 Found 436 hits with Last Name = 'dalvie' and Initial = 'd'
Found 436 hits with Last Name = 'dalvie' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433038
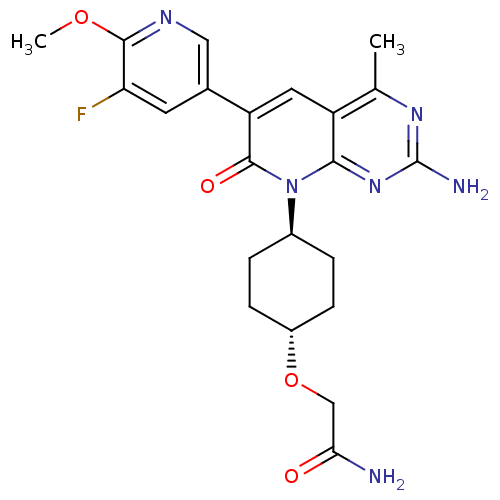
(CHEMBL2375957 | US8633204, 299)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wU:23.28,wD:20.21,(27.28,-44.28,;25.95,-43.51,;24.61,-44.28,;24.61,-45.82,;23.27,-46.58,;21.94,-45.81,;21.94,-44.27,;23.27,-43.5,;23.27,-41.96,;20.6,-46.56,;19.26,-45.78,;17.92,-46.56,;16.58,-45.8,;16.58,-44.26,;15.25,-46.57,;15.25,-48.11,;13.92,-48.88,;16.58,-48.88,;17.91,-48.12,;19.25,-48.89,;19.24,-50.43,;17.9,-51.19,;17.9,-52.72,;19.22,-53.51,;20.56,-52.74,;20.57,-51.2,;19.21,-55.04,;17.88,-55.81,;16.55,-55.03,;15.21,-55.79,;16.56,-53.49,;20.6,-48.12,;21.93,-48.89,)| Show InChI InChI=1S/C22H25FN6O4/c1-11-15-8-16(12-7-17(23)20(32-2)26-9-12)21(31)29(19(15)28-22(25)27-11)13-3-5-14(6-4-13)33-10-18(24)30/h7-9,13-14H,3-6,10H2,1-2H3,(H2,24,30)(H2,25,27,28)/t13-,14- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433032
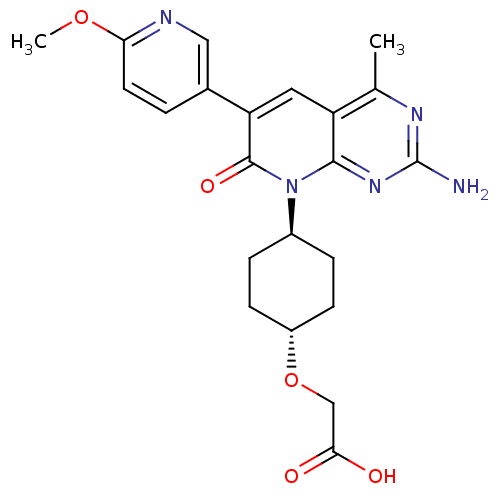
(CHEMBL2376096)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCC(O)=O)c1=O |r,wU:22.27,wD:19.20,(12.89,-1.02,;11.56,-.25,;10.23,-1.01,;8.89,-.24,;7.56,-1.01,;7.56,-2.54,;8.89,-3.32,;10.22,-2.55,;6.22,-3.3,;4.87,-2.52,;3.53,-3.3,;2.2,-2.53,;2.19,-.99,;.87,-3.3,;.87,-4.85,;-.48,-5.62,;2.2,-5.62,;3.53,-4.85,;4.87,-5.63,;4.86,-7.16,;3.52,-7.93,;3.51,-9.46,;4.84,-10.24,;6.18,-9.48,;6.19,-7.93,;4.83,-11.78,;3.49,-12.54,;2.16,-11.76,;2.16,-10.22,;.82,-12.52,;6.22,-4.86,;7.55,-5.63,)| Show InChI InChI=1S/C22H25N5O5/c1-12-16-9-17(13-3-8-18(31-2)24-10-13)21(30)27(20(16)26-22(23)25-12)14-4-6-15(7-5-14)32-11-19(28)29/h3,8-10,14-15H,4-7,11H2,1-2H3,(H,28,29)(H2,23,25,26)/t14-,15- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50387593
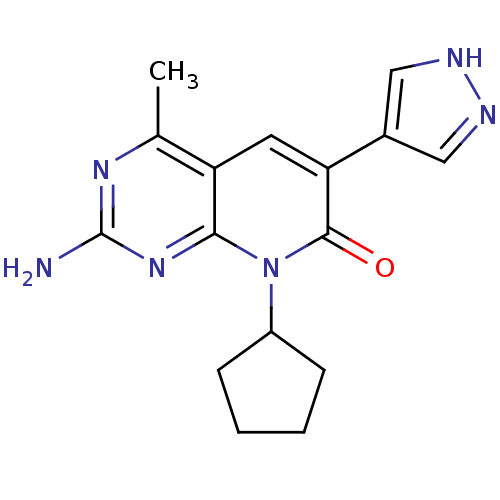
(CHEMBL2057726)Show SMILES Cc1nc(N)nc2n(C3CCCC3)c(=O)c(cc12)-c1cn[nH]c1 Show InChI InChI=1S/C16H18N6O/c1-9-12-6-13(10-7-18-19-8-10)15(23)22(11-4-2-3-5-11)14(12)21-16(17)20-9/h6-8,11H,2-5H2,1H3,(H,18,19)(H2,17,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 22: 5098-103 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.100
BindingDB Entry DOI: 10.7270/Q2CF9R58 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50380313
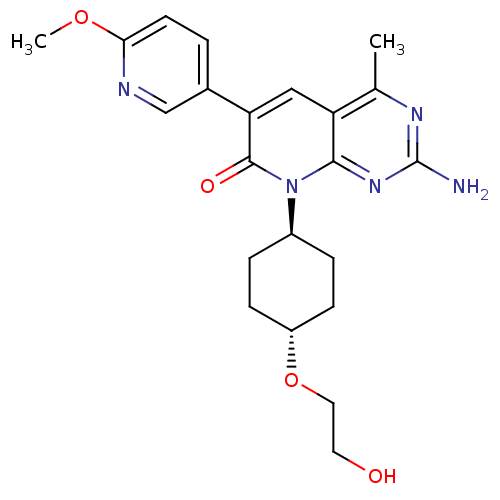
(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433050
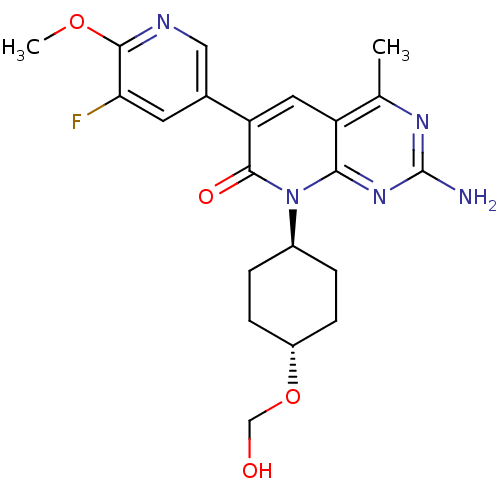
(CHEMBL2375968)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCO)c1=O |r,wU:23.28,wD:20.21,(26.25,-15.63,;24.91,-14.86,;23.58,-15.63,;23.58,-17.17,;22.24,-17.93,;20.91,-17.16,;20.91,-15.63,;22.24,-14.86,;22.24,-13.32,;19.57,-17.92,;18.23,-17.13,;16.89,-17.91,;15.55,-17.15,;15.55,-15.61,;14.22,-17.92,;14.22,-19.46,;12.89,-20.23,;15.55,-20.23,;16.88,-19.47,;18.22,-20.24,;18.21,-21.78,;16.88,-22.54,;16.87,-24.07,;18.19,-24.86,;19.53,-24.09,;19.54,-22.55,;18.18,-26.39,;16.85,-27.16,;15.52,-26.38,;19.57,-19.47,;20.9,-20.24,)| Show InChI InChI=1S/C21H24FN5O4/c1-11-15-8-16(12-7-17(22)19(30-2)24-9-12)20(29)27(18(15)26-21(23)25-11)13-3-5-14(6-4-13)31-10-28/h7-9,13-14,28H,3-6,10H2,1-2H3,(H2,23,25,26)/t13-,14- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433044
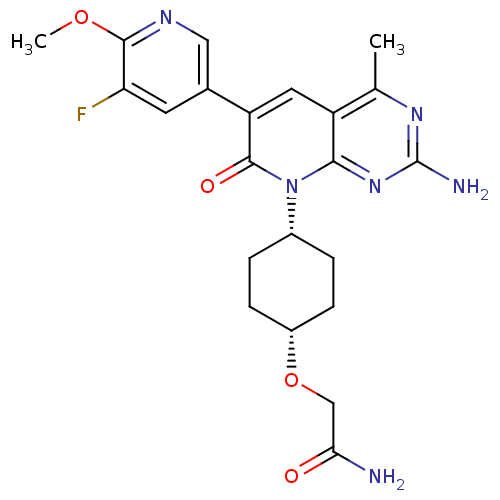
(CHEMBL2375963 | US8633204, 304)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wD:20.21,23.28,(27.43,-30.66,;26.1,-29.89,;24.76,-30.66,;24.76,-32.2,;23.42,-32.96,;22.09,-32.19,;22.09,-30.65,;23.42,-29.88,;23.42,-28.34,;20.75,-32.94,;19.4,-32.16,;18.06,-32.94,;16.73,-32.18,;16.73,-30.64,;15.4,-32.95,;15.4,-34.49,;14.07,-35.26,;16.73,-35.26,;18.06,-34.49,;19.4,-35.27,;19.39,-36.81,;20.72,-37.58,;20.71,-39.12,;19.37,-39.88,;18.05,-39.1,;18.05,-37.57,;19.36,-41.42,;18.02,-42.18,;16.7,-41.41,;15.36,-42.17,;16.71,-39.87,;20.75,-34.5,;22.08,-35.27,)| Show InChI InChI=1S/C22H25FN6O4/c1-11-15-8-16(12-7-17(23)20(32-2)26-9-12)21(31)29(19(15)28-22(25)27-11)13-3-5-14(6-4-13)33-10-18(24)30/h7-9,13-14H,3-6,10H2,1-2H3,(H2,24,30)(H2,25,27,28)/t13-,14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433049
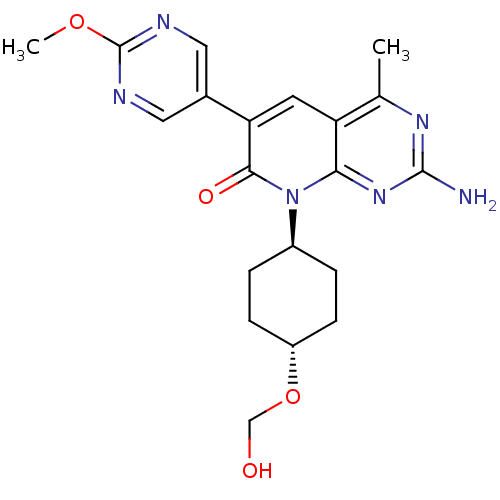
(CHEMBL2375365)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCO)c1=O |r,wU:22.27,wD:19.20,(39.75,-16,;38.42,-15.23,;37.08,-15.99,;37.08,-17.53,;35.75,-18.3,;34.41,-17.52,;34.42,-15.99,;35.74,-15.22,;33.08,-18.28,;31.73,-17.5,;30.39,-18.28,;29.05,-17.51,;29.05,-15.97,;27.73,-18.28,;27.72,-19.83,;26.39,-20.6,;29.06,-20.6,;30.39,-19.83,;31.72,-20.61,;31.72,-22.14,;30.38,-22.91,;30.37,-24.44,;31.7,-25.22,;33.04,-24.46,;33.05,-22.91,;31.69,-26.76,;30.35,-27.52,;29.02,-26.74,;33.07,-19.84,;34.4,-20.61,)| Show InChI InChI=1S/C20H24N6O4/c1-11-15-7-16(12-8-22-20(29-2)23-9-12)18(28)26(17(15)25-19(21)24-11)13-3-5-14(6-4-13)30-10-27/h7-9,13-14,27H,3-6,10H2,1-2H3,(H2,21,24,25)/t13-,14- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433031

(CHEMBL2376094)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCO)c1=O |r,wD:20.21,23.28,(38.17,-2.19,;36.84,-1.42,;35.5,-2.19,;35.5,-3.73,;34.16,-4.49,;32.83,-3.72,;32.83,-2.18,;34.16,-1.41,;34.16,.14,;31.49,-4.48,;30.15,-3.69,;28.81,-4.47,;27.47,-3.71,;27.47,-2.17,;26.14,-4.48,;26.14,-6.02,;24.81,-6.79,;27.48,-6.79,;28.8,-6.03,;30.14,-6.8,;30.13,-8.34,;31.46,-9.11,;31.45,-10.65,;30.11,-11.42,;28.79,-10.63,;28.8,-9.1,;30.1,-12.95,;28.77,-13.72,;27.44,-12.94,;31.49,-6.03,;32.82,-6.8,)| Show InChI InChI=1S/C21H24FN5O4/c1-11-15-8-16(12-7-17(22)19(30-2)24-9-12)20(29)27(18(15)26-21(23)25-11)13-3-5-14(6-4-13)31-10-28/h7-9,13-14,28H,3-6,10H2,1-2H3,(H2,23,25,26)/t13-,14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50387590
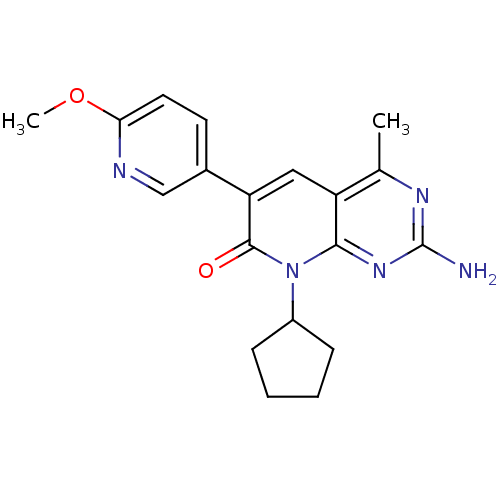
(CHEMBL2057725 | US8633204, 111)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n(C2CCCC2)c1=O Show InChI InChI=1S/C19H21N5O2/c1-11-14-9-15(12-7-8-16(26-2)21-10-12)18(25)24(13-5-3-4-6-13)17(14)23-19(20)22-11/h7-10,13H,3-6H2,1-2H3,(H2,20,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 22: 5098-103 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.100
BindingDB Entry DOI: 10.7270/Q2CF9R58 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433045
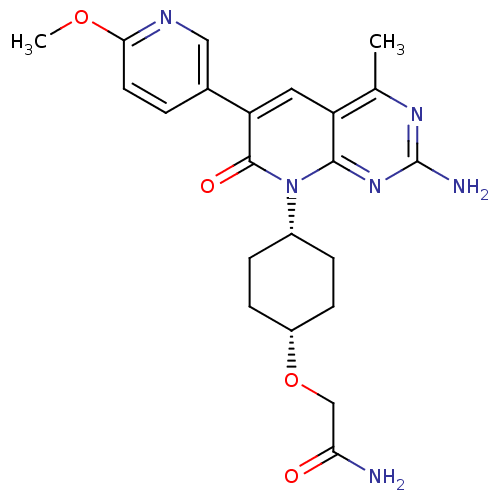
(CHEMBL2375964 | US8633204, 303)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wD:19.20,22.27,(14.55,-30.27,;13.22,-29.49,;11.88,-30.26,;10.54,-29.49,;9.21,-30.26,;9.21,-31.79,;10.54,-32.57,;11.88,-31.8,;7.87,-32.55,;6.53,-31.77,;5.19,-32.55,;3.85,-31.78,;3.85,-30.24,;2.52,-32.55,;2.52,-34.1,;1.19,-34.87,;3.86,-34.87,;5.19,-34.1,;6.52,-34.88,;6.51,-36.41,;7.85,-37.18,;7.83,-38.73,;6.5,-39.49,;5.17,-38.71,;5.18,-37.18,;6.49,-41.03,;5.15,-41.79,;3.82,-41.01,;2.48,-41.77,;3.83,-39.47,;7.87,-34.1,;9.2,-34.88,)| Show InChI InChI=1S/C22H26N6O4/c1-12-16-9-17(13-3-8-19(31-2)25-10-13)21(30)28(20(16)27-22(24)26-12)14-4-6-15(7-5-14)32-11-18(23)29/h3,8-10,14-15H,4-7,11H2,1-2H3,(H2,23,29)(H2,24,26,27)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433043

(CHEMBL2375961 | US8633204, 307)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wD:19.20,22.27,(40.28,-30.68,;38.94,-29.91,;37.61,-30.68,;37.6,-32.22,;36.27,-32.99,;34.94,-32.21,;34.94,-30.68,;36.27,-29.91,;33.6,-32.97,;32.25,-32.19,;30.91,-32.97,;29.58,-32.2,;29.57,-30.66,;28.25,-32.97,;28.25,-34.52,;26.91,-35.28,;29.58,-35.29,;30.91,-34.52,;32.25,-35.3,;32.24,-36.83,;33.57,-37.6,;33.56,-39.15,;32.22,-39.91,;30.89,-39.13,;30.9,-37.6,;32.21,-41.45,;30.87,-42.21,;29.55,-41.43,;28.21,-42.19,;29.56,-39.89,;33.6,-34.52,;34.93,-35.3,)| Show InChI InChI=1S/C21H25N7O4/c1-11-15-7-16(12-8-24-21(31-2)25-9-12)19(30)28(18(15)27-20(23)26-11)13-3-5-14(6-4-13)32-10-17(22)29/h7-9,13-14H,3-6,10H2,1-2H3,(H2,22,29)(H2,23,26,27)/t13-,14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433037
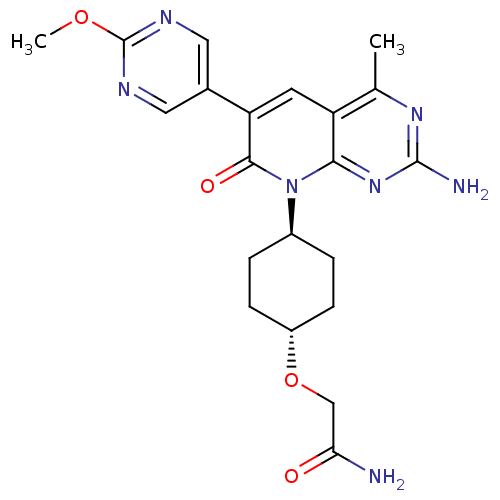
(CHEMBL2375956 | US8633204, 311)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wU:22.27,wD:19.20,(40.72,-44.52,;39.39,-43.75,;38.06,-44.52,;38.05,-46.06,;36.72,-46.82,;35.39,-46.05,;35.39,-44.51,;36.72,-43.74,;34.05,-46.8,;32.7,-46.02,;31.36,-46.8,;30.03,-46.04,;30.02,-44.5,;28.7,-46.81,;28.7,-48.35,;27.36,-49.12,;30.03,-49.12,;31.36,-48.35,;32.7,-49.13,;32.69,-50.67,;31.35,-51.43,;31.34,-52.96,;32.67,-53.74,;34.01,-52.98,;34.02,-51.44,;32.66,-55.28,;31.32,-56.04,;29.99,-55.26,;28.66,-56.03,;30,-53.73,;34.05,-48.36,;35.38,-49.13,)| Show InChI InChI=1S/C21H25N7O4/c1-11-15-7-16(12-8-24-21(31-2)25-9-12)19(30)28(18(15)27-20(23)26-11)13-3-5-14(6-4-13)32-10-17(22)29/h7-9,13-14H,3-6,10H2,1-2H3,(H2,22,29)(H2,23,26,27)/t13-,14- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433039

(CHEMBL2375958 | US8633204, 309)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wU:22.27,wD:19.20,(13.86,-44.58,;12.52,-43.81,;11.19,-44.58,;9.85,-43.81,;8.52,-44.58,;8.52,-46.11,;9.85,-46.88,;11.18,-46.12,;7.18,-46.87,;5.83,-46.08,;4.49,-46.86,;3.16,-46.1,;3.16,-44.56,;1.83,-46.87,;1.83,-48.41,;.5,-49.18,;3.16,-49.18,;4.49,-48.42,;5.83,-49.19,;5.82,-50.73,;4.48,-51.49,;4.48,-53.02,;5.8,-53.81,;7.14,-53.04,;7.15,-51.5,;5.79,-55.34,;4.45,-56.11,;3.13,-55.33,;1.79,-56.09,;3.14,-53.79,;7.18,-48.42,;8.51,-49.19,)| Show InChI InChI=1S/C22H26N6O4/c1-12-16-9-17(13-3-8-19(31-2)25-10-13)21(30)28(20(16)27-22(24)26-12)14-4-6-15(7-5-14)32-11-18(23)29/h3,8-10,14-15H,4-7,11H2,1-2H3,(H2,23,29)(H2,24,26,27)/t14-,15- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50387582

(CHEMBL2057736 | US8633204, 196)Show SMILES COc1ncc(cc1F)-c1cc2c(C)nc(N)nc2n(N2CCCC2)c1=O Show InChI InChI=1S/C18H19FN6O2/c1-10-12-8-13(11-7-14(19)16(27-2)21-9-11)17(26)25(24-5-3-4-6-24)15(12)23-18(20)22-10/h7-9H,3-6H2,1-2H3,(H2,20,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 22: 5098-103 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.100
BindingDB Entry DOI: 10.7270/Q2CF9R58 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50112173
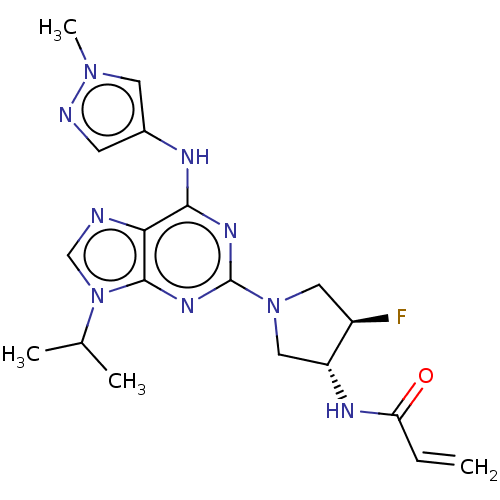
(CHEMBL3608429)Show SMILES CC(C)n1cnc2c(Nc3cnn(C)c3)nc(nc12)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C19H24FN9O/c1-5-15(30)24-14-9-28(8-13(14)20)19-25-17(23-12-6-22-27(4)7-12)16-18(26-19)29(10-21-16)11(2)3/h5-7,10-11,13-14H,1,8-9H2,2-4H3,(H,24,30)(H,23,25,26)/t13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159347
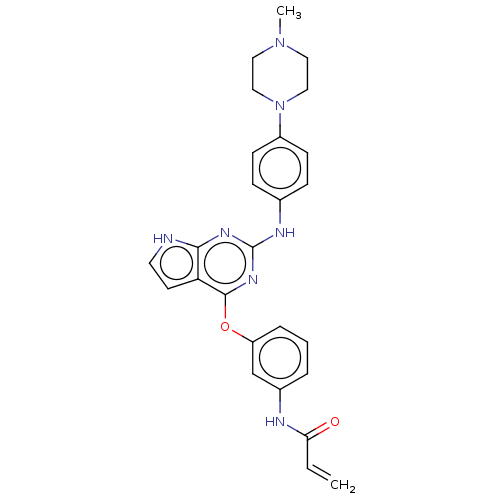
(CHEMBL3787662 | US9586965, Cpd 1)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C26H27N7O2/c1-3-23(34)28-19-5-4-6-21(17-19)35-25-22-11-12-27-24(22)30-26(31-25)29-18-7-9-20(10-8-18)33-15-13-32(2)14-16-33/h3-12,17H,1,13-16H2,2H3,(H,28,34)(H2,27,29,30,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450885
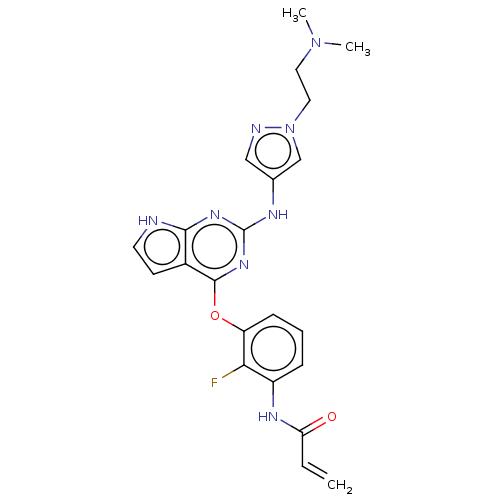
(CHEMBL4216749)Show SMILES CN(C)CCn1cc(Nc2nc(Oc3cccc(NC(=O)C=C)c3F)c3cc[nH]c3n2)cn1 Show InChI InChI=1S/C22H23FN8O2/c1-4-18(32)27-16-6-5-7-17(19(16)23)33-21-15-8-9-24-20(15)28-22(29-21)26-14-12-25-31(13-14)11-10-30(2)3/h4-9,12-13H,1,10-11H2,2-3H3,(H,27,32)(H2,24,26,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159358
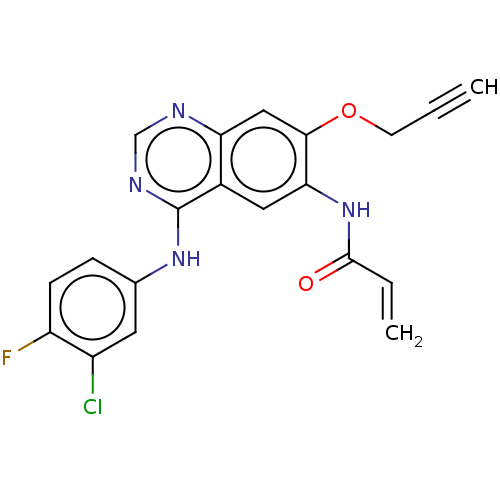
(CHEMBL3787386)Show SMILES Fc1ccc(Nc2ncnc3cc(OCC#C)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C20H14ClFN4O2/c1-3-7-28-18-10-16-13(9-17(18)26-19(27)4-2)20(24-11-23-16)25-12-5-6-15(22)14(21)8-12/h1,4-6,8-11H,2,7H2,(H,26,27)(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50433048

(CHEMBL2375967)Show SMILES Cc1nc(N)nc2n([C@H]3CC[C@@H](CC3)OCO)c(=O)c(cc12)-c1cn[nH]c1 |r,wU:11.14,wD:8.7,(43.23,-15.53,;43.23,-17.07,;41.9,-17.84,;41.9,-19.39,;40.57,-20.15,;43.23,-20.16,;44.56,-19.39,;45.9,-20.17,;45.89,-21.7,;44.56,-22.47,;44.55,-24,;45.88,-24.78,;47.21,-24.02,;47.23,-22.47,;45.87,-26.32,;44.53,-27.08,;43.2,-26.3,;47.25,-19.39,;48.58,-20.17,;47.25,-17.84,;45.91,-17.05,;44.57,-17.83,;48.59,-17.08,;49.98,-17.72,;51.02,-16.59,;50.26,-15.25,;48.76,-15.56,)| Show InChI InChI=1S/C18H22N6O3/c1-10-14-6-15(11-7-20-21-8-11)17(26)24(16(14)23-18(19)22-10)12-2-4-13(5-3-12)27-9-25/h6-8,12-13,25H,2-5,9H2,1H3,(H,20,21)(H2,19,22,23)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433030
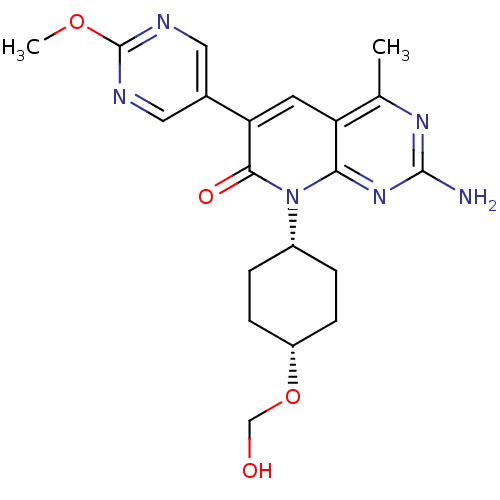
(CHEMBL2376093)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCO)c1=O |r,wD:19.20,22.27,(52.42,-2.69,;51.08,-1.92,;49.75,-2.69,;49.75,-4.23,;48.41,-4.99,;47.08,-4.21,;47.08,-2.68,;48.41,-1.91,;45.74,-4.97,;44.39,-4.19,;43.06,-4.97,;41.72,-4.21,;41.72,-2.67,;40.39,-4.98,;40.39,-6.52,;39.06,-7.29,;41.72,-7.29,;43.05,-6.52,;44.39,-7.3,;44.38,-8.84,;45.71,-9.6,;45.7,-11.15,;44.36,-11.91,;43.04,-11.13,;43.04,-9.6,;44.35,-13.45,;43.01,-14.21,;41.69,-13.43,;45.74,-6.53,;47.07,-7.3,)| Show InChI InChI=1S/C20H24N6O4/c1-11-15-7-16(12-8-22-20(29-2)23-9-12)18(28)26(17(15)25-19(21)24-11)13-3-5-14(6-4-13)30-10-27/h7-9,13-14,27H,3-6,10H2,1-2H3,(H2,21,24,25)/t13-,14+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50433045

(CHEMBL2375964 | US8633204, 303)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wD:19.20,22.27,(14.55,-30.27,;13.22,-29.49,;11.88,-30.26,;10.54,-29.49,;9.21,-30.26,;9.21,-31.79,;10.54,-32.57,;11.88,-31.8,;7.87,-32.55,;6.53,-31.77,;5.19,-32.55,;3.85,-31.78,;3.85,-30.24,;2.52,-32.55,;2.52,-34.1,;1.19,-34.87,;3.86,-34.87,;5.19,-34.1,;6.52,-34.88,;6.51,-36.41,;7.85,-37.18,;7.83,-38.73,;6.5,-39.49,;5.17,-38.71,;5.18,-37.18,;6.49,-41.03,;5.15,-41.79,;3.82,-41.01,;2.48,-41.77,;3.83,-39.47,;7.87,-34.1,;9.2,-34.88,)| Show InChI InChI=1S/C22H26N6O4/c1-12-16-9-17(13-3-8-19(31-2)25-10-13)21(30)28(20(16)27-22(24)26-12)14-4-6-15(7-5-14)32-11-18(23)29/h3,8-10,14-15H,4-7,11H2,1-2H3,(H2,23,29)(H2,24,26,27)/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450884
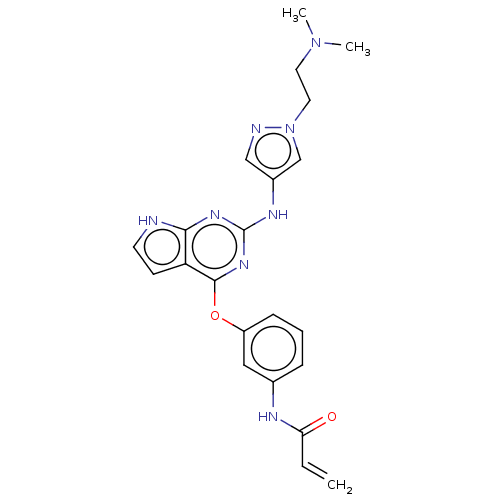
(CHEMBL4211782)Show SMILES CN(C)CCn1cc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cn1 Show InChI InChI=1S/C22H24N8O2/c1-4-19(31)25-15-6-5-7-17(12-15)32-21-18-8-9-23-20(18)27-22(28-21)26-16-13-24-30(14-16)11-10-29(2)3/h4-9,12-14H,1,10-11H2,2-3H3,(H,25,31)(H2,23,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159349
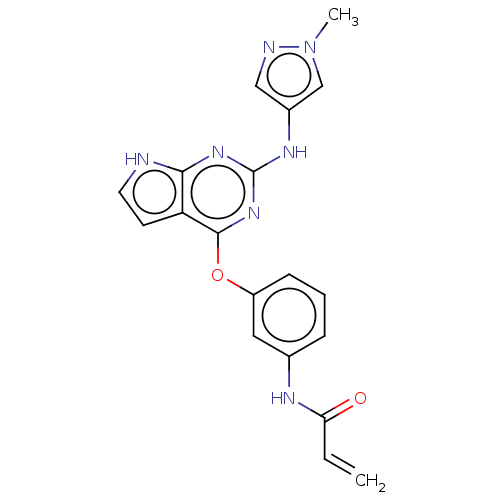
(CHEMBL3786962)Show SMILES Cn1cc(Nc2nc(Oc3cccc(NC(=O)C=C)c3)c3cc[nH]c3n2)cn1 Show InChI InChI=1S/C19H17N7O2/c1-3-16(27)22-12-5-4-6-14(9-12)28-18-15-7-8-20-17(15)24-19(25-18)23-13-10-21-26(2)11-13/h3-11H,1H2,2H3,(H,22,27)(H2,20,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159352
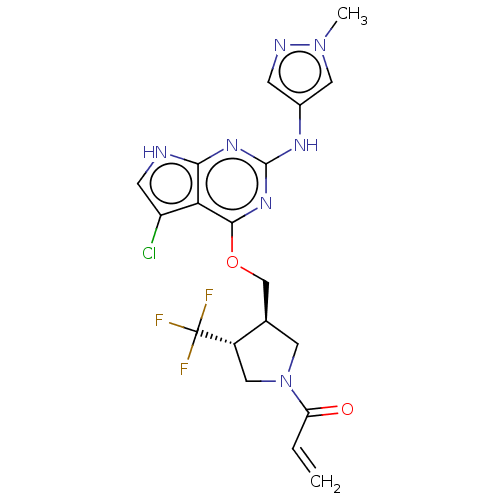
(CHEMBL3786802)Show SMILES Cn1cc(Nc2nc(OC[C@H]3CN(C[C@@H]3C(F)(F)F)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C19H19ClF3N7O2/c1-3-14(31)30-6-10(12(8-30)19(21,22)23)9-32-17-15-13(20)5-24-16(15)27-18(28-17)26-11-4-25-29(2)7-11/h3-5,7,10,12H,1,6,8-9H2,2H3,(H2,24,26,27,28)/t10-,12+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159353

(CHEMBL3787220)Show SMILES Cn1cc(Nc2nc(OC[C@H]3CN(C[C@@H]3F)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C18H19ClFN7O2/c1-3-14(28)27-6-10(13(20)8-27)9-29-17-15-12(19)5-21-16(15)24-18(25-17)23-11-4-22-26(2)7-11/h3-5,7,10,13H,1,6,8-9H2,2H3,(H2,21,23,24,25)/t10-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450887
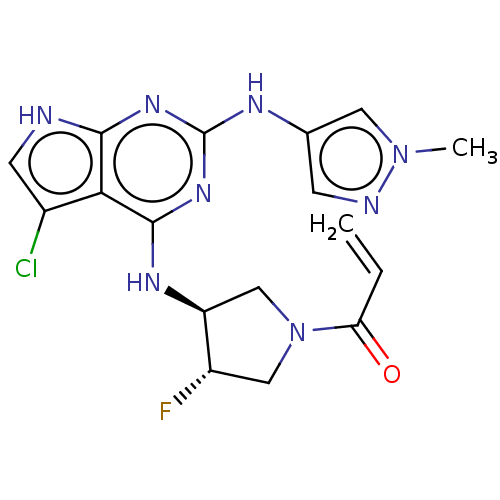
(CHEMBL4211367)Show SMILES Cn1cc(Nc2nc(N[C@H]3CN(C[C@@H]3F)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C17H18ClFN8O/c1-3-13(28)27-7-11(19)12(8-27)23-16-14-10(18)5-20-15(14)24-17(25-16)22-9-4-21-26(2)6-9/h3-6,11-12H,1,7-8H2,2H3,(H3,20,22,23,24,25)/t11-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433048

(CHEMBL2375967)Show SMILES Cc1nc(N)nc2n([C@H]3CC[C@@H](CC3)OCO)c(=O)c(cc12)-c1cn[nH]c1 |r,wU:11.14,wD:8.7,(43.23,-15.53,;43.23,-17.07,;41.9,-17.84,;41.9,-19.39,;40.57,-20.15,;43.23,-20.16,;44.56,-19.39,;45.9,-20.17,;45.89,-21.7,;44.56,-22.47,;44.55,-24,;45.88,-24.78,;47.21,-24.02,;47.23,-22.47,;45.87,-26.32,;44.53,-27.08,;43.2,-26.3,;47.25,-19.39,;48.58,-20.17,;47.25,-17.84,;45.91,-17.05,;44.57,-17.83,;48.59,-17.08,;49.98,-17.72,;51.02,-16.59,;50.26,-15.25,;48.76,-15.56,)| Show InChI InChI=1S/C18H22N6O3/c1-10-14-6-15(11-7-20-21-8-11)17(26)24(16(14)23-18(19)22-10)12-2-4-13(5-3-12)27-9-25/h6-8,12-13,25H,2-5,9H2,1H3,(H,20,21)(H2,19,22,23)/t12-,13- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50387589
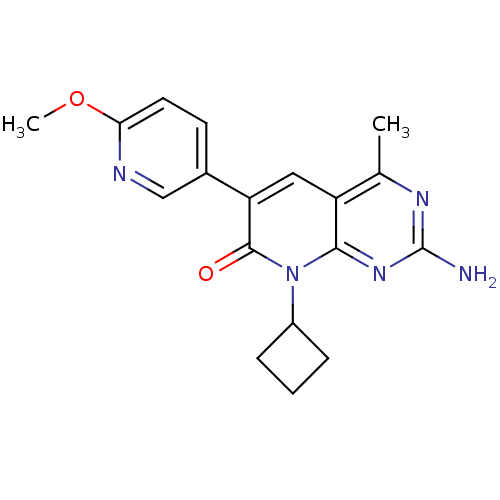
(CHEMBL2057727 | US8633204, 206)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n(C2CCC2)c1=O Show InChI InChI=1S/C18H19N5O2/c1-10-13-8-14(11-6-7-15(25-2)20-9-11)17(24)23(12-4-3-5-12)16(13)22-18(19)21-10/h6-9,12H,3-5H2,1-2H3,(H2,19,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 22: 5098-103 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.100
BindingDB Entry DOI: 10.7270/Q2CF9R58 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159360
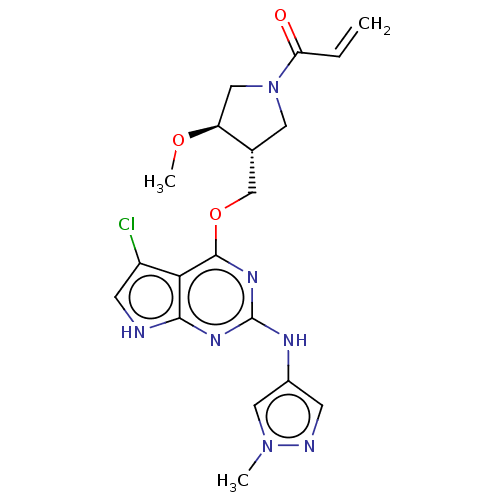
(CHEMBL3786098)Show SMILES CO[C@H]1CN(C[C@@H]1COc1nc(Nc2cnn(C)c2)nc2[nH]cc(Cl)c12)C(=O)C=C |r| Show InChI InChI=1S/C19H22ClN7O3/c1-4-15(28)27-7-11(14(9-27)29-3)10-30-18-16-13(20)6-21-17(16)24-19(25-18)23-12-5-22-26(2)8-12/h4-6,8,11,14H,1,7,9-10H2,2-3H3,(H2,21,23,24,25)/t11-,14+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450886
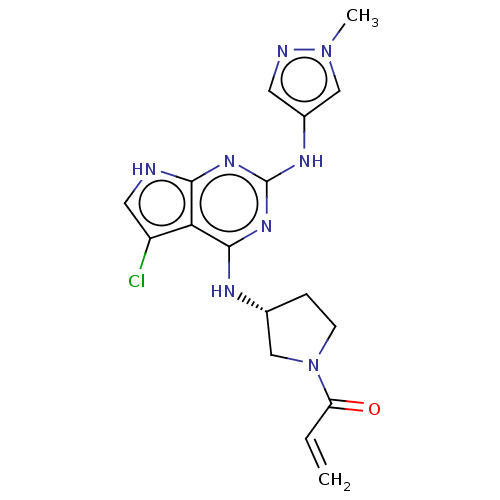
(CHEMBL4206481)Show SMILES Cn1cc(Nc2nc(N[C@@H]3CCN(C3)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C17H19ClN8O/c1-3-13(27)26-5-4-10(9-26)21-16-14-12(18)7-19-15(14)23-17(24-16)22-11-6-20-25(2)8-11/h3,6-8,10H,1,4-5,9H2,2H3,(H3,19,21,22,23,24)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159356
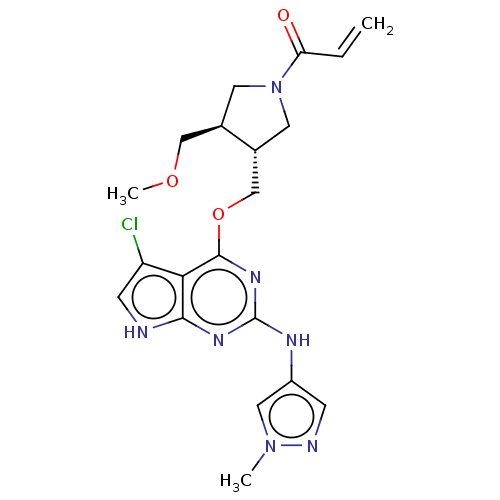
(CHEMBL3786523)Show SMILES COC[C@H]1CN(C[C@@H]1COc1nc(Nc2cnn(C)c2)nc2[nH]cc(Cl)c12)C(=O)C=C |r| Show InChI InChI=1S/C20H24ClN7O3/c1-4-16(29)28-7-12(10-30-3)13(8-28)11-31-19-17-15(21)6-22-18(17)25-20(26-19)24-14-5-23-27(2)9-14/h4-6,9,12-13H,1,7-8,10-11H2,2-3H3,(H2,22,24,25,26)/t12-,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50433030

(CHEMBL2376093)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCO)c1=O |r,wD:19.20,22.27,(52.42,-2.69,;51.08,-1.92,;49.75,-2.69,;49.75,-4.23,;48.41,-4.99,;47.08,-4.21,;47.08,-2.68,;48.41,-1.91,;45.74,-4.97,;44.39,-4.19,;43.06,-4.97,;41.72,-4.21,;41.72,-2.67,;40.39,-4.98,;40.39,-6.52,;39.06,-7.29,;41.72,-7.29,;43.05,-6.52,;44.39,-7.3,;44.38,-8.84,;45.71,-9.6,;45.7,-11.15,;44.36,-11.91,;43.04,-11.13,;43.04,-9.6,;44.35,-13.45,;43.01,-14.21,;41.69,-13.43,;45.74,-6.53,;47.07,-7.3,)| Show InChI InChI=1S/C20H24N6O4/c1-11-15-7-16(12-8-22-20(29-2)23-9-12)18(28)26(17(15)25-19(21)24-11)13-3-5-14(6-4-13)30-10-27/h7-9,13-14,27H,3-6,10H2,1-2H3,(H2,21,24,25)/t13-,14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50433042
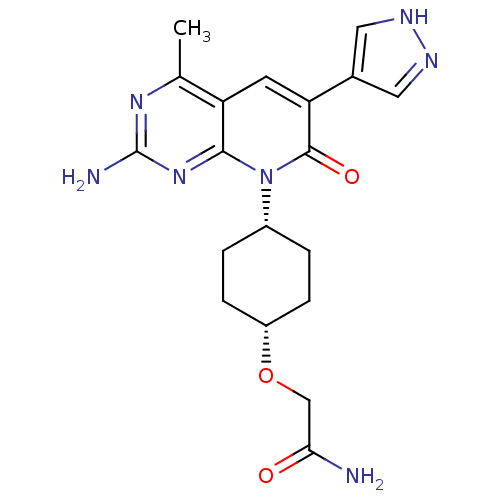
(CHEMBL2375962)Show SMILES Cc1nc(N)nc2n([C@@H]3CC[C@@H](CC3)OCC(N)=O)c(=O)c(cc12)-c1cn[nH]c1 |r,wD:8.7,11.14,(43.18,-30.37,;43.19,-31.91,;41.86,-32.68,;41.85,-34.23,;40.52,-34.99,;43.19,-35,;44.52,-34.23,;45.86,-35.01,;45.85,-36.54,;47.18,-37.31,;47.17,-38.86,;45.83,-39.62,;44.5,-38.84,;44.51,-37.31,;45.82,-41.16,;44.48,-41.92,;43.15,-41.14,;41.81,-41.9,;43.16,-39.6,;47.21,-34.23,;48.54,-35.01,;47.21,-32.68,;45.86,-31.89,;44.52,-32.67,;48.55,-31.92,;49.94,-32.56,;50.98,-31.43,;50.22,-30.09,;48.71,-30.4,)| Show InChI InChI=1S/C19H23N7O3/c1-10-14-6-15(11-7-22-23-8-11)18(28)26(17(14)25-19(21)24-10)12-2-4-13(5-3-12)29-9-16(20)27/h6-8,12-13H,2-5,9H2,1H3,(H2,20,27)(H,22,23)(H2,21,24,25)/t12-,13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433033
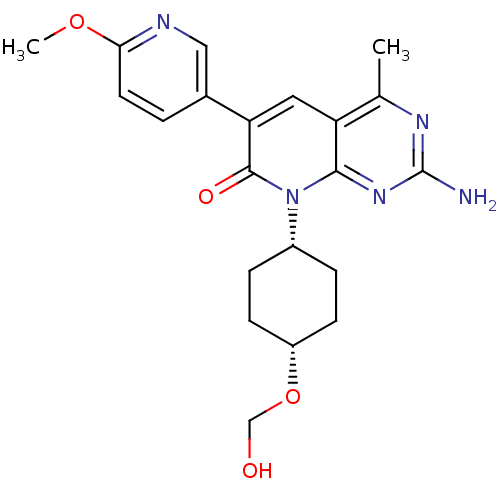
(CHEMBL2376095)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@@H]2CC[C@@H](CC2)OCO)c1=O |r,wD:19.20,22.27,(23.9,-1.62,;22.57,-.85,;21.24,-1.62,;19.89,-.85,;18.57,-1.62,;18.56,-3.15,;19.9,-3.93,;21.23,-3.16,;17.23,-3.91,;15.88,-3.12,;14.54,-3.9,;13.21,-3.14,;13.2,-1.6,;11.88,-3.91,;11.87,-5.45,;10.54,-6.22,;13.21,-6.22,;14.54,-5.46,;15.88,-6.23,;15.87,-7.77,;17.2,-8.54,;17.19,-10.08,;15.85,-10.85,;14.52,-10.07,;14.53,-8.53,;15.84,-12.39,;14.5,-13.15,;13.17,-12.37,;17.22,-5.46,;18.56,-6.24,)| Show InChI InChI=1S/C21H25N5O4/c1-12-16-9-17(13-3-8-18(29-2)23-10-13)20(28)26(19(16)25-21(22)24-12)14-4-6-15(7-5-14)30-11-27/h3,8-10,14-15,27H,4-7,11H2,1-2H3,(H2,22,24,25)/t14-,15+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50433049

(CHEMBL2375365)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCO)c1=O |r,wU:22.27,wD:19.20,(39.75,-16,;38.42,-15.23,;37.08,-15.99,;37.08,-17.53,;35.75,-18.3,;34.41,-17.52,;34.42,-15.99,;35.74,-15.22,;33.08,-18.28,;31.73,-17.5,;30.39,-18.28,;29.05,-17.51,;29.05,-15.97,;27.73,-18.28,;27.72,-19.83,;26.39,-20.6,;29.06,-20.6,;30.39,-19.83,;31.72,-20.61,;31.72,-22.14,;30.38,-22.91,;30.37,-24.44,;31.7,-25.22,;33.04,-24.46,;33.05,-22.91,;31.69,-26.76,;30.35,-27.52,;29.02,-26.74,;33.07,-19.84,;34.4,-20.61,)| Show InChI InChI=1S/C20H24N6O4/c1-11-15-7-16(12-8-22-20(29-2)23-9-12)18(28)26(17(15)25-19(21)24-11)13-3-5-14(6-4-13)30-10-27/h7-9,13-14,27H,3-6,10H2,1-2H3,(H2,21,24,25)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433034
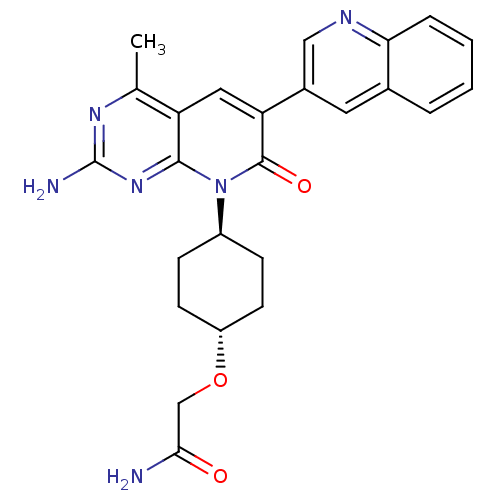
(CHEMBL2375953 | US8633204, 310)Show SMILES Cc1nc(N)nc2n([C@H]3CC[C@@H](CC3)OCC(N)=O)c(=O)c(cc12)-c1cnc2ccccc2c1 |r,wU:11.14,wD:8.7,(66.59,-45.15,;66.6,-46.69,;65.27,-47.46,;65.26,-49,;63.93,-49.77,;66.6,-49.77,;67.93,-49.01,;69.27,-49.78,;69.26,-51.32,;67.92,-52.08,;67.91,-53.62,;69.24,-54.4,;70.58,-53.63,;70.59,-52.09,;69.23,-55.94,;67.89,-56.7,;66.56,-55.92,;65.22,-56.68,;66.57,-54.38,;70.62,-49.01,;71.95,-49.78,;70.62,-47.46,;69.27,-46.67,;67.93,-47.45,;71.96,-46.7,;73.29,-47.47,;74.62,-46.71,;74.62,-45.17,;75.95,-44.41,;75.96,-42.86,;74.62,-42.09,;73.29,-42.86,;73.29,-44.39,;71.96,-45.16,)| Show InChI InChI=1S/C25H26N6O3/c1-14-19-11-20(16-10-15-4-2-3-5-21(15)28-12-16)24(33)31(23(19)30-25(27)29-14)17-6-8-18(9-7-17)34-13-22(26)32/h2-5,10-12,17-18H,6-9,13H2,1H3,(H2,26,32)(H2,27,29,30)/t17-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450871

(CHEMBL4205392)Show SMILES CCn1cnc2c(Nc3cn(C)nc3OC)nc(nc12)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C19H24FN9O2/c1-5-14(30)22-12-9-29(7-11(12)20)19-24-16(15-17(25-19)28(6-2)10-21-15)23-13-8-27(3)26-18(13)31-4/h5,8,10-12H,1,6-7,9H2,2-4H3,(H,22,30)(H,23,24,25)/t11-,12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433036

(CHEMBL2375955)Show SMILES Cc1nc(N)nc2n([C@H]3CC[C@@H](CC3)OCC(N)=O)c(=O)c(cc12)-c1cn[nH]c1 |r,wU:11.14,wD:8.7,(44.02,-44.59,;44.03,-46.13,;42.7,-46.9,;42.69,-48.44,;41.36,-49.21,;44.03,-49.21,;45.36,-48.45,;46.7,-49.22,;46.69,-50.76,;45.35,-51.52,;45.34,-53.06,;46.67,-53.84,;48.01,-53.07,;48.02,-51.53,;46.66,-55.38,;45.32,-56.14,;43.99,-55.36,;42.65,-56.12,;44,-53.82,;48.05,-48.45,;49.38,-49.23,;48.05,-46.9,;46.7,-46.11,;45.36,-46.89,;49.39,-46.14,;50.78,-46.78,;51.82,-45.64,;51.06,-44.31,;49.55,-44.61,)| Show InChI InChI=1S/C19H23N7O3/c1-10-14-6-15(11-7-22-23-8-11)18(28)26(17(14)25-19(21)24-10)12-2-4-13(5-3-12)29-9-16(20)27/h6-8,12-13H,2-5,9H2,1H3,(H2,20,27)(H,22,23)(H2,21,24,25)/t12-,13- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433042

(CHEMBL2375962)Show SMILES Cc1nc(N)nc2n([C@@H]3CC[C@@H](CC3)OCC(N)=O)c(=O)c(cc12)-c1cn[nH]c1 |r,wD:8.7,11.14,(43.18,-30.37,;43.19,-31.91,;41.86,-32.68,;41.85,-34.23,;40.52,-34.99,;43.19,-35,;44.52,-34.23,;45.86,-35.01,;45.85,-36.54,;47.18,-37.31,;47.17,-38.86,;45.83,-39.62,;44.5,-38.84,;44.51,-37.31,;45.82,-41.16,;44.48,-41.92,;43.15,-41.14,;41.81,-41.9,;43.16,-39.6,;47.21,-34.23,;48.54,-35.01,;47.21,-32.68,;45.86,-31.89,;44.52,-32.67,;48.55,-31.92,;49.94,-32.56,;50.98,-31.43,;50.22,-30.09,;48.71,-30.4,)| Show InChI InChI=1S/C19H23N7O3/c1-10-14-6-15(11-7-22-23-8-11)18(28)26(17(14)25-19(21)24-10)12-2-4-13(5-3-12)29-9-16(20)27/h6-8,12-13H,2-5,9H2,1H3,(H2,20,27)(H,22,23)(H2,21,24,25)/t12-,13+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50387588
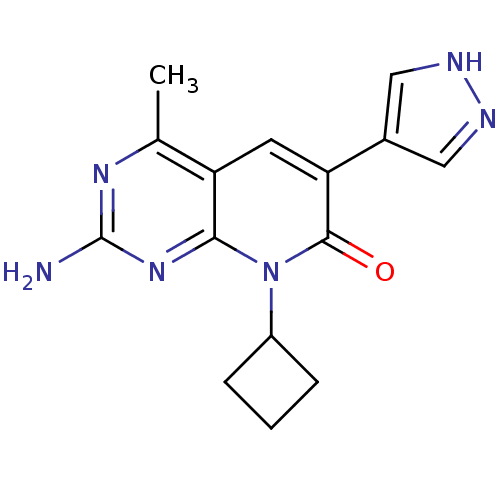
(CHEMBL2057728 | US8633204, 205)Show SMILES Cc1nc(N)nc2n(C3CCC3)c(=O)c(cc12)-c1cn[nH]c1 Show InChI InChI=1S/C15H16N6O/c1-8-11-5-12(9-6-17-18-7-9)14(22)21(10-3-2-4-10)13(11)20-15(16)19-8/h5-7,10H,2-4H2,1H3,(H,17,18)(H2,16,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 22: 5098-103 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.100
BindingDB Entry DOI: 10.7270/Q2CF9R58 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433040

(CHEMBL2375959 | US8633204, 308)Show SMILES Cc1nc(N)nc2n([C@@H]3CC[C@@H](CC3)OCC(N)=O)c(=O)c(cc12)-c1cnc2ccccc2c1 |r,wD:8.7,11.14,(67.89,-30.91,;67.89,-32.45,;66.56,-33.22,;66.56,-34.76,;65.23,-35.53,;67.9,-35.53,;69.23,-34.77,;70.56,-35.54,;70.56,-37.08,;71.89,-37.85,;71.88,-39.39,;70.54,-40.16,;69.21,-39.38,;69.22,-37.84,;70.53,-41.7,;69.19,-42.46,;67.86,-41.68,;66.52,-42.44,;67.87,-40.14,;71.91,-34.77,;73.24,-35.54,;71.91,-33.21,;70.57,-32.43,;69.23,-33.21,;73.25,-32.46,;74.59,-33.23,;75.92,-32.47,;75.92,-30.93,;77.25,-30.16,;77.25,-28.62,;75.91,-27.85,;74.59,-28.62,;74.58,-30.15,;73.25,-30.92,)| Show InChI InChI=1S/C25H26N6O3/c1-14-19-11-20(16-10-15-4-2-3-5-21(15)28-12-16)24(33)31(23(19)30-25(27)29-14)17-6-8-18(9-7-17)34-13-22(26)32/h2-5,10-12,17-18H,6-9,13H2,1H3,(H2,26,32)(H2,27,29,30)/t17-,18+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50433029

(CHEMBL2376092)Show SMILES Cc1nc(N)nc2n([C@@H]3CC[C@@H](CC3)OCO)c(=O)c(cc12)-c1cn[nH]c1 |r,wD:8.7,11.14,(55.51,-1.14,;55.51,-2.68,;54.18,-3.45,;54.18,-5,;52.85,-5.77,;55.52,-5.77,;56.85,-5,;58.18,-5.78,;58.18,-7.31,;59.51,-8.08,;59.5,-9.63,;58.16,-10.39,;56.83,-9.61,;56.84,-8.08,;58.15,-11.93,;56.81,-12.69,;55.48,-11.91,;59.53,-5.01,;60.86,-5.78,;59.53,-3.45,;58.19,-2.67,;56.85,-3.45,;60.87,-2.69,;62.26,-3.33,;63.3,-2.2,;62.55,-.86,;61.04,-1.17,)| Show InChI InChI=1S/C18H22N6O3/c1-10-14-6-15(11-7-20-21-8-11)17(26)24(16(14)23-18(19)22-10)12-2-4-13(5-3-12)27-9-25/h6-8,12-13,25H,2-5,9H2,1H3,(H,20,21)(H2,19,22,23)/t12-,13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50433046
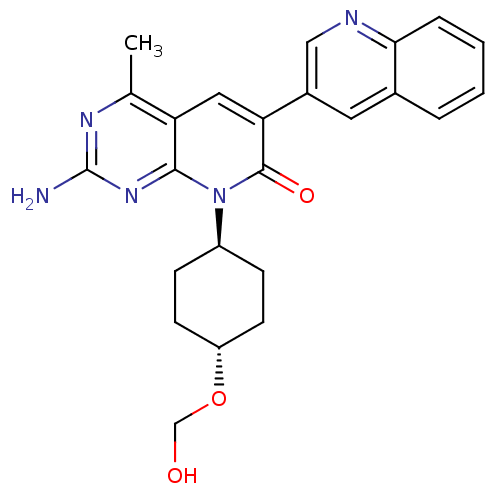
(CHEMBL2375965)Show SMILES Cc1nc(N)nc2n([C@H]3CC[C@@H](CC3)OCO)c(=O)c(cc12)-c1cnc2ccccc2c1 |r,wU:11.14,wD:8.7,(65.77,-15.8,;65.77,-17.34,;64.44,-18.11,;64.44,-19.65,;63.11,-20.42,;65.77,-20.42,;67.1,-19.65,;68.44,-20.43,;68.43,-21.96,;67.09,-22.73,;67.09,-24.26,;68.41,-25.04,;69.75,-24.28,;69.76,-22.73,;68.4,-26.58,;67.07,-27.34,;65.74,-26.56,;69.79,-19.66,;71.12,-20.43,;69.79,-18.1,;68.45,-17.32,;67.11,-18.1,;71.13,-17.34,;72.46,-18.12,;73.8,-17.36,;73.79,-15.82,;75.12,-15.05,;75.13,-13.51,;73.79,-12.74,;72.46,-13.51,;72.46,-15.04,;71.13,-15.81,)| Show InChI InChI=1S/C24H25N5O3/c1-14-19-11-20(16-10-15-4-2-3-5-21(15)26-12-16)23(31)29(22(19)28-24(25)27-14)17-6-8-18(9-7-17)32-13-30/h2-5,10-12,17-18,30H,6-9,13H2,1H3,(H2,25,27,28)/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159351
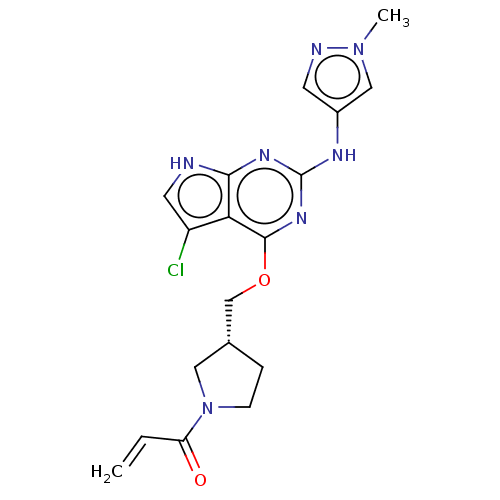
(CHEMBL3786858)Show SMILES Cn1cc(Nc2nc(OC[C@@H]3CCN(C3)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C18H20ClN7O2/c1-3-14(27)26-5-4-11(8-26)10-28-17-15-13(19)7-20-16(15)23-18(24-17)22-12-6-21-25(2)9-12/h3,6-7,9,11H,1,4-5,8,10H2,2H3,(H2,20,22,23,24)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50159354
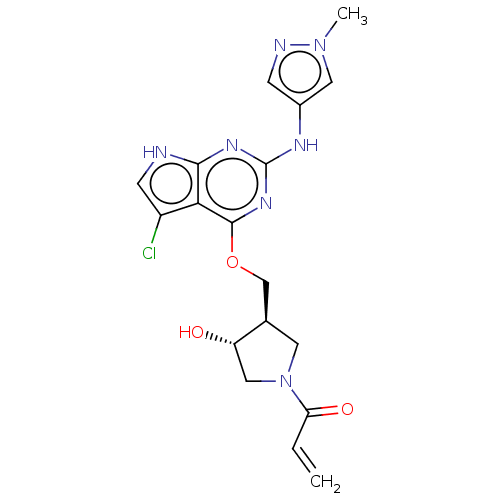
(CHEMBL3786220)Show SMILES Cn1cc(Nc2nc(OC[C@H]3CN(C[C@@H]3O)C(=O)C=C)c3c(Cl)c[nH]c3n2)cn1 |r| Show InChI InChI=1S/C18H20ClN7O3/c1-3-14(28)26-6-10(13(27)8-26)9-29-17-15-12(19)5-20-16(15)23-18(24-17)22-11-4-21-25(2)7-11/h3-5,7,10,13,27H,1,6,8-9H2,2H3,(H2,20,22,23,24)/t10-,13+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50433046

(CHEMBL2375965)Show SMILES Cc1nc(N)nc2n([C@H]3CC[C@@H](CC3)OCO)c(=O)c(cc12)-c1cnc2ccccc2c1 |r,wU:11.14,wD:8.7,(65.77,-15.8,;65.77,-17.34,;64.44,-18.11,;64.44,-19.65,;63.11,-20.42,;65.77,-20.42,;67.1,-19.65,;68.44,-20.43,;68.43,-21.96,;67.09,-22.73,;67.09,-24.26,;68.41,-25.04,;69.75,-24.28,;69.76,-22.73,;68.4,-26.58,;67.07,-27.34,;65.74,-26.56,;69.79,-19.66,;71.12,-20.43,;69.79,-18.1,;68.45,-17.32,;67.11,-18.1,;71.13,-17.34,;72.46,-18.12,;73.8,-17.36,;73.79,-15.82,;75.12,-15.05,;75.13,-13.51,;73.79,-12.74,;72.46,-13.51,;72.46,-15.04,;71.13,-15.81,)| Show InChI InChI=1S/C24H25N5O3/c1-14-19-11-20(16-10-15-4-2-3-5-21(15)26-12-16)23(31)29(22(19)28-24(25)27-14)17-6-8-18(9-7-17)32-13-30/h2-5,10-12,17-18,30H,6-9,13H2,1H3,(H2,25,27,28)/t17-,18- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50450868
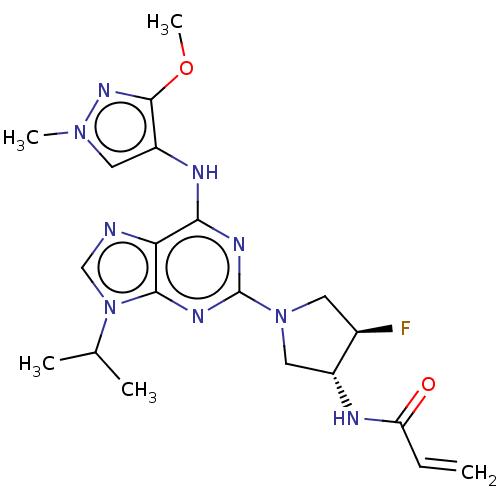
(CHEMBL4216679)Show SMILES COc1nn(C)cc1Nc1nc(nc2n(cnc12)C(C)C)N1C[C@@H](F)[C@@H](C1)NC(=O)C=C |r| Show InChI InChI=1S/C20H26FN9O2/c1-6-15(31)23-13-9-29(7-12(13)21)20-25-17(24-14-8-28(4)27-19(14)32-5)16-18(26-20)30(10-22-16)11(2)3/h6,8,10-13H,1,7,9H2,2-5H3,(H,23,31)(H,24,25,26)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuxi AppTec
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) |
J Med Chem 60: 3002-3019 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01894
BindingDB Entry DOI: 10.7270/Q25X2CJZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50433032

(CHEMBL2376096)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCC(O)=O)c1=O |r,wU:22.27,wD:19.20,(12.89,-1.02,;11.56,-.25,;10.23,-1.01,;8.89,-.24,;7.56,-1.01,;7.56,-2.54,;8.89,-3.32,;10.22,-2.55,;6.22,-3.3,;4.87,-2.52,;3.53,-3.3,;2.2,-2.53,;2.19,-.99,;.87,-3.3,;.87,-4.85,;-.48,-5.62,;2.2,-5.62,;3.53,-4.85,;4.87,-5.63,;4.86,-7.16,;3.52,-7.93,;3.51,-9.46,;4.84,-10.24,;6.18,-9.48,;6.19,-7.93,;4.83,-11.78,;3.49,-12.54,;2.16,-11.76,;2.16,-10.22,;.82,-12.52,;6.22,-4.86,;7.55,-5.63,)| Show InChI InChI=1S/C22H25N5O5/c1-12-16-9-17(13-3-8-18(31-2)24-10-13)21(30)27(20(16)26-22(23)25-12)14-4-6-15(7-5-14)32-11-19(28)29/h3,8-10,14-15H,4-7,11H2,1-2H3,(H,28,29)(H2,23,25,26)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50433037

(CHEMBL2375956 | US8633204, 311)Show SMILES COc1ncc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCC(N)=O)c1=O |r,wU:22.27,wD:19.20,(40.72,-44.52,;39.39,-43.75,;38.06,-44.52,;38.05,-46.06,;36.72,-46.82,;35.39,-46.05,;35.39,-44.51,;36.72,-43.74,;34.05,-46.8,;32.7,-46.02,;31.36,-46.8,;30.03,-46.04,;30.02,-44.5,;28.7,-46.81,;28.7,-48.35,;27.36,-49.12,;30.03,-49.12,;31.36,-48.35,;32.7,-49.13,;32.69,-50.67,;31.35,-51.43,;31.34,-52.96,;32.67,-53.74,;34.01,-52.98,;34.02,-51.44,;32.66,-55.28,;31.32,-56.04,;29.99,-55.26,;28.66,-56.03,;30,-53.73,;34.05,-48.36,;35.38,-49.13,)| Show InChI InChI=1S/C21H25N7O4/c1-11-15-7-16(12-8-24-21(31-2)25-9-12)19(30)28(18(15)27-20(23)26-11)13-3-5-14(6-4-13)32-10-17(22)29/h7-9,13-14H,3-6,10H2,1-2H3,(H2,22,29)(H2,23,26,27)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
Bioorg Med Chem Lett 23: 2787-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.020
BindingDB Entry DOI: 10.7270/Q2NZ890X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data