

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92374 (RY Analogue, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 685 | -35.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92376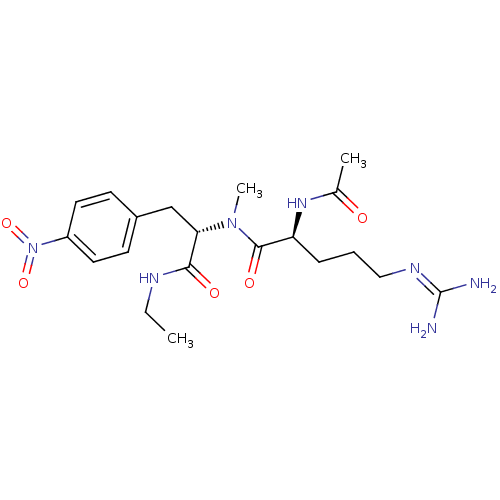 (RY Analogue, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92372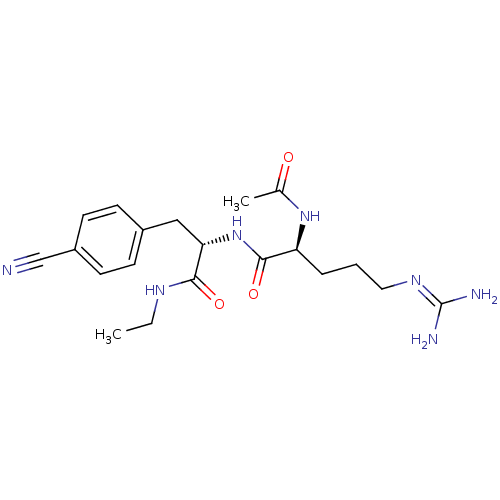 (RY Analogue, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60E+4 | -24.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92373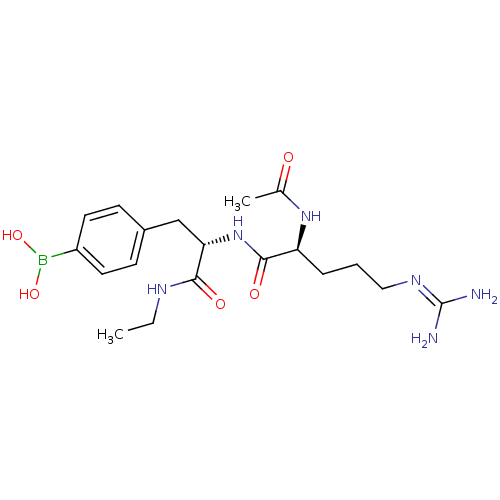 (RY Analogue, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.90E+4 | -24.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92368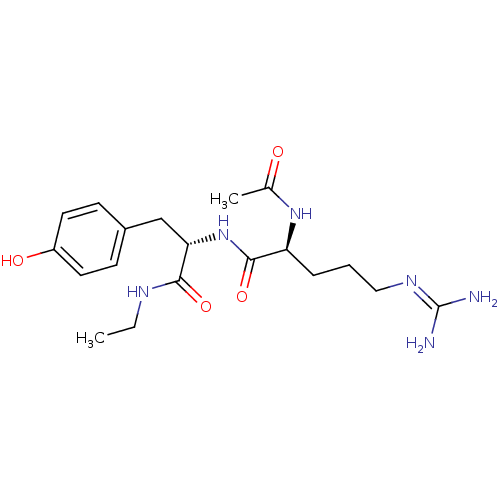 (RY Analogue, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.40E+4 | -23.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92371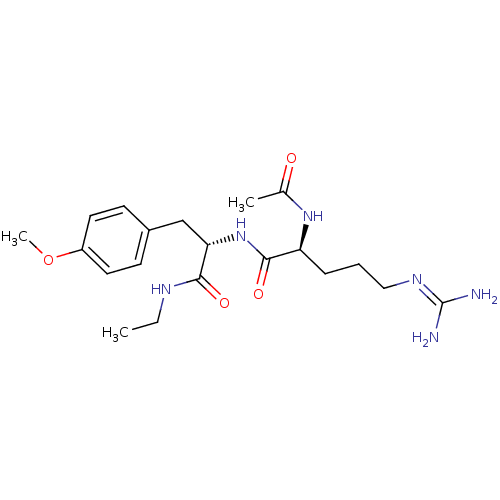 (RY Analogue, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.70E+4 | -23.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92370 (RY Analogue, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.35E+5 | -22.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92375 (RY Analogue, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.45E+5 | -21.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92369 (RY Analogue, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.39E+5 | -19.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||