,24,26)(H2,21,25,29)(H2,20,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in human U-87 cells assessed as inhibition of phosphorylation of AKT at S473 |
Bioorg Med Chem Lett 21: 2064-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.007
BindingDB Entry DOI: 10.7270/Q2FT8MBW |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50003175
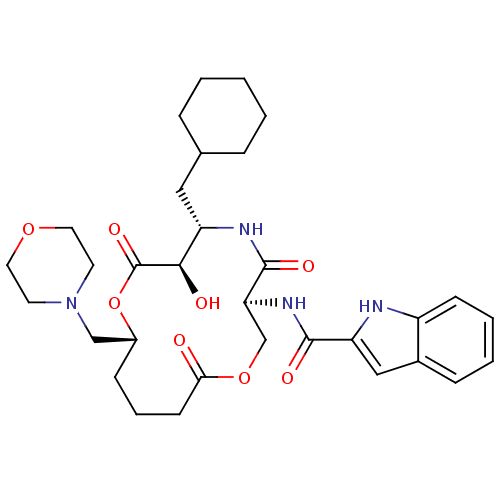
(1H-Indole-2-carboxylic acid (6-cyclohexylmethyl-7-...)Show SMILES O[C@@H]1[C@H](CC2CCCCC2)NC(=O)[C@H](COC(=O)CCC[C@H](CN2CCOCC2)OC1=O)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C32H44N4O8/c37-28-12-6-10-23(19-36-13-15-42-16-14-36)44-32(41)29(38)25(17-21-7-2-1-3-8-21)34-31(40)27(20-43-28)35-30(39)26-18-22-9-4-5-11-24(22)33-26/h4-5,9,11,18,21,23,25,27,29,33,38H,1-3,6-8,10,12-17,19-20H2,(H,34,40)(H,35,39)/t23-,25+,27+,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human renin inhibition (at pH 7.4) |
J Med Chem 35: 3755-73 (1992)
BindingDB Entry DOI: 10.7270/Q26W9BPZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data