

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287527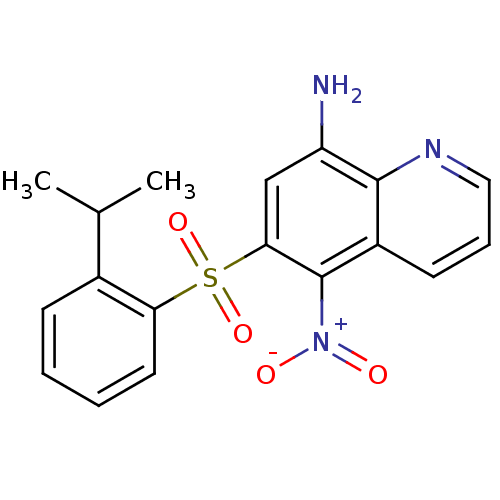 (6-(2-Isopropyl-benzenesulfonyl)-5-nitro-quinolin-8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287536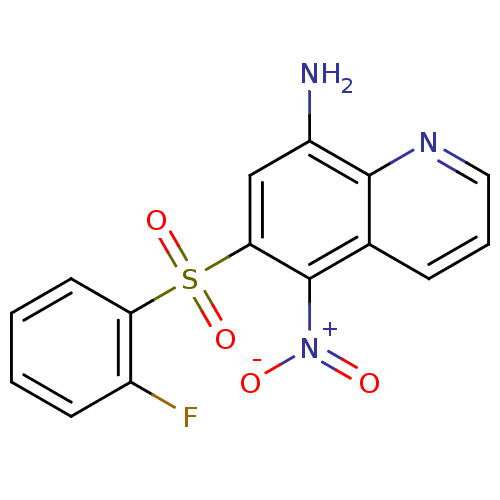 (6-(2-Fluoro-benzenesulfonyl)-5-nitro-quinolin-8-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287529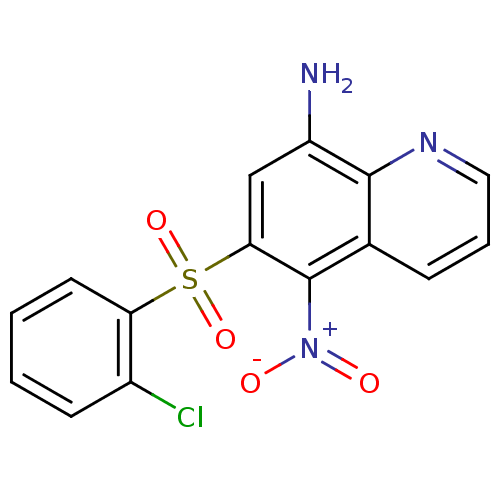 (6-(2-Chloro-benzenesulfonyl)-5-nitro-quinolin-8-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287541 (5-Nitro-6-(toluene-2-sulfonyl)-quinolin-8-ylamine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287538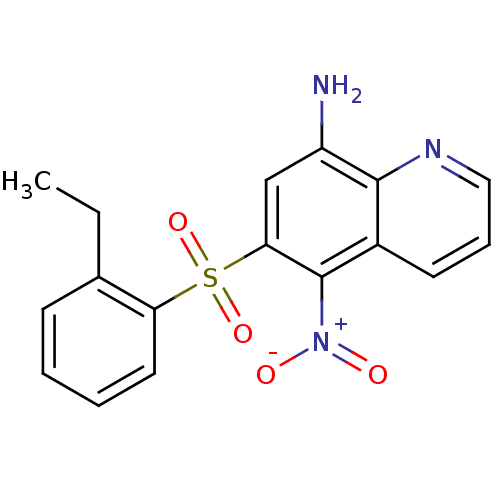 (6-(2-Ethyl-benzenesulfonyl)-5-nitro-quinolin-8-yla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287534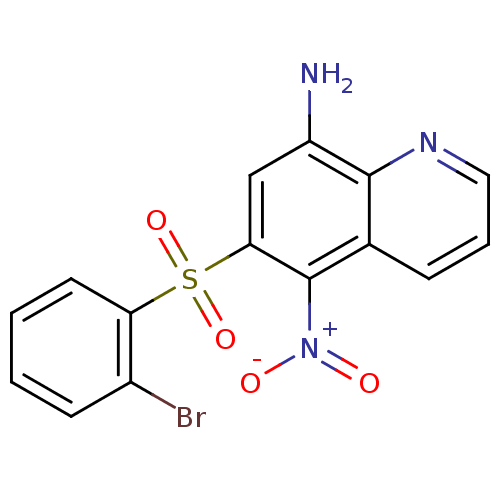 (6-(2-Bromo-benzenesulfonyl)-5-nitro-quinolin-8-yla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287526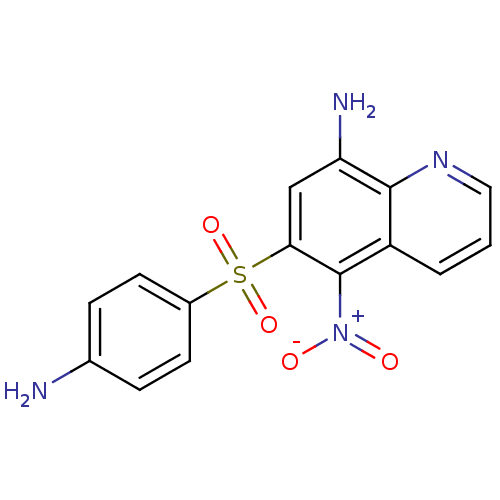 (6-(4-Amino-benzenesulfonyl)-5-nitro-quinolin-8-yla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287539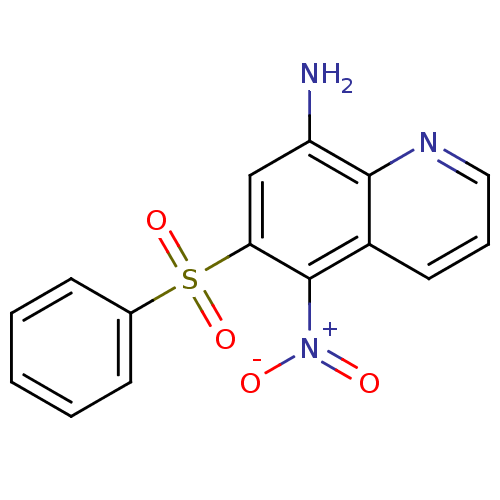 (6-Benzenesulfonyl-5-nitro-quinolin-8-ylamine | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 297 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287530 (5-Nitro-6-(toluene-4-sulfonyl)-quinolin-8-ylamine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 594 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287525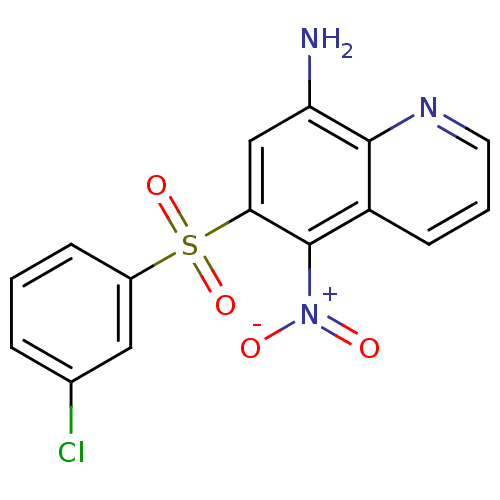 (6-(3-Chloro-benzenesulfonyl)-5-nitro-quinolin-8-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 892 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287528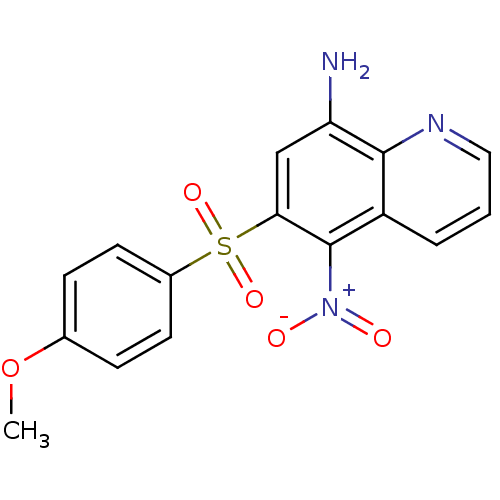 (6-(4-Methoxy-benzenesulfonyl)-5-nitro-quinolin-8-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287533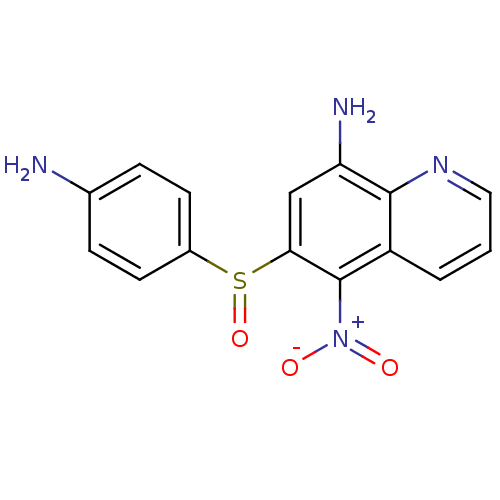 (6-(4-Amino-benzenesulfinyl)-5-nitro-quinolin-8-yla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287532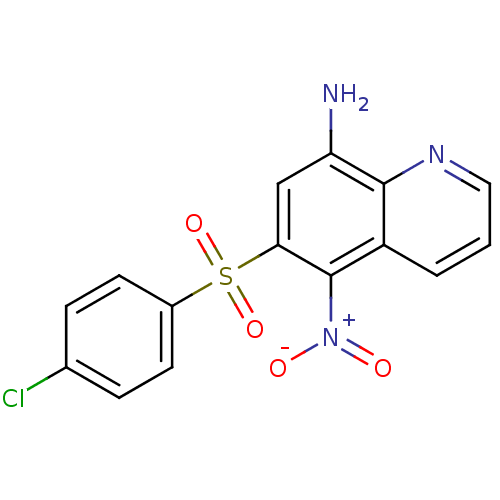 (6-(4-Chloro-benzenesulfonyl)-5-nitro-quinolin-8-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287531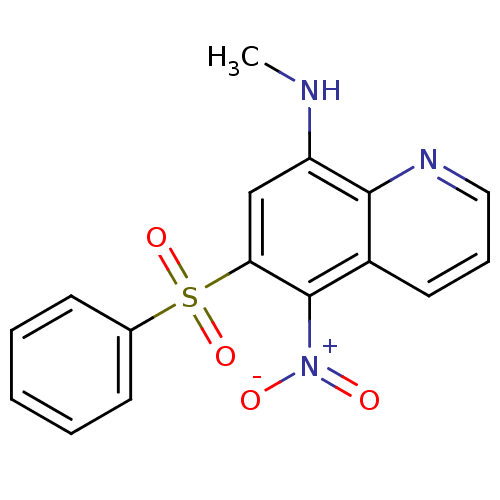 ((6-Benzenesulfonyl-5-nitro-quinolin-8-yl)-methyl-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287540 (CHEMBL54289 | N-(8-Amino-5-nitro-quinolin-6-yl)-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287542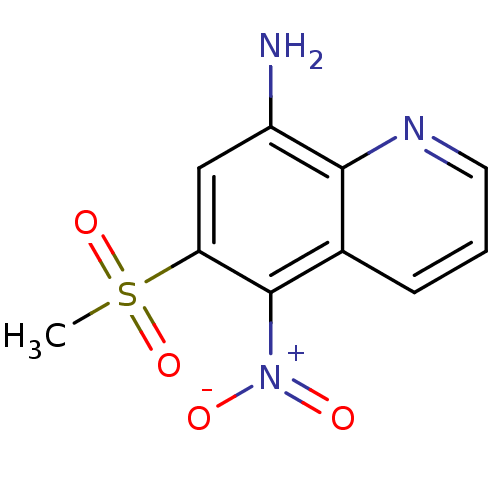 (6-Methanesulfonyl-5-nitro-quinolin-8-ylamine | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287537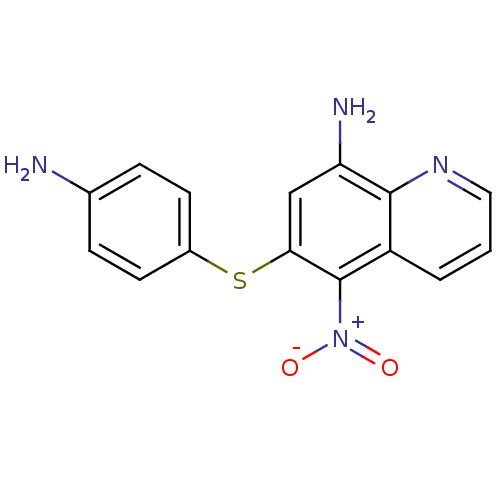 (6-(4-Amino-phenylsulfanyl)-5-nitro-quinolin-8-ylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (RAT) | BDBM50287526 (6-(4-Amino-benzenesulfonyl)-5-nitro-quinolin-8-yla...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y2 (NPY2) receptor from rat hippocampi. | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287524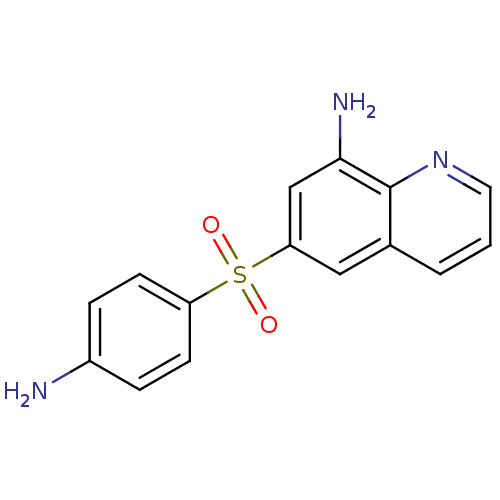 (6-(4-Amino-benzenesulfonyl)-quinolin-8-ylamine | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287535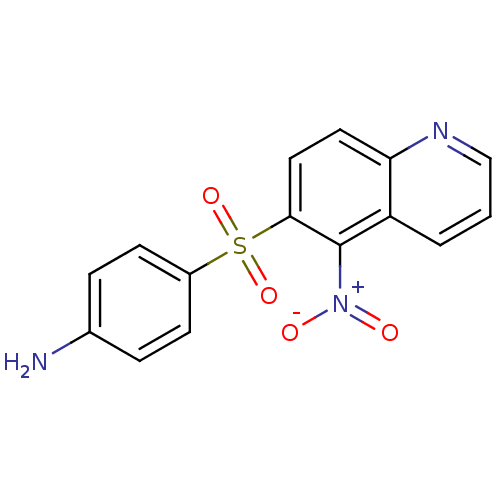 (4-(5-Nitro-quinoline-6-sulfonyl)-phenylamine | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287522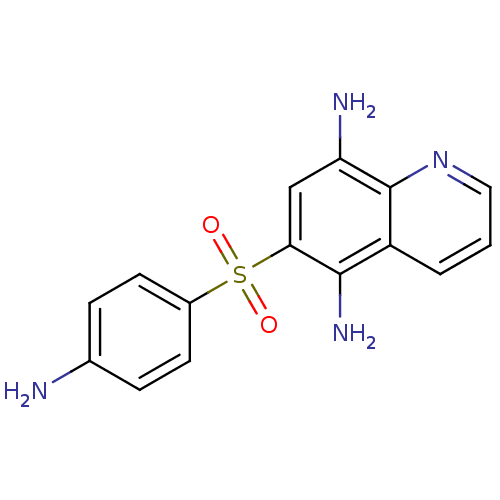 (6-(4-Amino-benzenesulfonyl)-quinoline-5,8-diamine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50287523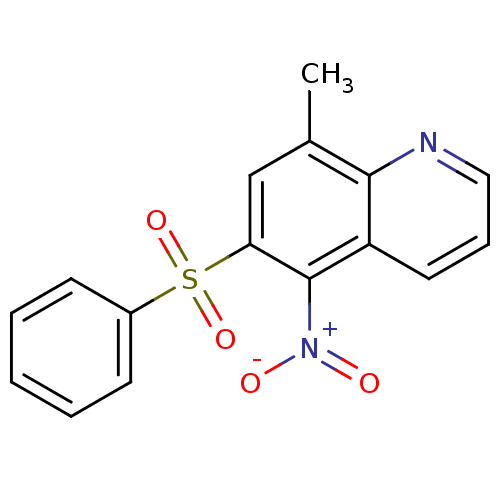 (6-Benzenesulfonyl-8-methyl-5-nitro-quinoline | CHE...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against neuropeptide Y1 (NPY1) receptor from SK-N-MC membranes using [125I]-PYY as radioligand | Bioorg Med Chem Lett 6: 1809-1814 (1996) Article DOI: 10.1016/0960-894X(96)00319-8 BindingDB Entry DOI: 10.7270/Q2M045FM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50011204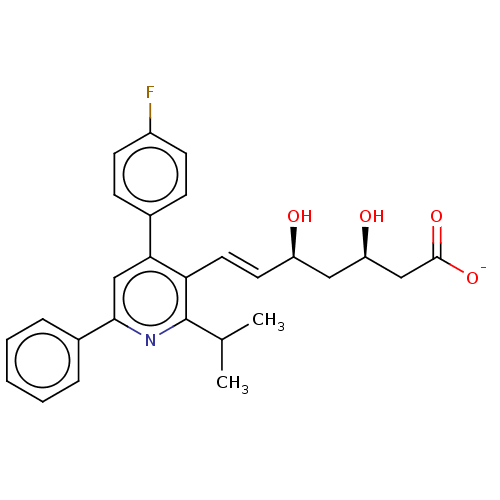 (CHEMBL2368094 | Sodium; 7-[4-(4-fluoro-phenyl)-2-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50006407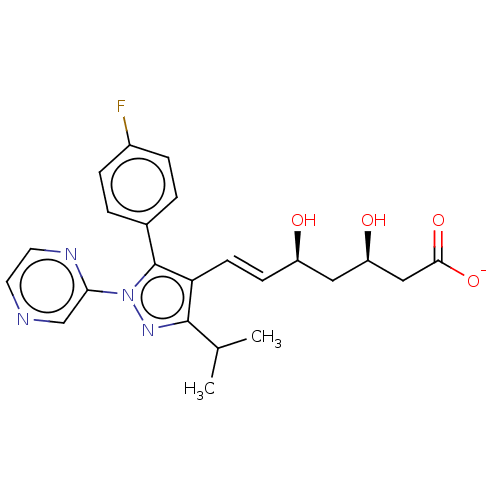 (CHEMBL2367472 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50006409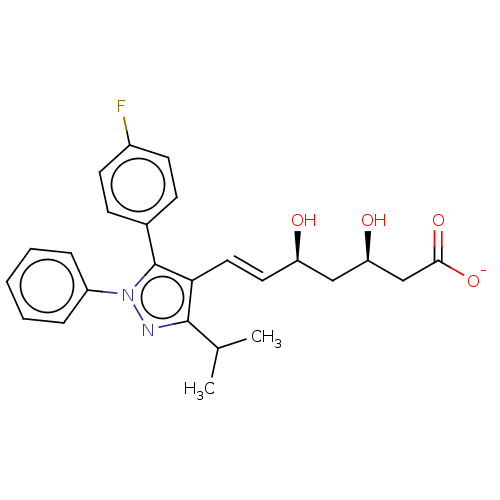 (CHEMBL2367478 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50011213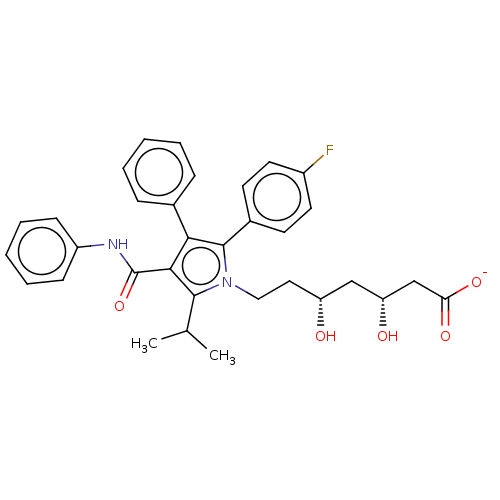 (CHEMBL3349878 | Sodium; 7-[2-(4-fluoro-phenyl)-5-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50011211 (CHEMBL2368089 | Sodium; 7-[4-(4-fluoro-phenyl)-2-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054300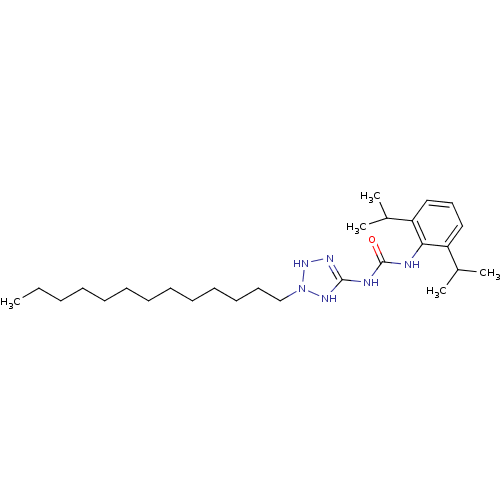 (1-(2,6-Diisopropyl-phenyl)-3-(2-tridecyl-2,3-dihyd...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054305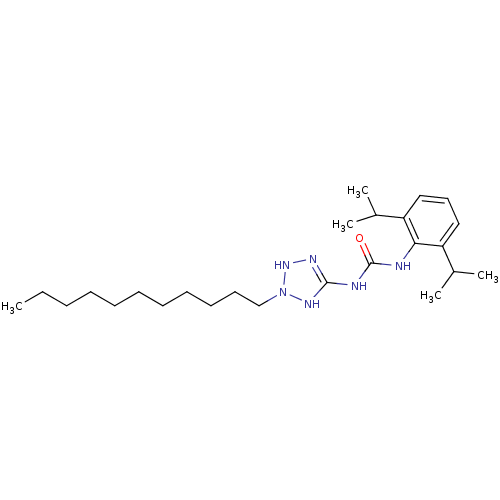 (1-(2,6-Diisopropyl-phenyl)-3-(2-undecyl-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50011208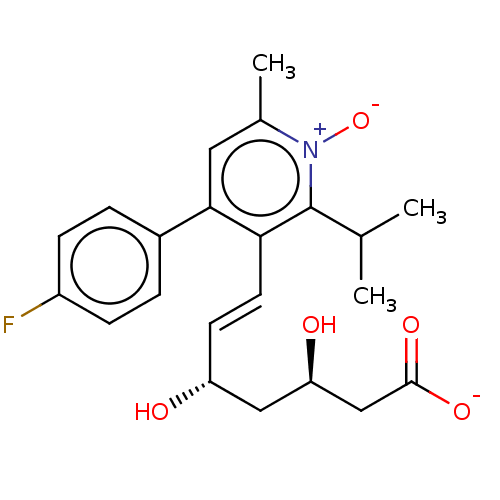 (7-[4-(4-Fluoro-phenyl)-2-isopropyl-6-methyl-1-oxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50006410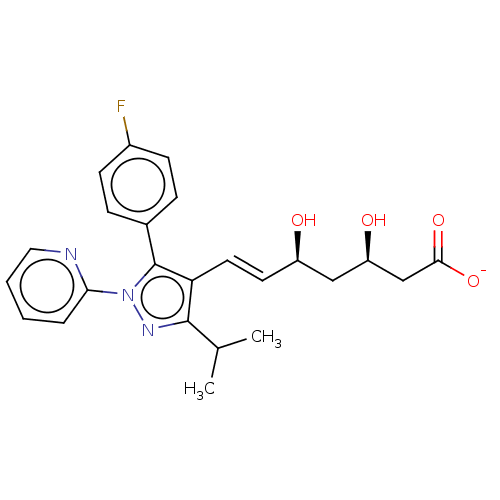 (CHEMBL2367477 | Sodium; 7-[5-(4-fluoro-phenyl)-3-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054288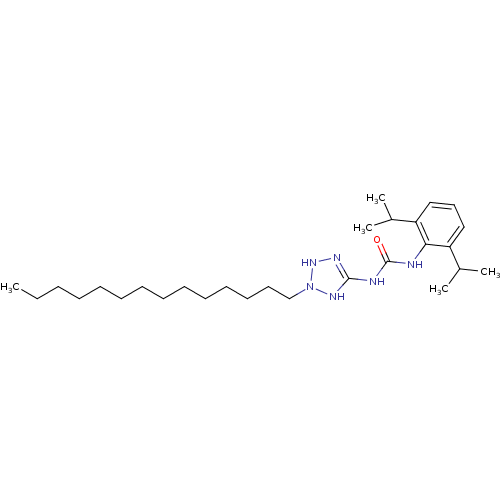 (1-(2,6-Diisopropyl-phenyl)-3-(2-tetradecyl-2,3-dih...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054279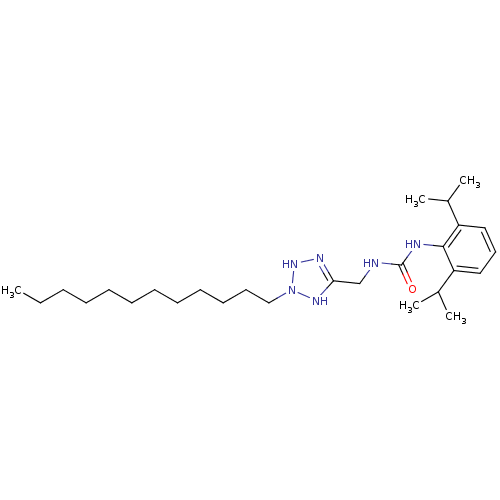 (1-(2,6-Diisopropyl-phenyl)-3-(2-dodecyl-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054281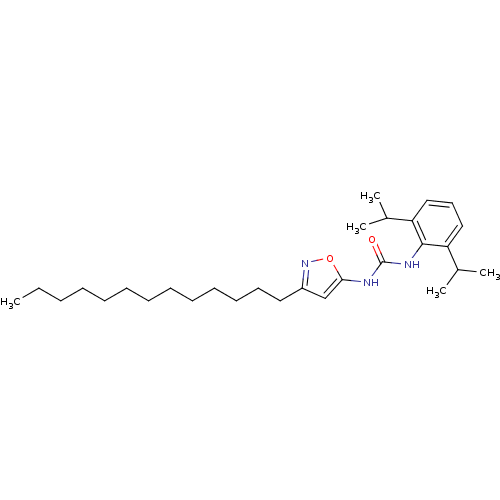 (1-(2,6-Diisopropyl-phenyl)-3-(3-tridecyl-isoxazol-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50011206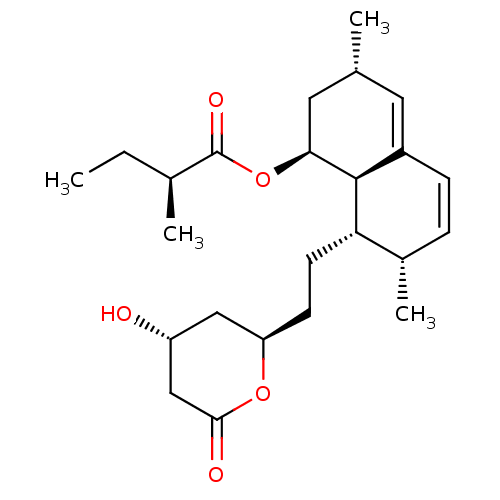 (2-Methyl-butyric acid 8-[2-(4-hydroxy-6-oxo-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50014345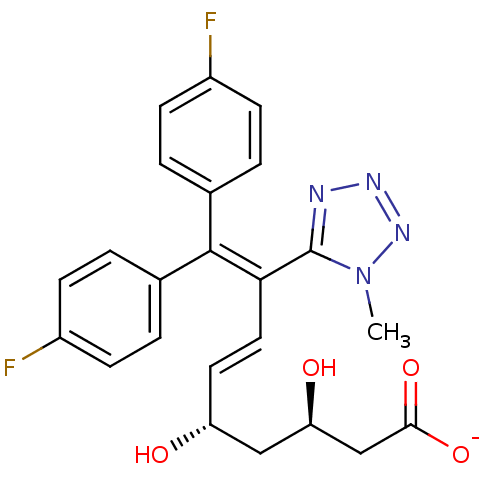 (CHEMBL11804 | Sodium; (E)-(3R,5S)-9,9-bis-(4-fluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054284 (1-(2-Decyl-2,3-dihydro-1H-tetrazol-5-yl)-3-(2,6-di...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054302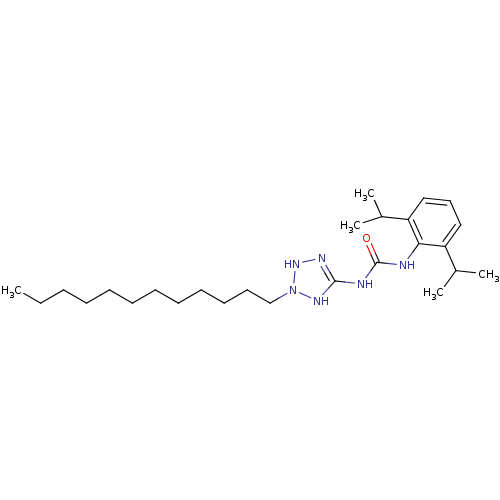 (1-(2,6-Diisopropyl-phenyl)-3-(2-dodecyl-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054278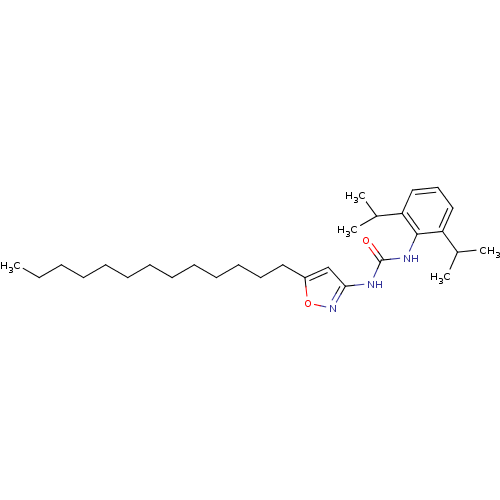 (1-(2,6-Diisopropyl-phenyl)-3-(5-tridecyl-isoxazol-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054297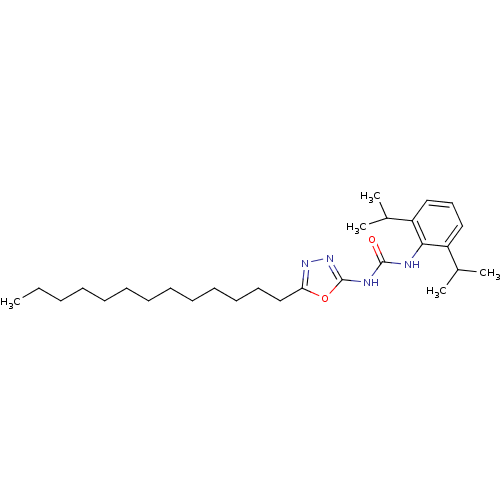 (1-(2,6-Diisopropyl-phenyl)-3-(5-tridecyl-[1,3,4]ox...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054294 (1-(2,6-Diisopropyl-phenyl)-3-(3-dodecyl-isoxazol-5...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50011203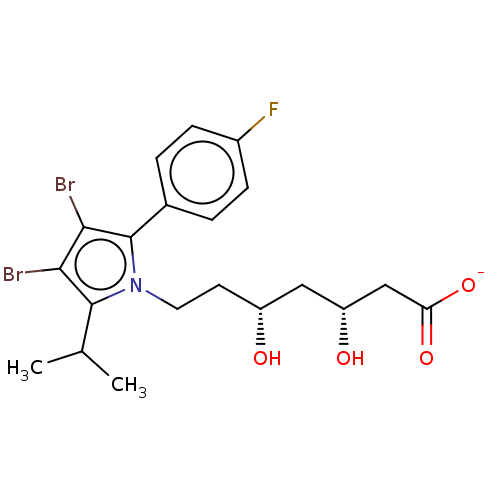 (CHEMBL3349880 | Sodium; 7-[3,4-dibromo-2-(4-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054304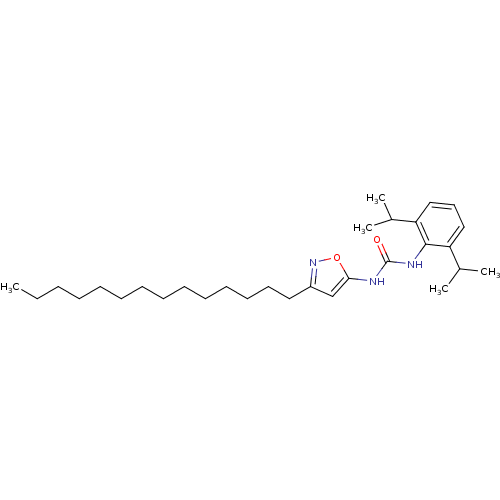 (1-(2,6-Diisopropyl-phenyl)-3-(3-tetradecyl-isoxazo...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054277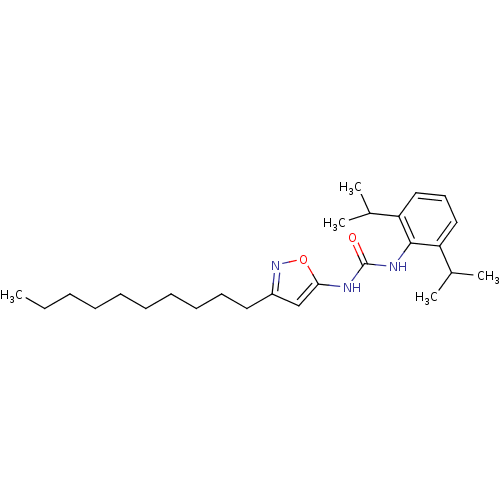 (1-(3-Decyl-isoxazol-5-yl)-3-(2,6-diisopropyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054312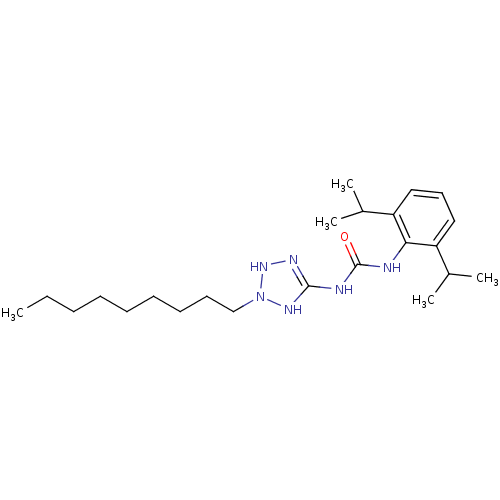 (1-(2,6-Diisopropyl-phenyl)-3-(2-nonyl-2,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054308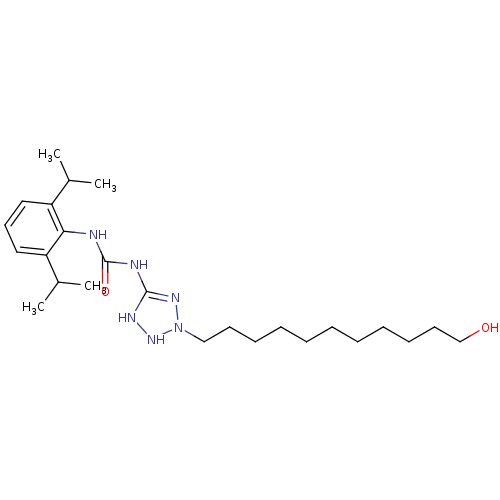 (1-(2,6-Diisopropyl-phenyl)-3-[2-(11-hydroxy-undecy...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Rattus norvegicus (rat)) | BDBM50368147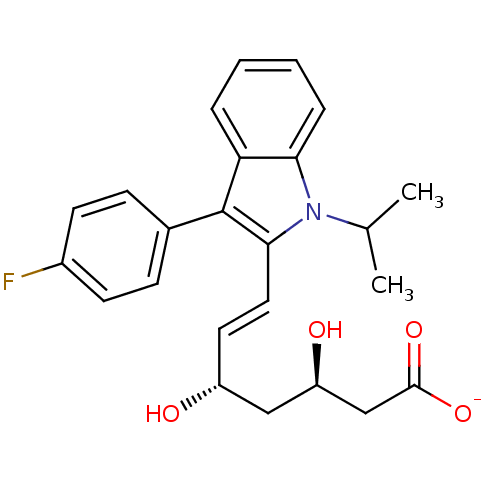 ((+)-(3R,5S)-fluvastatin | (3R,5S)-fluvastatin | (3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver. | J Med Chem 34: 463-6 (1991) BindingDB Entry DOI: 10.7270/Q2MS3TC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054289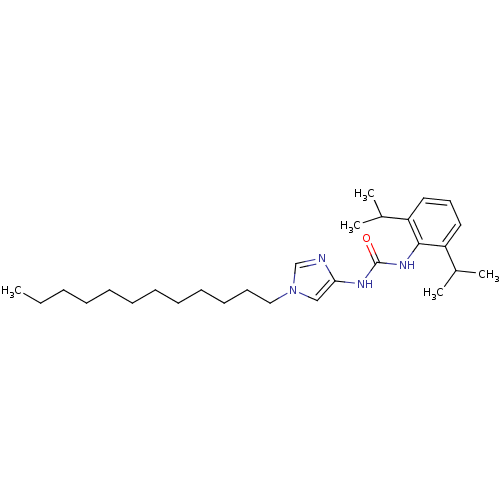 (1-(2,6-Diisopropyl-phenyl)-3-(1-dodecyl-1H-imidazo...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054298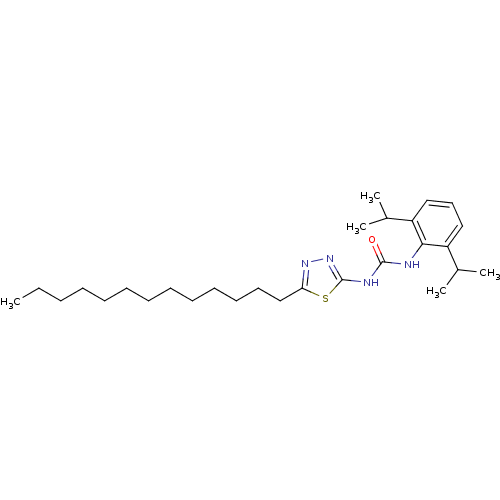 (1-(2,6-Diisopropyl-phenyl)-3-(5-tridecyl-[1,3,4]th...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50054313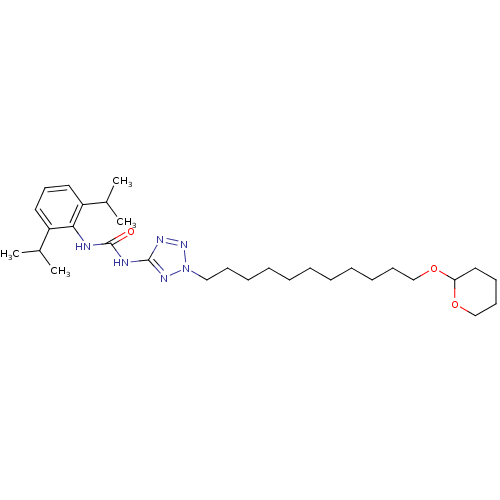 (1-(2,6-Diisopropyl-phenyl)-3-{2-[11-(tetrahydro-py...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Acyl coenzyme A:cholesterol acyltransferase 1 inhibition in vitro measured in rabbit intestinal microsomes | J Med Chem 39: 4382-95 (1996) Article DOI: 10.1021/jm960404v BindingDB Entry DOI: 10.7270/Q2571B3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 80 total ) | Next | Last >> |