

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50213845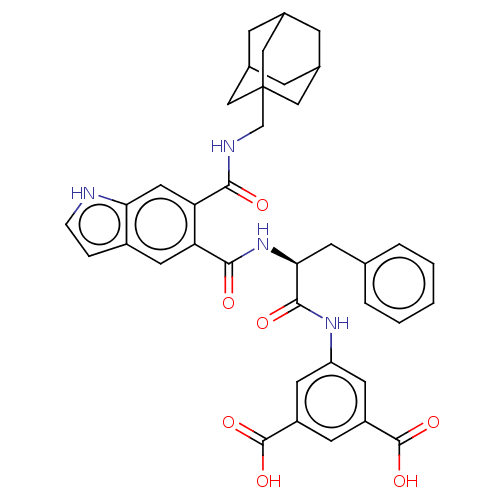 (CHEMBL14557) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50470629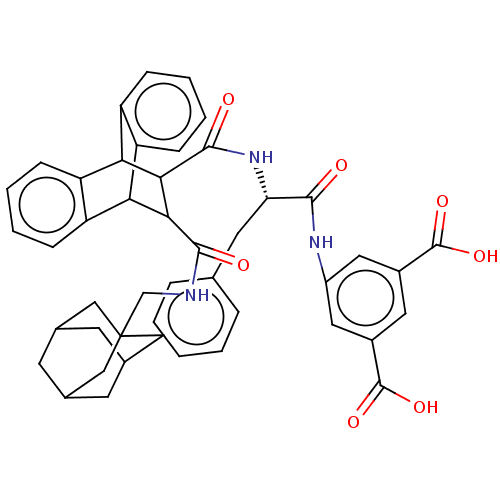 (CHEMBL342616) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217235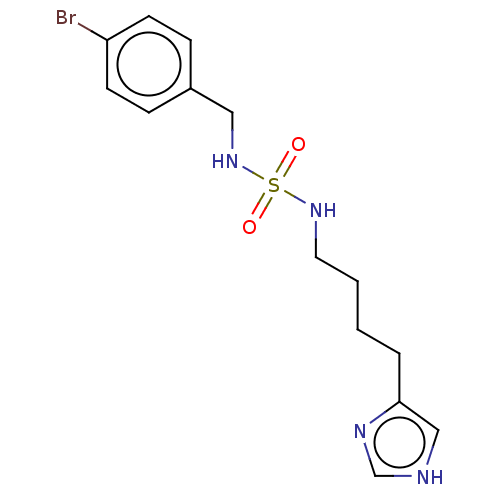 (CHEMBL104827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217241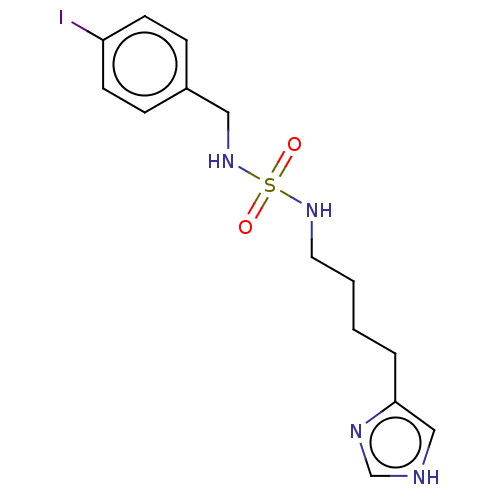 (CHEMBL104366) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217236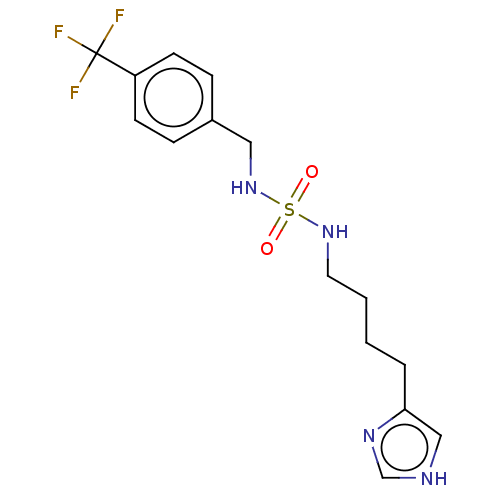 (CHEMBL105744) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217238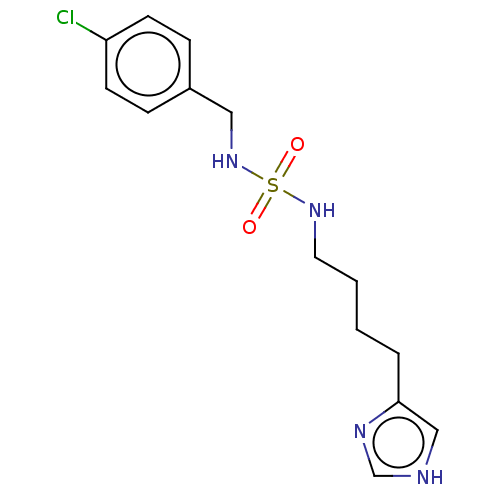 (CHEMBL107083) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217249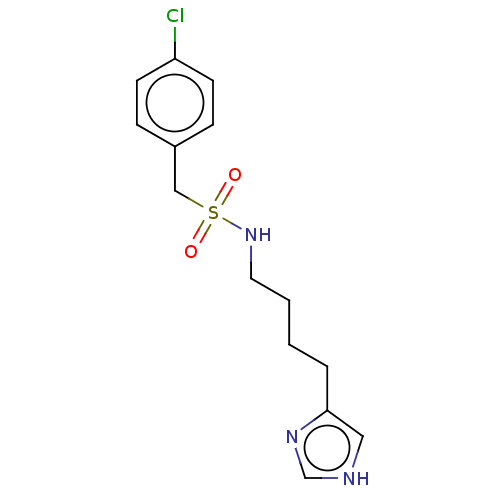 (CHEMBL104646) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217234 (CHEMBL106342) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50421390 (CHEMBL24313) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217237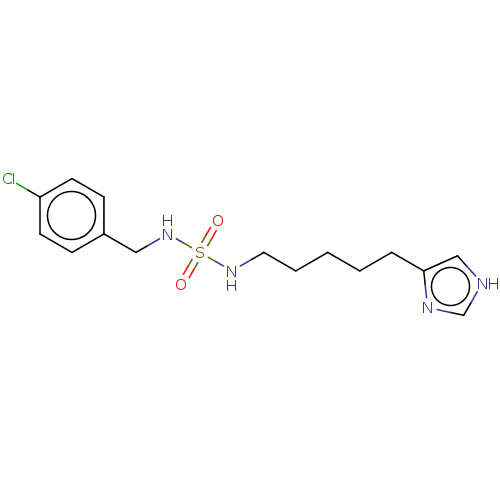 (CHEMBL105748) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217242 (CHEMBL105495) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217239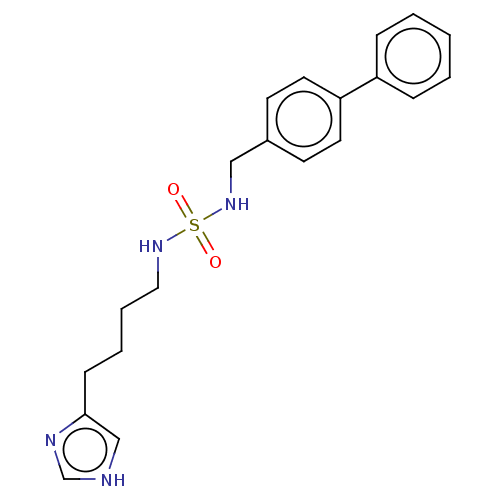 (CHEMBL105161) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217243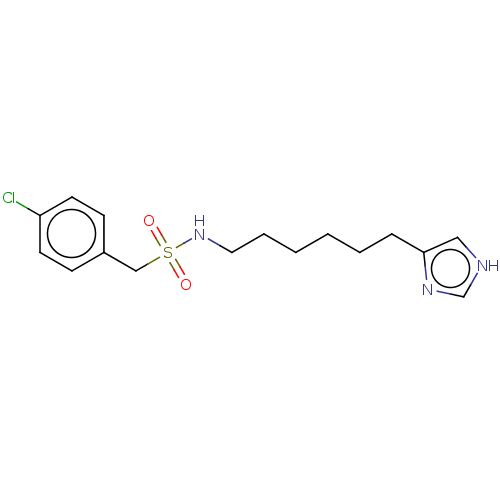 (CHEMBL104522) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217248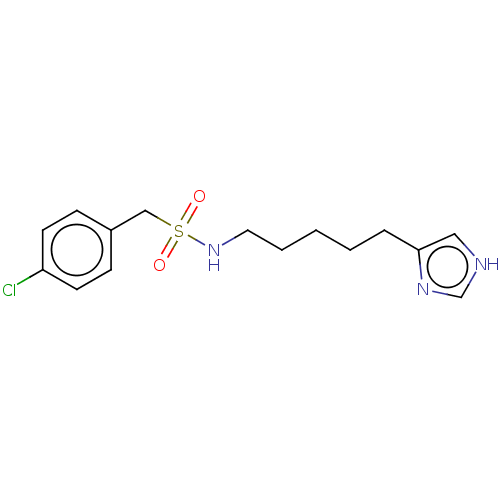 (CHEMBL322779) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50471064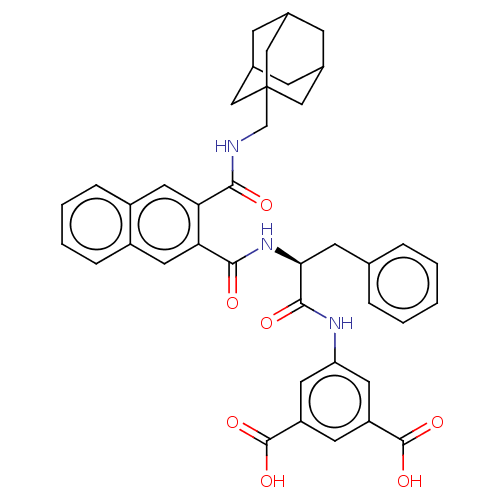 (CHEMBL301810) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217240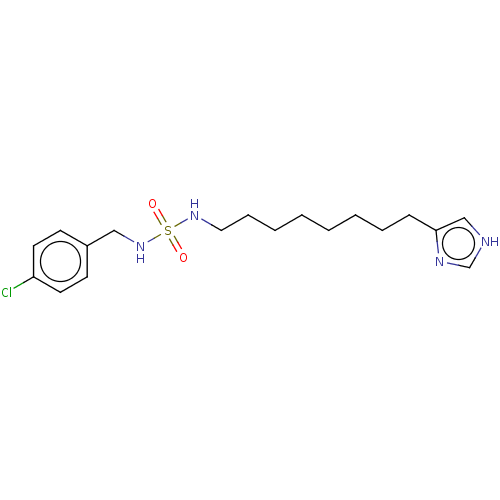 (CHEMBL104717) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217244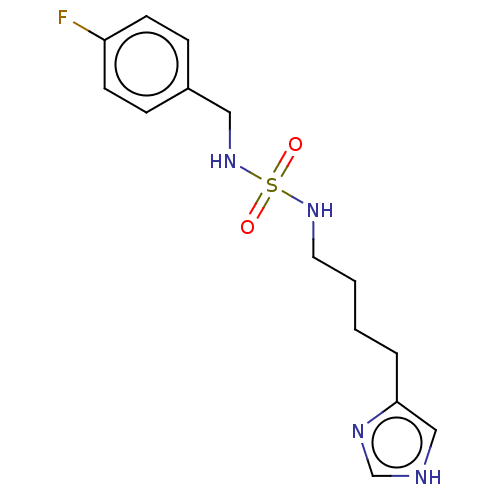 (CHEMBL423320) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475510 (CHEMBL2067967) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475518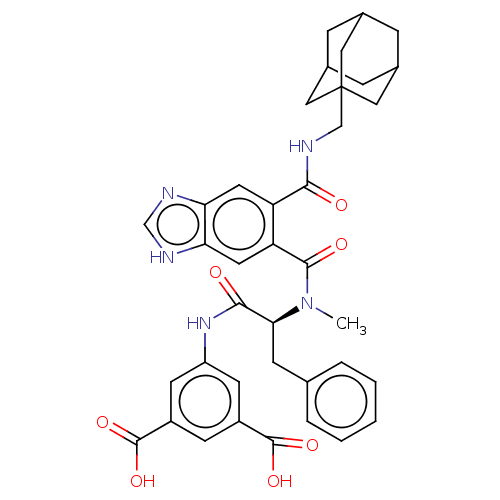 (CHEMBL2067951) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217245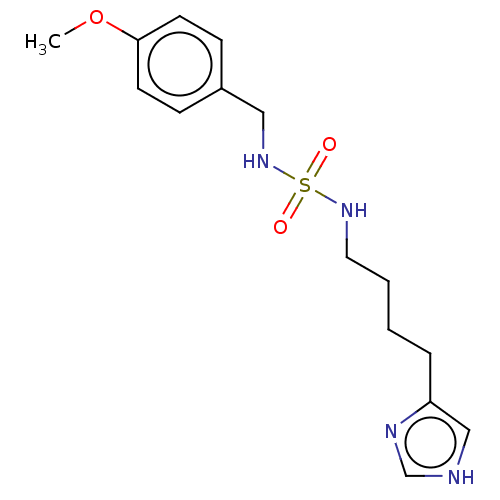 (CHEMBL106278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475513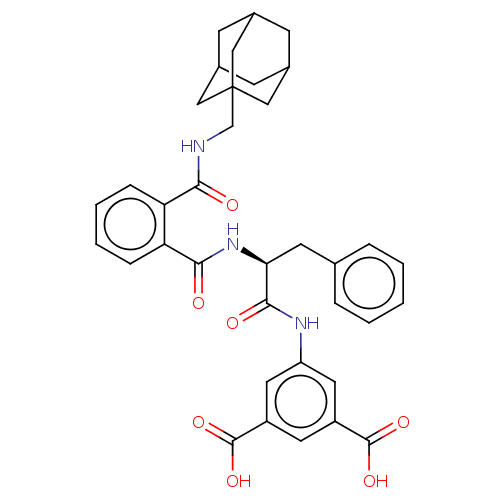 (CHEMBL2067963) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217250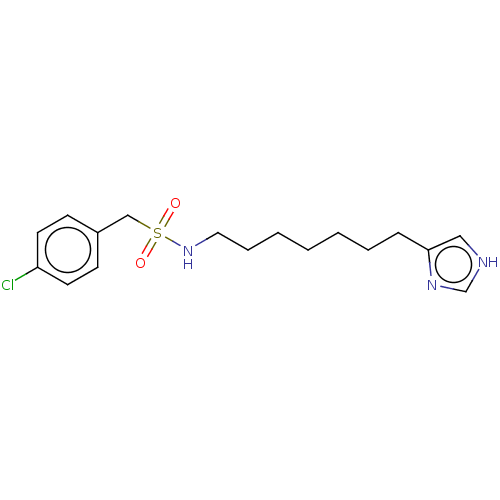 (CHEMBL317420) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217251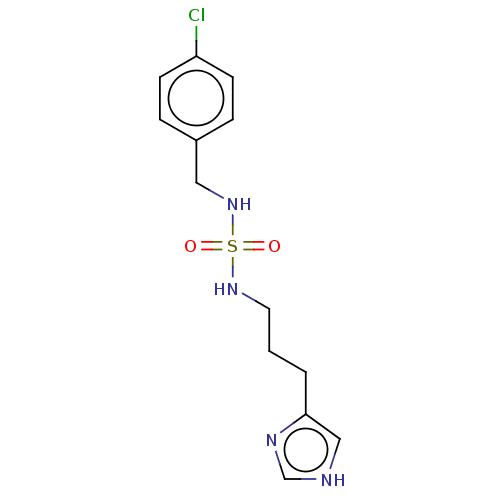 (CHEMBL320432) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217247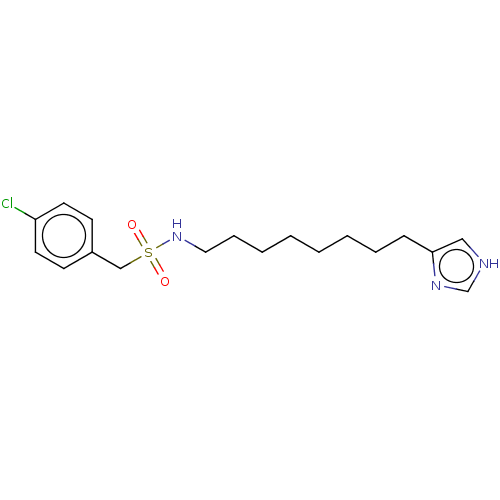 (CHEMBL104320) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217233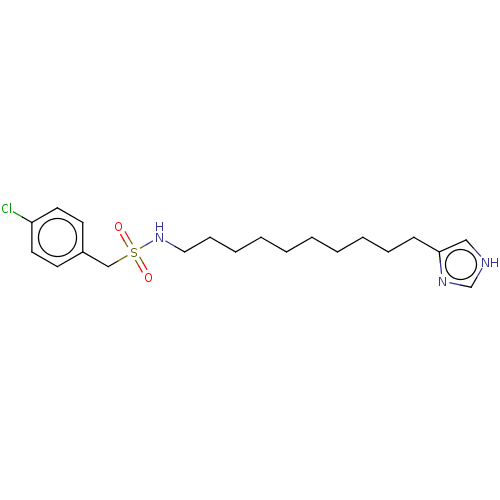 (CHEMBL104300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475522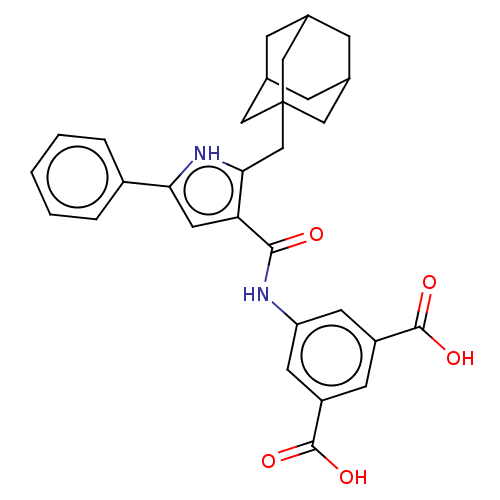 (CHEMBL2067955) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475516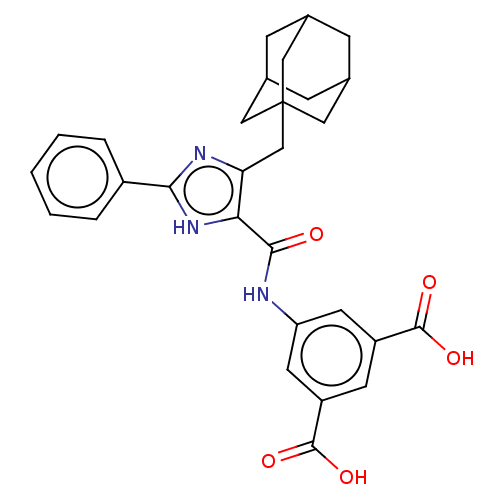 (CHEMBL2067957) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475508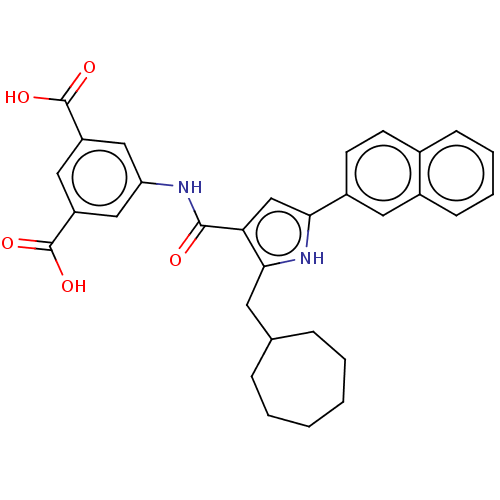 (CHEMBL2067956) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475507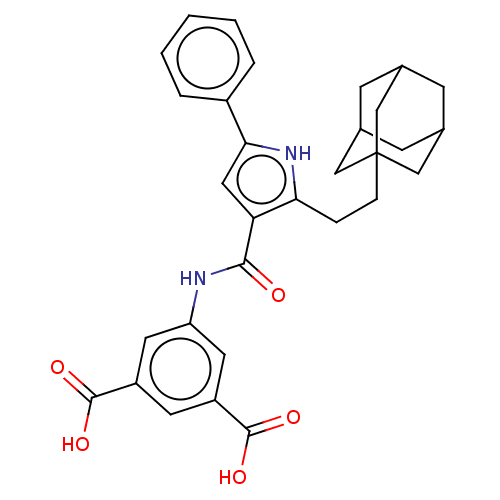 (CHEMBL2067961) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475521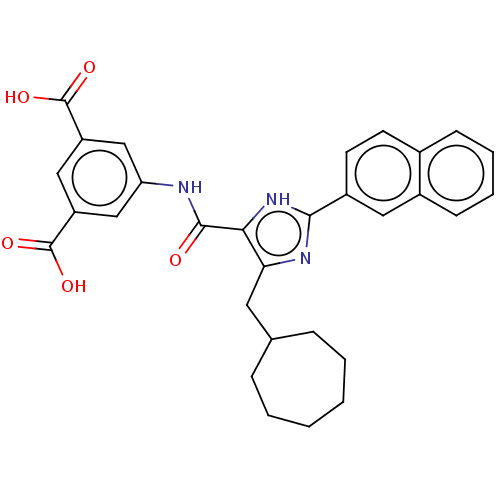 (CHEMBL2067950) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475515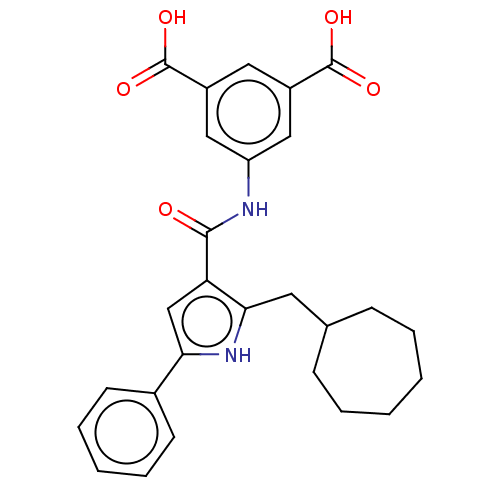 (CHEMBL2067948) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475512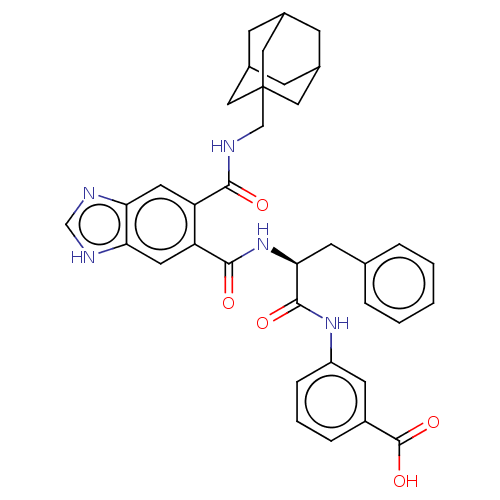 (CHEMBL2067962) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475520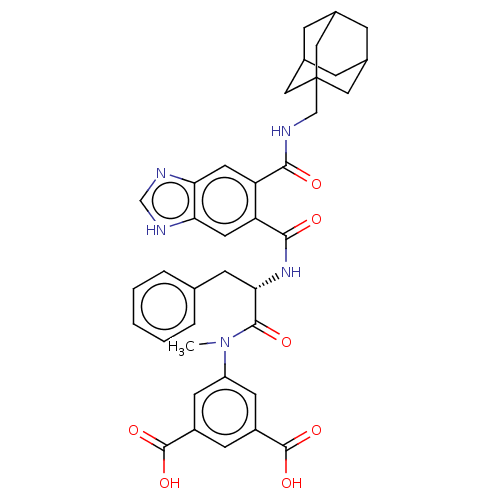 (CHEMBL2067958) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475519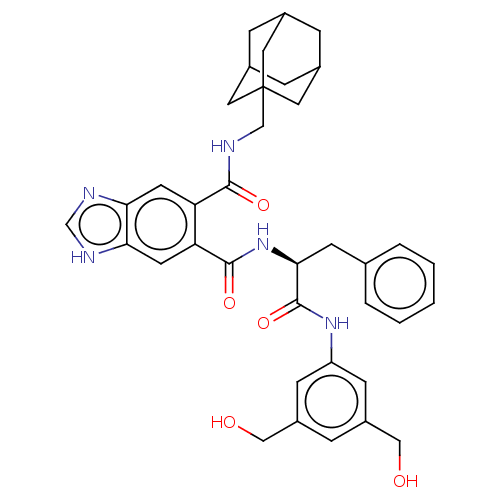 (CHEMBL2067969) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475514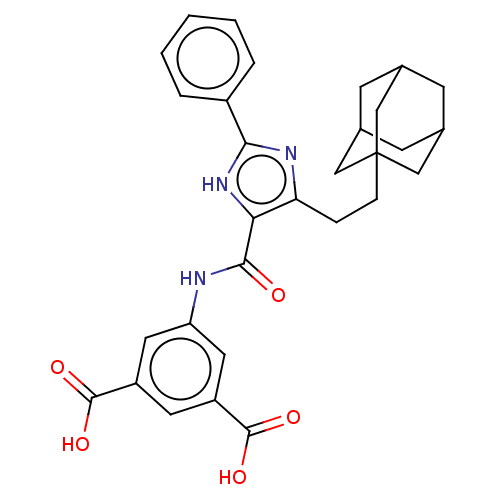 (CHEMBL2067964) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475524 (CHEMBL2067960) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50471064 (CHEMBL301810) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to guinea pig pancreas membrane Cholecystokinin 1 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50217246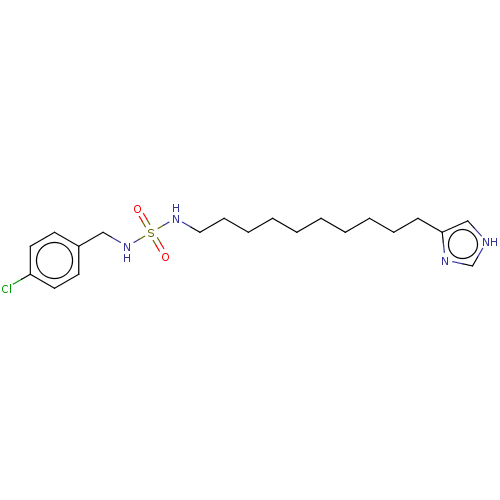 (CHEMBL107073) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The James Black Foundation Curated by ChEMBL | Assay Description Ability to displace [3H]R-alpha-methylhistamine from histamine H3 receptor in guinea pig ileum longitudinal muscle myenteric plexus(LMMP) membranes | Bioorg Med Chem Lett 9: 3103-8 (1999) BindingDB Entry DOI: 10.7270/Q2QN68ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475523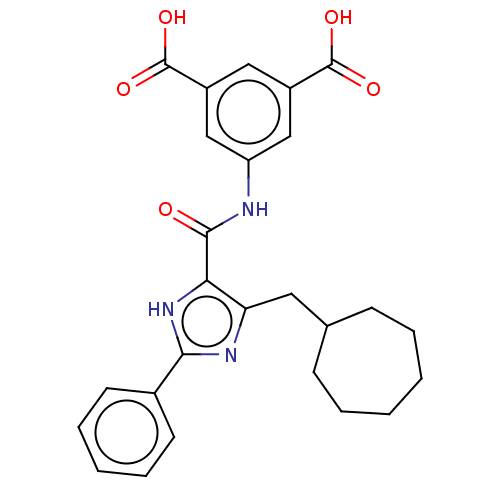 (CHEMBL2067965) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475517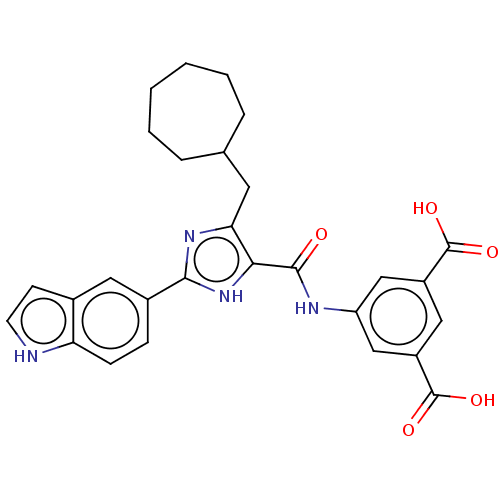 (CHEMBL2067954) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475525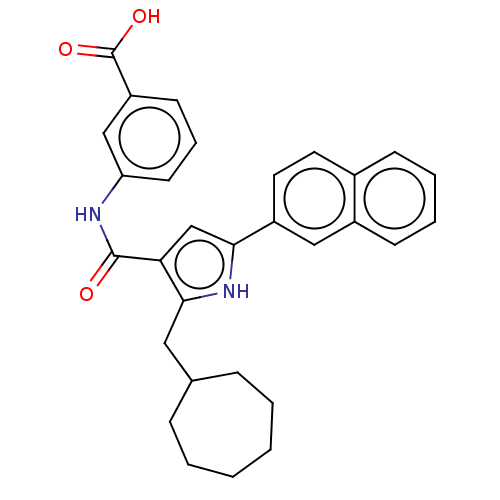 (CHEMBL2067971) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50421390 (CHEMBL24313) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to guinea pig pancreas membrane Cholecystokinin 1 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50470629 (CHEMBL342616) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to guinea pig pancreas membrane Cholecystokinin 1 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50475510 (CHEMBL2067967) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to guinea pig pancreas membrane Cholecystokinin 1 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50475524 (CHEMBL2067960) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to guinea pig pancreas membrane Cholecystokinin 1 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50475508 (CHEMBL2067956) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to guinea pig pancreas membrane Cholecystokinin 1 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50475516 (CHEMBL2067957) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to guinea pig pancreas membrane Cholecystokinin 1 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50475509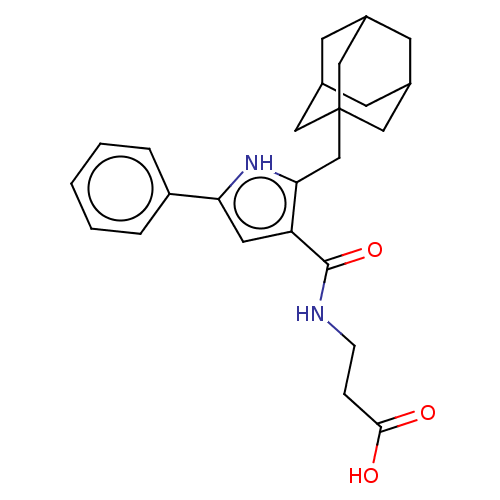 (CHEMBL2067947) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to mouse cortical membrane Cholecystokinin 2 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50475511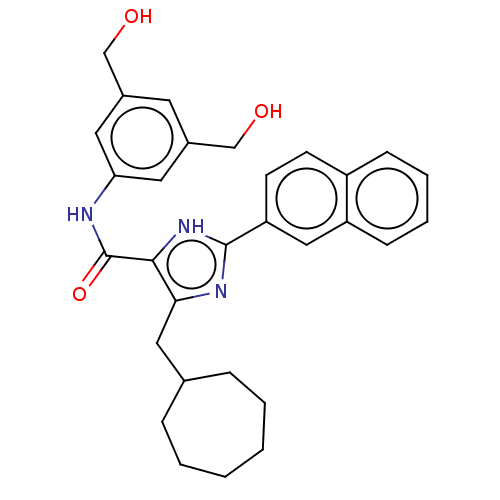 (CHEMBL2067953) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to guinea pig pancreas membrane Cholecystokinin 1 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50475513 (CHEMBL2067963) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation Curated by ChEMBL | Assay Description Inhibition of 20 pM [125I]BH-CCK-8S binding to guinea pig pancreas membrane Cholecystokinin 1 receptor | J Med Chem 48: 6790-802 (2005) Article DOI: 10.1021/jm049069y BindingDB Entry DOI: 10.7270/Q2BC428F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 70 total ) | Next | Last >> |