

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50403297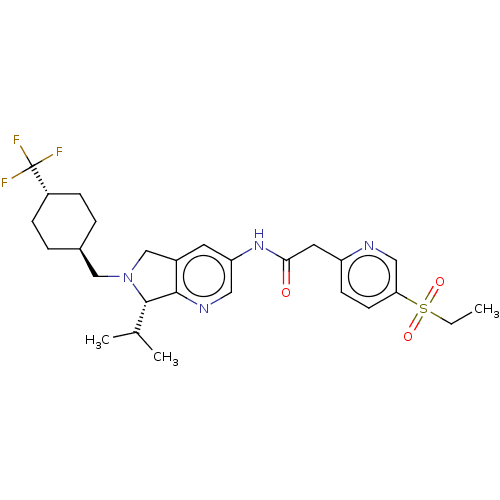 (CHEMBL5288854) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The calpain inhibitory activity(I 50) was measured as ability to enter the platelet to inhibit calpain after cell lysis(assay 2) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50594741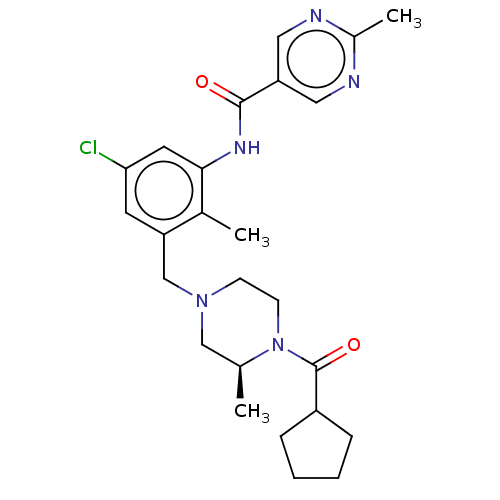 (CHEMBL5205088) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50403296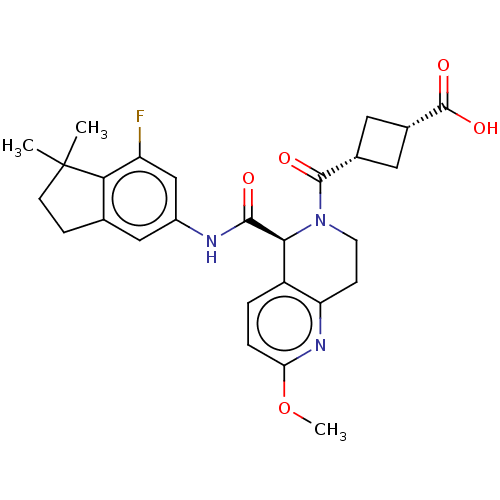 (CHEMBL5284935) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Compound was evaluated for the inhibition of Escherichia coli Thymidylate Synthase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50403300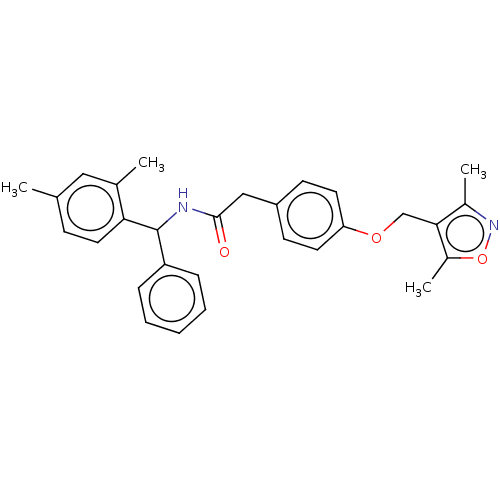 (CHEMBL5282505) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibitory activity against Escherichia coli dihydrofolate reductase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50403299 (CHEMBL5276895) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50403296 (CHEMBL5284935) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Compound was evaluated for the inhibition of Escherichia coli Thymidylate Synthase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50106301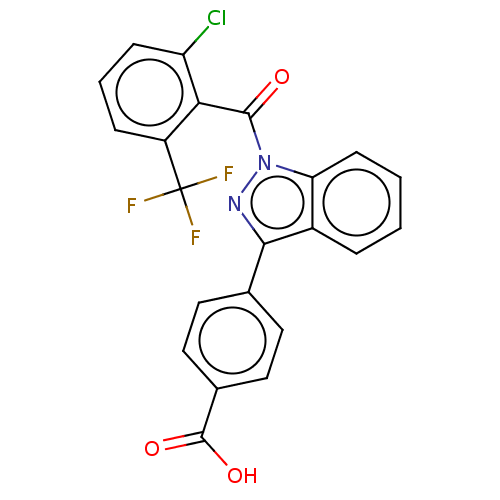 (CHEMBL3598140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound was evaluated for the inhibition of Escherichia coli Thymidylate Synthase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50403298 (CHEMBL5187263) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50594741 (CHEMBL5205088) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50594741 (CHEMBL5205088) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The ability of compound to inhibit calpain in a preparation of lysed platelets was measured with a caseinolytic assay(assay 1) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50594741 (CHEMBL5205088) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound was evaluated for the inhibition of Escherichia coli Thymidylate Synthase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-17A (Homo sapiens (Human)) | BDBM50403297 (CHEMBL5288854) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | n/a | n/a | 221 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description The calpain inhibitory activity(I 50) was measured as ability to enter the platelet to inhibit calpain after cell lysis(assay 2) | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM68281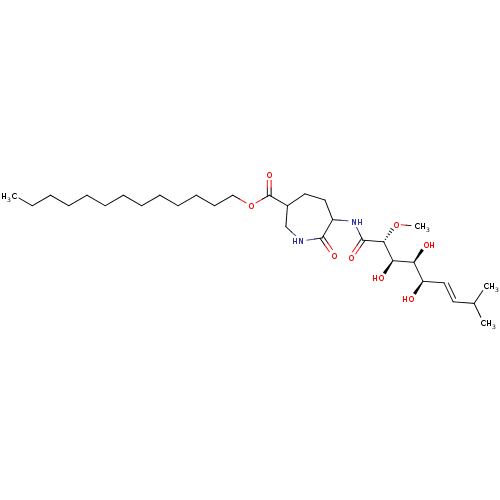 (Bengamide A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM68286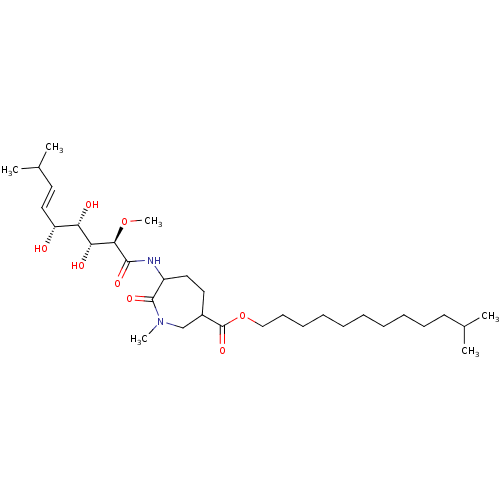 (Bengamide N | Bengamide O) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50068270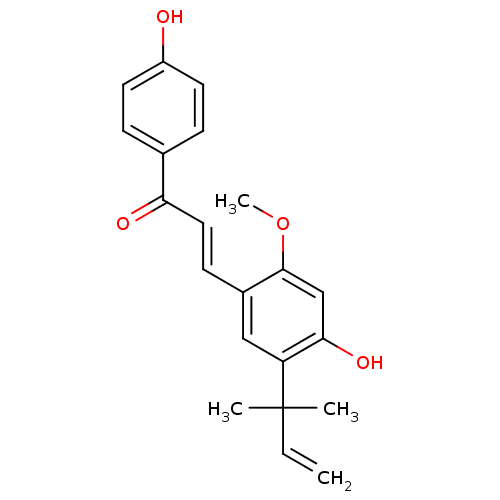 ((E)-3-(4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guizhou Medcial University Curated by ChEMBL | Assay Description Inhibition of IDH1 R132C mutant (unknown origin) by enzymatic assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126825 BindingDB Entry DOI: 10.7270/Q2P55S1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM68285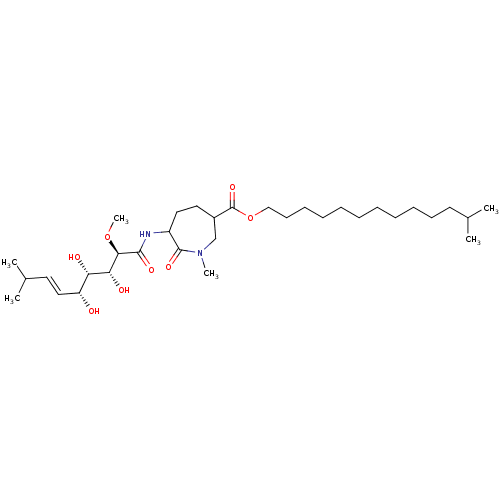 (Bengamide M) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM68281 (Bengamide A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM68282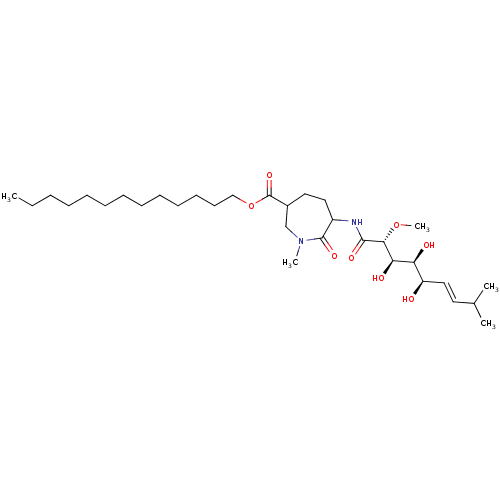 (Bengamide B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM68283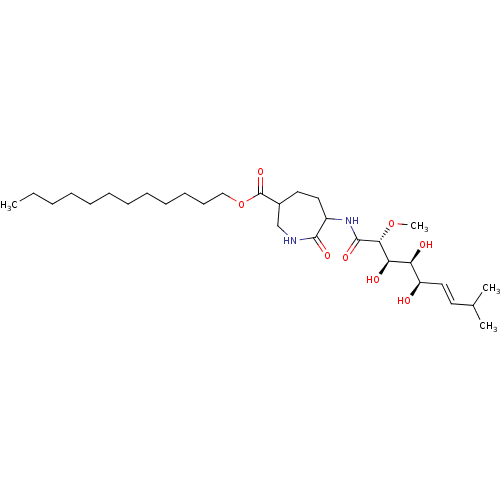 (Bengamide G) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM68282 (Bengamide B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM68284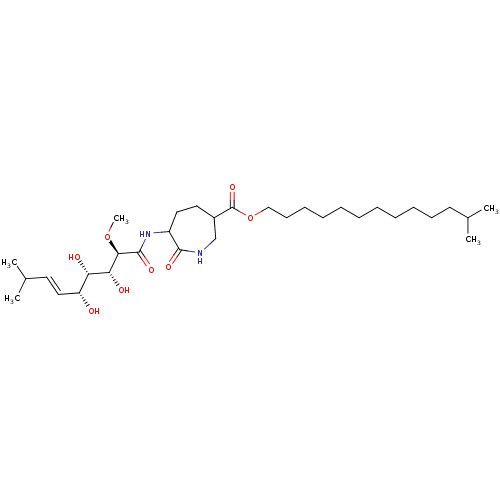 (Bengamide L) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM68286 (Bengamide N | Bengamide O) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM68286 (Bengamide N | Bengamide O) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM68283 (Bengamide G) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM68284 (Bengamide L) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM68285 (Bengamide M) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM68286 (Bengamide N | Bengamide O) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Johns Hopkins University | Assay Description The determined effects of seven bengamide analogs on the enzymatic activity of both recombinated human MetAP1 and MetAP2 in vitro. | Chem Biol 14: 764-74 (2007) Article DOI: 10.1016/j.chembiol.2007.05.010 BindingDB Entry DOI: 10.7270/Q2NC5ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50068270 ((E)-3-(4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Guizhou Medcial University Curated by ChEMBL | Assay Description Inhibition of IDH1 R132H mutant (unknown origin) by enzymatic assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126825 BindingDB Entry DOI: 10.7270/Q2P55S1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50068270 ((E)-3-(4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Guizhou Medcial University Curated by ChEMBL | Assay Description Inhibition of IDH1 (unknown origin) by enzymatic assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126825 BindingDB Entry DOI: 10.7270/Q2P55S1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50068270 ((E)-3-(4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a |
Guizhou Medcial University Curated by ChEMBL | Assay Description Binding affinity to IDH1 R132H mutant (unknown origin) by surface plasmon resonance assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126825 BindingDB Entry DOI: 10.7270/Q2P55S1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50068270 ((E)-3-(4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a |
Guizhou Medcial University Curated by ChEMBL | Assay Description Binding affinity to IDH1 (unknown origin) by surface plasmon resonance assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126825 BindingDB Entry DOI: 10.7270/Q2P55S1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM50068270 ((E)-3-(4-hydroxy-2-methoxy-5-(2-methylbut-3-en-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a |
Guizhou Medcial University Curated by ChEMBL | Assay Description Binding affinity to IDH1 R132C mutant (unknown origin) by surface plasmon resonance assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2019.126825 BindingDB Entry DOI: 10.7270/Q2P55S1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50153594 (CHEMBL3774855) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound was evaluated for the inhibition of Escherichia coli Thymidylate Synthase | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||