 Found 1798 hits with Last Name = 'davies' and Initial = 'g'
Found 1798 hits with Last Name = 'davies' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000041

((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...)Show InChI InChI=1S/C19H24N2O/c1-22-18-12-6-5-10-16(18)14-21-17-11-7-13-20-19(17)15-8-3-2-4-9-15/h2-6,8-10,12,17,19-21H,7,11,13-14H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human NK1 receptor was determined |
Bioorg Med Chem Lett 5: 2671-2676 (1995)
Article DOI: 10.1016/0960-894X(95)00481-8
BindingDB Entry DOI: 10.7270/Q2V40V5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-[acyl-carrier-protein] reductase [NADH]
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50425950
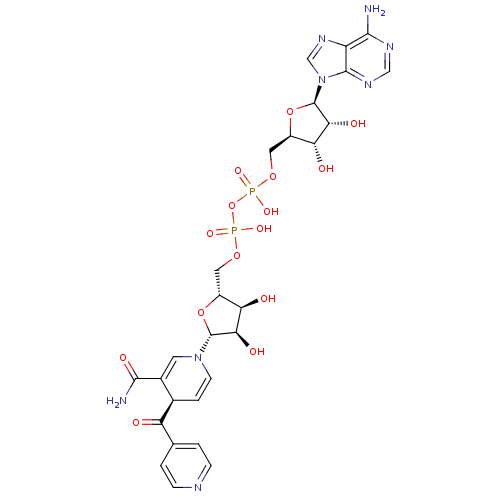
(CHEMBL2311561 | Isoniazid-NAD)Show SMILES NC(=O)C1=CN(C=C[C@H]1C(=O)c1ccncc1)[C@@H]1O[C@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r,c:6,t:3| Show InChI InChI=1S/C27H32N8O15P2/c28-23-17-25(32-10-31-23)35(11-33-17)27-22(40)20(38)16(49-27)9-47-52(44,45)50-51(42,43)46-8-15-19(37)21(39)26(48-15)34-6-3-13(14(7-34)24(29)41)18(36)12-1-4-30-5-2-12/h1-7,10-11,13,15-16,19-22,26-27,37-40H,8-9H2,(H2,29,41)(H,42,43)(H,44,45)(H2,28,31,32)/t13-,15-,16-,19-,20-,21-,22-,26-,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca India Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of wild type Mycobacterium tuberculosis inhA |
J Med Chem 56: 8533-42 (2013)
Article DOI: 10.1021/jm4012033
BindingDB Entry DOI: 10.7270/Q2SB4769 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000041

((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...)Show InChI InChI=1S/C19H24N2O/c1-22-18-12-6-5-10-16(18)14-21-17-11-7-13-20-19(17)15-8-3-2-4-9-15/h2-6,8-10,12,17,19-21H,7,11,13-14H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity measured by displacement of tritiated radiolabeled substance P from cloned human NK1 receptor expressed in CHO cell membranes |
Bioorg Med Chem Lett 5: 2671-2676 (1995)
Article DOI: 10.1016/0960-894X(95)00481-8
BindingDB Entry DOI: 10.7270/Q2V40V5B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394065
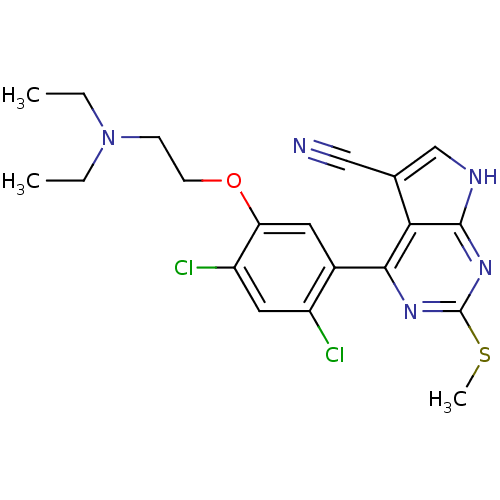
(CHEMBL2158626)Show SMILES CCN(CC)CCOc1cc(c(Cl)cc1Cl)-c1nc(SC)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C20H21Cl2N5OS/c1-4-27(5-2)6-7-28-16-8-13(14(21)9-15(16)22)18-17-12(10-23)11-24-19(17)26-20(25-18)29-3/h8-9,11H,4-7H2,1-3H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394058
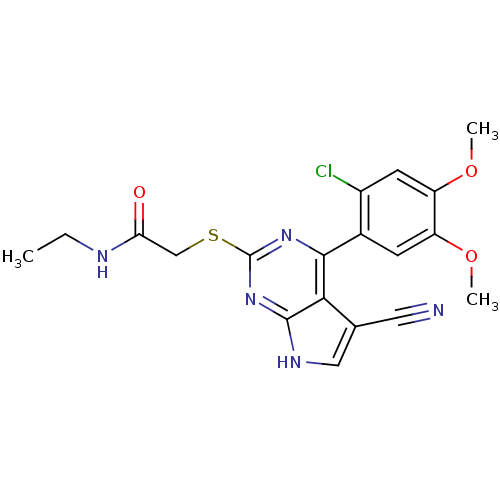
(CHEMBL2158570)Show SMILES CCNC(=O)CSc1nc(-c2cc(OC)c(OC)cc2Cl)c2c(c[nH]c2n1)C#N Show InChI InChI=1S/C19H18ClN5O3S/c1-4-22-15(26)9-29-19-24-17(16-10(7-21)8-23-18(16)25-19)11-5-13(27-2)14(28-3)6-12(11)20/h5-6,8H,4,9H2,1-3H3,(H,22,26)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394056
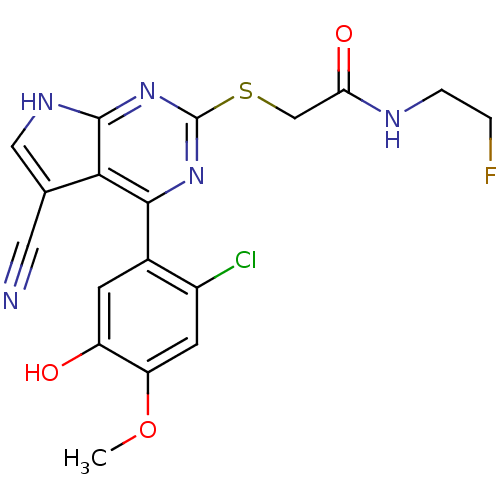
(CHEMBL2158577)Show SMILES COc1cc(Cl)c(cc1O)-c1nc(SCC(=O)NCCF)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C18H15ClFN5O3S/c1-28-13-5-11(19)10(4-12(13)26)16-15-9(6-21)7-23-17(15)25-18(24-16)29-8-14(27)22-3-2-20/h4-5,7,26H,2-3,8H2,1H3,(H,22,27)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394064
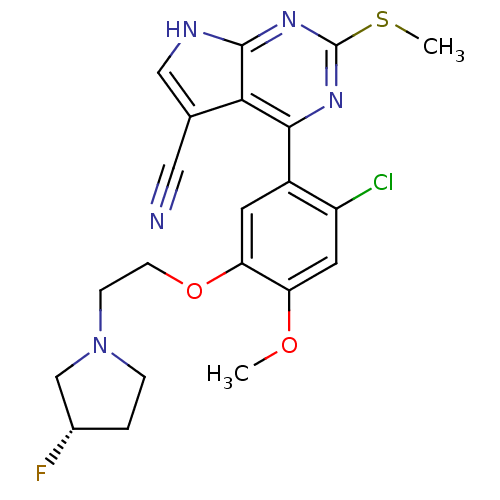
(CHEMBL2158627)Show SMILES COc1cc(Cl)c(cc1OCCN1CC[C@H](F)C1)-c1nc(SC)nc2[nH]cc(C#N)c12 |r| Show InChI InChI=1S/C21H21ClFN5O2S/c1-29-16-8-15(22)14(7-17(16)30-6-5-28-4-3-13(23)11-28)19-18-12(9-24)10-25-20(18)27-21(26-19)31-2/h7-8,10,13H,3-6,11H2,1-2H3,(H,25,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394059
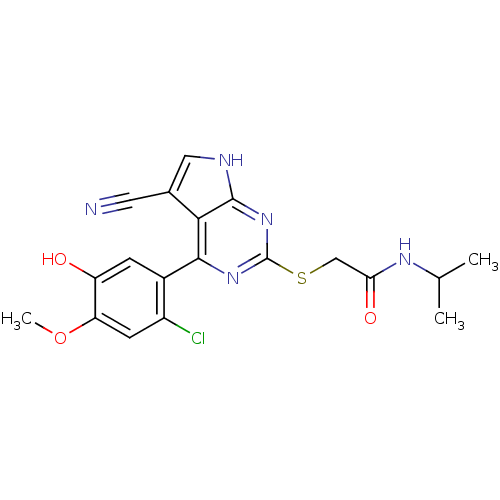
(CHEMBL2158569)Show SMILES COc1cc(Cl)c(cc1O)-c1nc(SCC(=O)NC(C)C)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H18ClN5O3S/c1-9(2)23-15(27)8-29-19-24-17(16-10(6-21)7-22-18(16)25-19)11-4-13(26)14(28-3)5-12(11)20/h4-5,7,9,26H,8H2,1-3H3,(H,23,27)(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394066
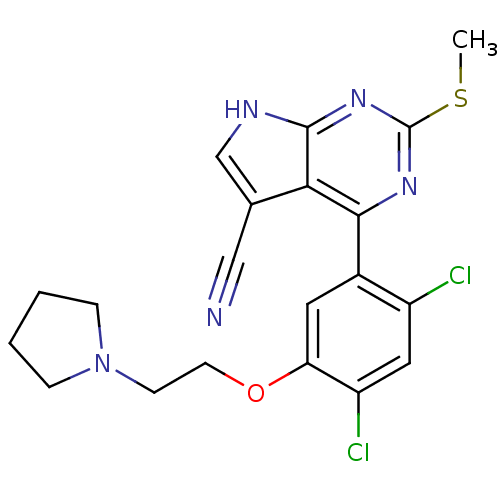
(CHEMBL2158625)Show SMILES CSc1nc(-c2cc(OCCN3CCCC3)c(Cl)cc2Cl)c2c(c[nH]c2n1)C#N Show InChI InChI=1S/C20H19Cl2N5OS/c1-29-20-25-18(17-12(10-23)11-24-19(17)26-20)13-8-16(15(22)9-14(13)21)28-7-6-27-4-2-3-5-27/h8-9,11H,2-7H2,1H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394057
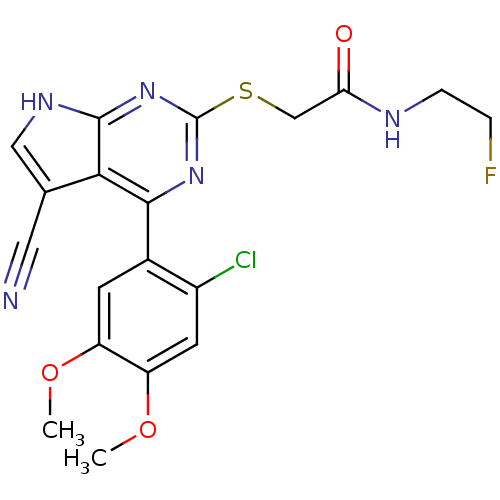
(CHEMBL2158576)Show SMILES COc1cc(Cl)c(cc1OC)-c1nc(SCC(=O)NCCF)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C19H17ClFN5O3S/c1-28-13-5-11(12(20)6-14(13)29-2)17-16-10(7-22)8-24-18(16)26-19(25-17)30-9-15(27)23-4-3-21/h5-6,8H,3-4,9H2,1-2H3,(H,23,27)(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394062
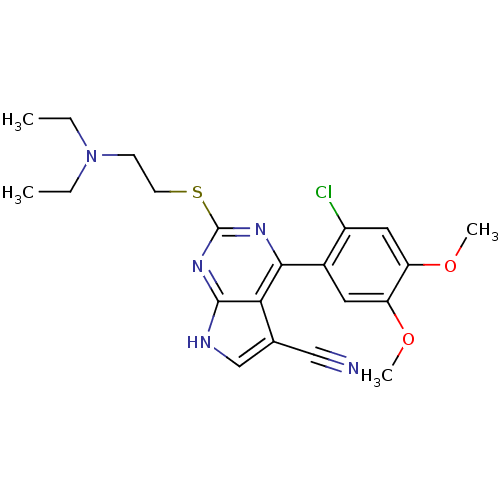
(CHEMBL2158630)Show SMILES CCN(CC)CCSc1nc(-c2cc(OC)c(OC)cc2Cl)c2c(c[nH]c2n1)C#N Show InChI InChI=1S/C21H24ClN5O2S/c1-5-27(6-2)7-8-30-21-25-19(18-13(11-23)12-24-20(18)26-21)14-9-16(28-3)17(29-4)10-15(14)22/h9-10,12H,5-8H2,1-4H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394055
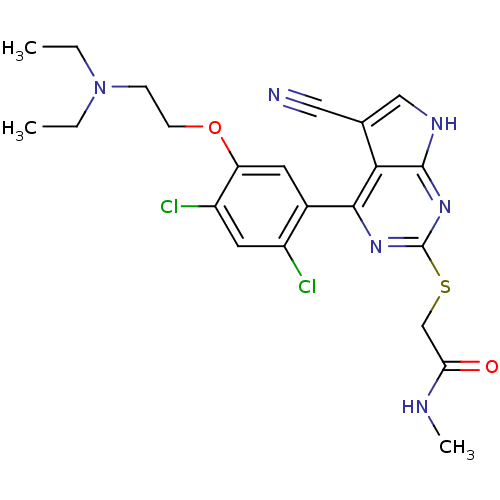
(CHEMBL2158563)Show SMILES CCN(CC)CCOc1cc(c(Cl)cc1Cl)-c1nc(SCC(=O)NC)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C22H24Cl2N6O2S/c1-4-30(5-2)6-7-32-17-8-14(15(23)9-16(17)24)20-19-13(10-25)11-27-21(19)29-22(28-20)33-12-18(31)26-3/h8-9,11H,4-7,12H2,1-3H3,(H,26,31)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394063
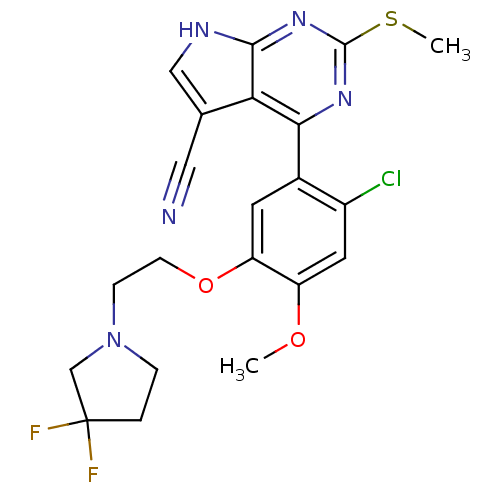
(CHEMBL2158628)Show SMILES COc1cc(Cl)c(cc1OCCN1CCC(F)(F)C1)-c1nc(SC)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C21H20ClF2N5O2S/c1-30-15-8-14(22)13(7-16(15)31-6-5-29-4-3-21(23,24)11-29)18-17-12(9-25)10-26-19(17)28-20(27-18)32-2/h7-8,10H,3-6,11H2,1-2H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236511
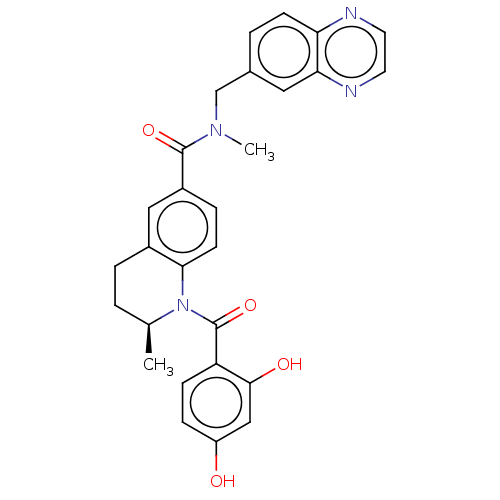
(CHEMBL3718319)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)C(=O)N(C)Cc1ccc2nccnc2c1 |r| Show InChI InChI=1S/C28H26N4O4/c1-17-3-5-19-14-20(6-10-25(19)32(17)28(36)22-8-7-21(33)15-26(22)34)27(35)31(2)16-18-4-9-23-24(13-18)30-12-11-29-23/h4,6-15,17,33-34H,3,5,16H2,1-2H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity towards human Dopamine receptor D2 (long) by [3H]-spiperone displacement. |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50323697

((3AR,5R,6S,7R,7AR)-2-(ETHYLAMINO)-5-(HYDROXYMETHYL...)Show SMILES CCNC1=N[C@H]2[C@H](O[C@H](CO)[C@@H](O)[C@@H]2O)S1 |r,t:3| Show InChI InChI=1S/C9H16N2O4S/c1-2-10-9-11-5-7(14)6(13)4(3-12)15-8(5)16-9/h4-8,12-14H,2-3H2,1H3,(H,10,11)/t4-,5-,6-,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human O-GlcNAcase |
Nat Chem Biol 4: 483-90 (2008)
Article DOI: 10.1038/nchembio.96
BindingDB Entry DOI: 10.7270/Q28C9WGB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236516
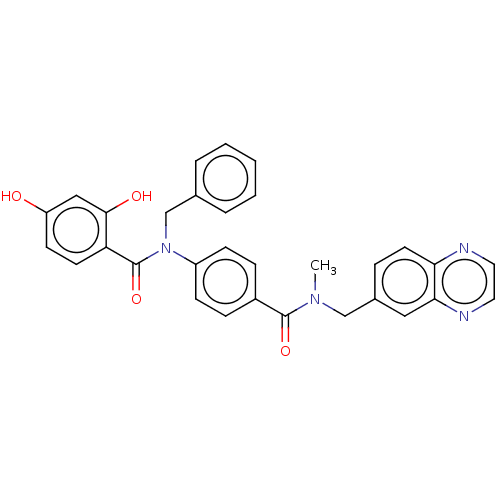
(CHEMBL3731789)Show SMILES CN(Cc1ccc2nccnc2c1)C(=O)c1ccc(cc1)N(Cc1ccccc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C31H26N4O4/c1-34(19-22-7-14-27-28(17-22)33-16-15-32-27)30(38)23-8-10-24(11-9-23)35(20-21-5-3-2-4-6-21)31(39)26-13-12-25(36)18-29(26)37/h2-18,36-37H,19-20H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236530
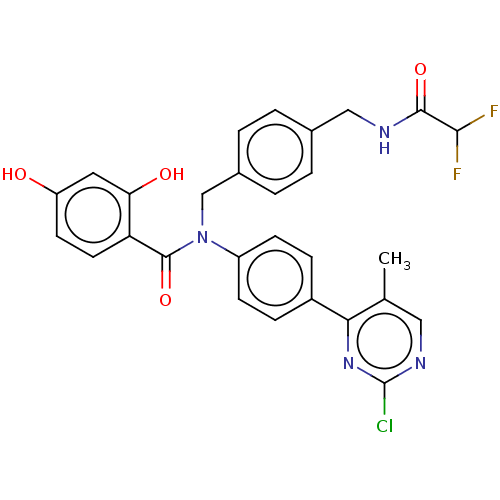
(CHEMBL3727577)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccc(CNC(=O)C(F)F)cc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C28H23ClF2N4O4/c1-16-13-33-28(29)34-24(16)19-6-8-20(9-7-19)35(27(39)22-11-10-21(36)12-23(22)37)15-18-4-2-17(3-5-18)14-32-26(38)25(30)31/h2-13,25,36-37H,14-15H2,1H3,(H,32,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM265209
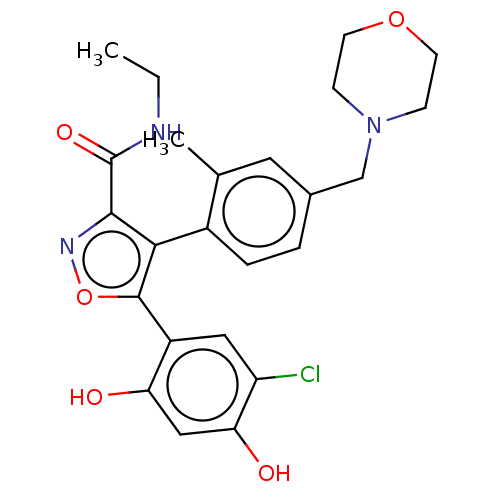
(US10413550, Example 41j | US11234987, Example 41j ...)Show SMILES CCNC(=O)c1noc(c1-c1ccc(CN2CCOCC2)cc1C)-c1cc(Cl)c(O)cc1O |(3.1,-6.74,;2.33,-5.41,;3.1,-4.07,;2.33,-2.74,;3.1,-1.41,;.79,-2.74,;-.12,-3.99,;-1.58,-3.51,;-1.58,-1.97,;-.12,-1.49,;.28,-.01,;-.81,1.08,;-.41,2.57,;1.08,2.97,;1.48,4.46,;2.96,4.85,;4.05,3.77,;5.54,4.16,;5.94,5.65,;4.85,6.74,;3.36,6.34,;2.17,1.88,;1.77,.39,;2.86,-.7,;-2.67,-.88,;-2.27,.61,;-3.36,1.7,;-2.96,3.18,;-4.85,1.3,;-5.94,2.39,;-5.25,-.19,;-4.16,-1.28,;-4.56,-2.77,)| Show InChI InChI=1S/C24H26ClN3O5/c1-3-26-24(31)22-21(23(33-27-22)17-11-18(25)20(30)12-19(17)29)16-5-4-15(10-14(16)2)13-28-6-8-32-9-7-28/h4-5,10-12,29-30H,3,6-9,13H2,1-2H3,(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236519
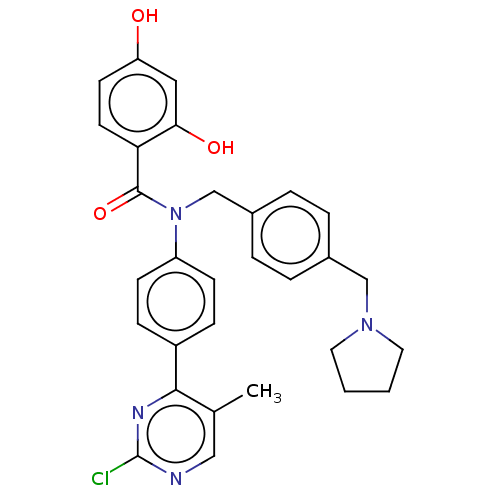
(CHEMBL3727843)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccc(CN2CCCC2)cc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C30H29ClN4O3/c1-20-17-32-30(31)33-28(20)23-8-10-24(11-9-23)35(29(38)26-13-12-25(36)16-27(26)37)19-22-6-4-21(5-7-22)18-34-14-2-3-15-34/h4-13,16-17,36-37H,2-3,14-15,18-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine transporter (DAT) of cynomolgus monkey caudate-putamen |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236514
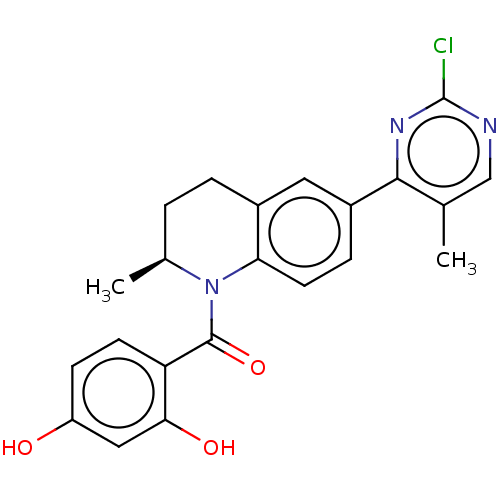
(CHEMBL4100504)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc(Cl)ncc1C |r| Show InChI InChI=1S/C22H20ClN3O3/c1-12-11-24-22(23)25-20(12)15-5-8-18-14(9-15)4-3-13(2)26(18)21(29)17-7-6-16(27)10-19(17)28/h5-11,13,27-28H,3-4H2,1-2H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50213785
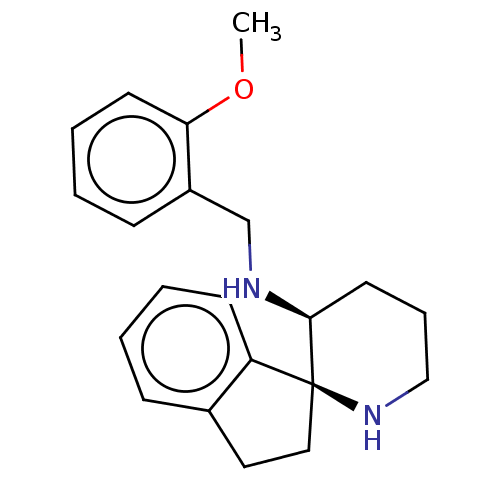
(CHEMBL91475)Show InChI InChI=1S/C21H26N2O/c1-24-19-10-5-3-8-17(19)15-22-20-11-6-14-23-21(20)13-12-16-7-2-4-9-18(16)21/h2-5,7-10,20,22-23H,6,11-15H2,1H3/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity measured by displacement of tritiated radiolabeled substance P from cloned human NK1 receptor expressed in CHO cell membranes |
Bioorg Med Chem Lett 5: 2671-2676 (1995)
Article DOI: 10.1016/0960-894X(95)00481-8
BindingDB Entry DOI: 10.7270/Q2V40V5B |
More data for this
Ligand-Target Pair | |
Beta-mannosidase
(Bacteroides thetaiotaomicron) | BDBM36374

((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@H](O)c2nc(CCc3ccccc3)cn12 Show InChI InChI=1S/C16H20N2O4/c19-9-12-13(20)14(21)15(22)16-17-11(8-18(12)16)7-6-10-4-2-1-3-5-10/h1-5,8,12-15,19-22H,6-7,9H2/t12-,13-,14+,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | -43.0 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Medical School, Newcastle University
| Assay Description
Putative mannosidase assay using wild-type protein Btman2A. Enzyme inhibition assay. |
Nat Chem Biol 4: 306-12 (2008)
Article DOI: 10.1038/nchembio.81
BindingDB Entry DOI: 10.7270/Q2X34VS6 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394061
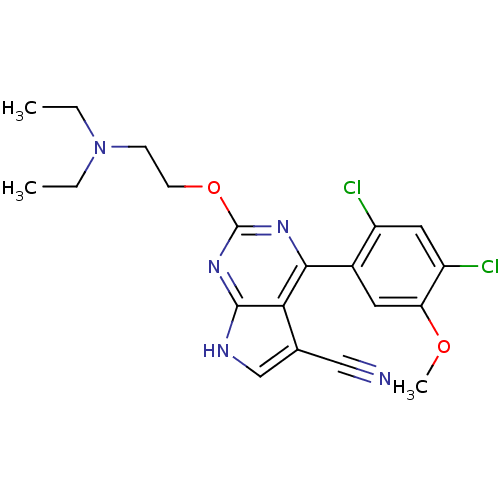
(CHEMBL2158008)Show SMILES CCN(CC)CCOc1nc(-c2cc(OC)c(Cl)cc2Cl)c2c(c[nH]c2n1)C#N Show InChI InChI=1S/C20H21Cl2N5O2/c1-4-27(5-2)6-7-29-20-25-18(17-12(10-23)11-24-19(17)26-20)13-8-16(28-3)15(22)9-14(13)21/h8-9,11H,4-7H2,1-3H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236518
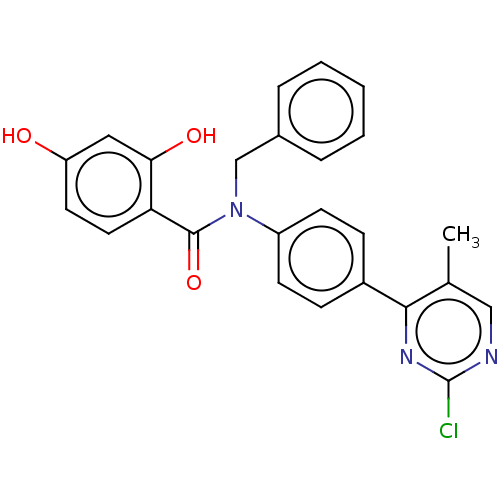
(CHEMBL3732469)Show SMILES Cc1cnc(Cl)nc1-c1ccc(cc1)N(Cc1ccccc1)C(=O)c1ccc(O)cc1O Show InChI InChI=1S/C25H20ClN3O3/c1-16-14-27-25(26)28-23(16)18-7-9-19(10-8-18)29(15-17-5-3-2-4-6-17)24(32)21-12-11-20(30)13-22(21)31/h2-14,30-31H,15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Beta-mannosidase
(Bacteroides thetaiotaomicron) | BDBM36375

((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@H](O)c2nc(CNc3ccccc3)cn12 Show InChI InChI=1S/C15H19N3O4/c19-8-11-12(20)13(21)14(22)15-17-10(7-18(11)15)6-16-9-4-2-1-3-5-9/h1-5,7,11-14,16,19-22H,6,8H2/t11-,12-,13+,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 72 | -42.4 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Medical School, Newcastle University
| Assay Description
Putative mannosidase assay using wild-type protein Btman2A. Enzyme inhibition assay. |
Nat Chem Biol 4: 306-12 (2008)
Article DOI: 10.1038/nchembio.81
BindingDB Entry DOI: 10.7270/Q2X34VS6 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236521
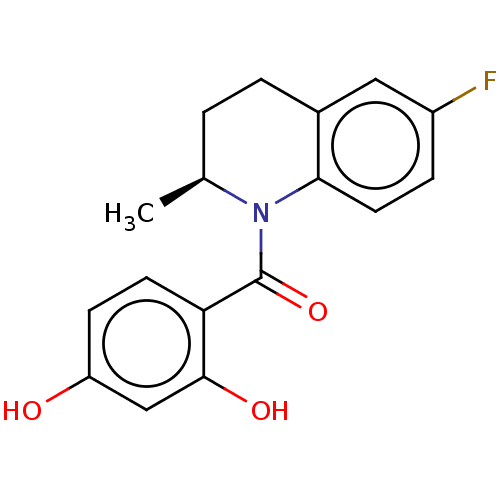
(CHEMBL3715843)Show SMILES C[C@H]1CCc2cc(F)ccc2N1C(=O)c1ccc(O)cc1O |r| Show InChI InChI=1S/C17H16FNO3/c1-10-2-3-11-8-12(18)4-7-15(11)19(10)17(22)14-6-5-13(20)9-16(14)21/h4-10,20-21H,2-3H2,1H3/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PDHK1 (unknown origin) by isothermal titration calorimetry |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236512
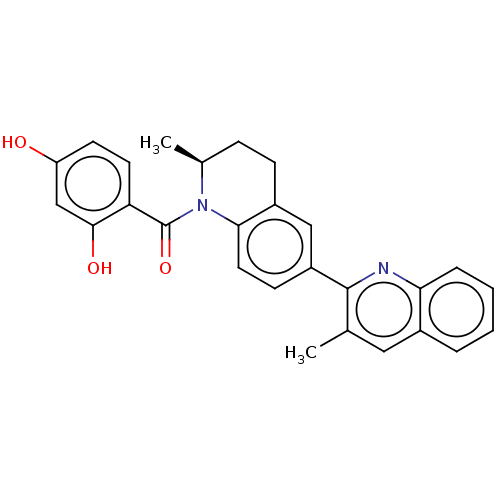
(CHEMBL3716663)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc2ccccc2cc1C |r| Show InChI InChI=1S/C27H24N2O3/c1-16-13-18-5-3-4-6-23(18)28-26(16)20-9-12-24-19(14-20)8-7-17(2)29(24)27(32)22-11-10-21(30)15-25(22)31/h3-6,9-15,17,30-31H,7-8H2,1-2H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM265209

(US10413550, Example 41j | US11234987, Example 41j ...)Show SMILES CCNC(=O)c1noc(c1-c1ccc(CN2CCOCC2)cc1C)-c1cc(Cl)c(O)cc1O |(3.1,-6.74,;2.33,-5.41,;3.1,-4.07,;2.33,-2.74,;3.1,-1.41,;.79,-2.74,;-.12,-3.99,;-1.58,-3.51,;-1.58,-1.97,;-.12,-1.49,;.28,-.01,;-.81,1.08,;-.41,2.57,;1.08,2.97,;1.48,4.46,;2.96,4.85,;4.05,3.77,;5.54,4.16,;5.94,5.65,;4.85,6.74,;3.36,6.34,;2.17,1.88,;1.77,.39,;2.86,-.7,;-2.67,-.88,;-2.27,.61,;-3.36,1.7,;-2.96,3.18,;-4.85,1.3,;-5.94,2.39,;-5.25,-.19,;-4.16,-1.28,;-4.56,-2.77,)| Show InChI InChI=1S/C24H26ClN3O5/c1-3-26-24(31)22-21(23(33-27-22)17-11-18(25)20(30)12-19(17)29)16-5-4-15(10-14(16)2)13-28-6-8-32-9-7-28/h4-5,10-12,29-30H,3,6-9,13H2,1-2H3,(H,26,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PDHK1 (unknown origin) by isothermal titration calorimetry |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial
(Homo sapiens (Human)) | BDBM50236526
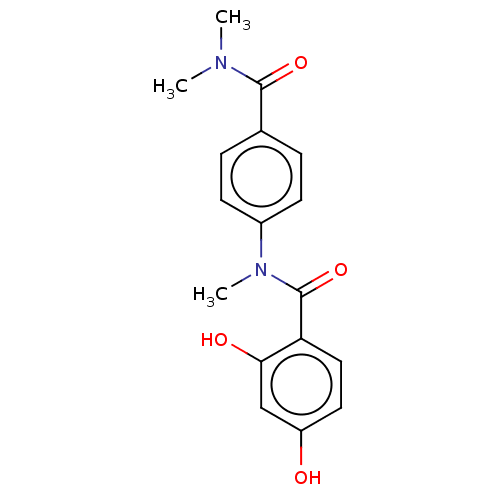
(CHEMBL4089806)Show InChI InChI=1S/C17H18N2O4/c1-18(2)16(22)11-4-6-12(7-5-11)19(3)17(23)14-9-8-13(20)10-15(14)21/h4-10,20-21H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK2 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394060
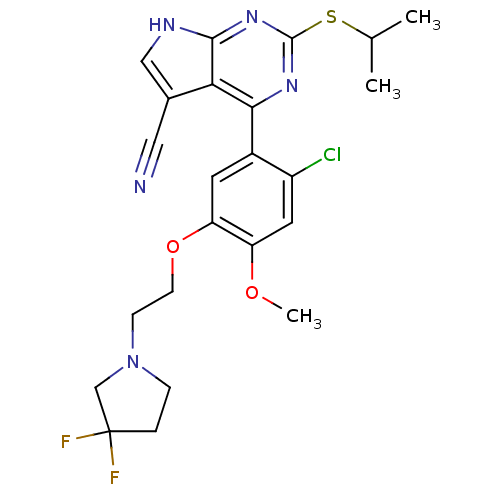
(CHEMBL2158565)Show SMILES COc1cc(Cl)c(cc1OCCN1CCC(F)(F)C1)-c1nc(SC(C)C)nc2[nH]cc(C#N)c12 Show InChI InChI=1S/C23H24ClF2N5O2S/c1-13(2)34-22-29-20(19-14(10-27)11-28-21(19)30-22)15-8-18(17(32-3)9-16(15)24)33-7-6-31-5-4-23(25,26)12-31/h8-9,11,13H,4-7,12H2,1-3H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50236525

(CHEMBL4097485)Show SMILES CN(C(=O)c1ccc(O)cc1O)c1ccc(cc1)C(=O)N1CC(Oc2ccccc12)C(O)=O Show InChI InChI=1S/C24H20N2O7/c1-25(23(30)17-11-10-16(27)12-19(17)28)15-8-6-14(7-9-15)22(29)26-13-21(24(31)32)33-20-5-3-2-4-18(20)26/h2-12,21,27-28H,13H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of fluorescein-labelled VER160364 from HSP90A (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394079
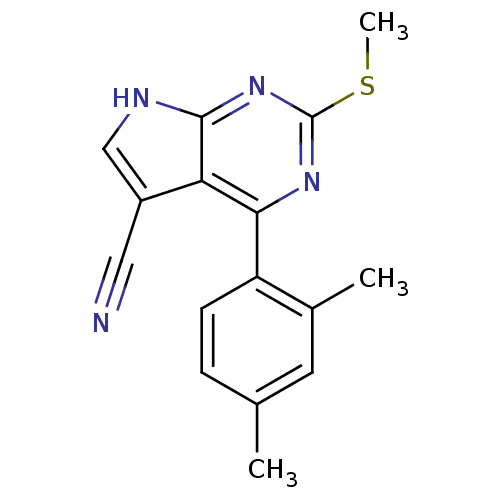
(CHEMBL2158581)Show InChI InChI=1S/C16H14N4S/c1-9-4-5-12(10(2)6-9)14-13-11(7-17)8-18-15(13)20-16(19-14)21-3/h4-6,8H,1-3H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236523
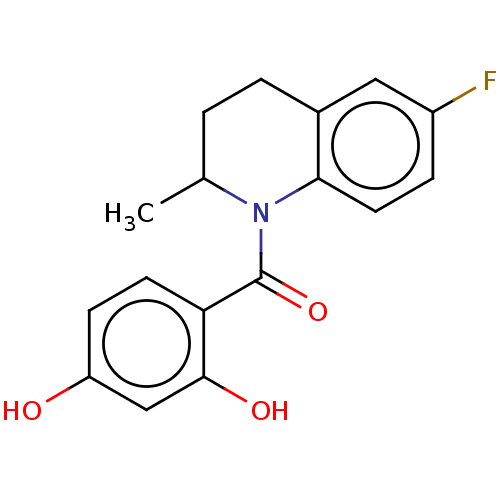
(CHEMBL3714988)Show InChI InChI=1S/C17H16FNO3/c1-10-2-3-11-8-12(18)4-7-15(11)19(10)17(22)14-6-5-13(20)9-16(14)21/h4-10,20-21H,2-3H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]WIN-35428 binding to dopamine transporter (DAT) of cynomolgus monkey caudate-putamen |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394077
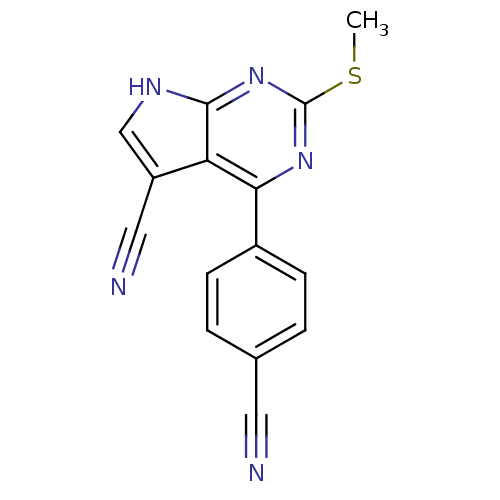
(CHEMBL2158583)Show InChI InChI=1S/C15H9N5S/c1-21-15-19-13(10-4-2-9(6-16)3-5-10)12-11(7-17)8-18-14(12)20-15/h2-5,8H,1H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236517
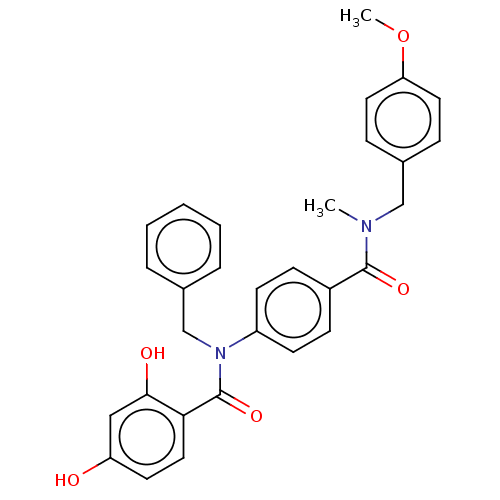
(CHEMBL3732579)Show SMILES COc1ccc(CN(C)C(=O)c2ccc(cc2)N(Cc2ccccc2)C(=O)c2ccc(O)cc2O)cc1 Show InChI InChI=1S/C30H28N2O5/c1-31(19-22-8-15-26(37-2)16-9-22)29(35)23-10-12-24(13-11-23)32(20-21-6-4-3-5-7-21)30(36)27-17-14-25(33)18-28(27)34/h3-18,33-34H,19-20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236525

(CHEMBL4097485)Show SMILES CN(C(=O)c1ccc(O)cc1O)c1ccc(cc1)C(=O)N1CC(Oc2ccccc12)C(O)=O Show InChI InChI=1S/C24H20N2O7/c1-25(23(30)17-11-10-16(27)12-19(17)28)15-8-6-14(7-9-15)22(29)26-13-21(24(31)32)33-20-5-3-2-4-18(20)26/h2-12,21,27-28H,13H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PDHK1 (unknown origin) by isothermal titration calorimetry |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394078
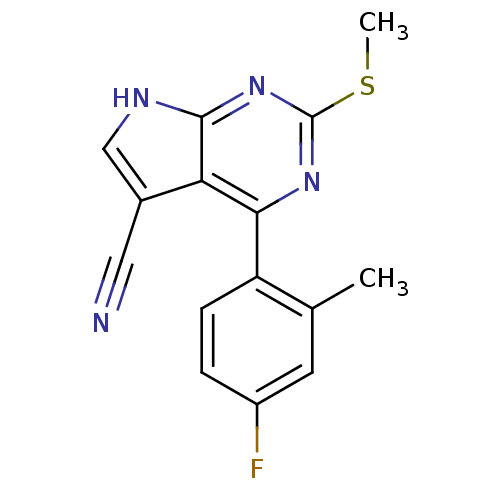
(CHEMBL2158582)Show InChI InChI=1S/C15H11FN4S/c1-8-5-10(16)3-4-11(8)13-12-9(6-17)7-18-14(12)20-15(19-13)21-2/h3-5,7H,1-2H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 358 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Alpha-1,2-mannosidase, putative
(Bacteroides thetaiotaomicron ) | BDBM36373

((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...)Show InChI InChI=1S/C8H12N2O4/c11-3-4-5(12)6(13)7(14)8-9-1-2-10(4)8/h1-2,4-7,11-14H,3H2/t4-,5-,6+,7+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3965 assessed as reduction of mannose release using 4NP-mannopyranoside substrate |
Nat Chem Biol 6: 125-32 (2010)
Article DOI: 10.1038/nchembio.278
BindingDB Entry DOI: 10.7270/Q2ZW1PQB |
More data for this
Ligand-Target Pair | |
Beta-mannosidase
(Bacteroides thetaiotaomicron) | BDBM36376

((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@H](O)c2nc(COc3ccccc3)cn12 Show InChI InChI=1S/C15H18N2O5/c18-7-11-12(19)13(20)14(21)15-16-9(6-17(11)15)8-22-10-4-2-1-3-5-10/h1-6,11-14,18-21H,7-8H2/t11-,12-,13+,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 401 | -38.0 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Medical School, Newcastle University
| Assay Description
Putative mannosidase assay using wild-type protein Btman2A. Enzyme inhibition assay. |
Nat Chem Biol 4: 306-12 (2008)
Article DOI: 10.1038/nchembio.81
BindingDB Entry DOI: 10.7270/Q2X34VS6 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50323695
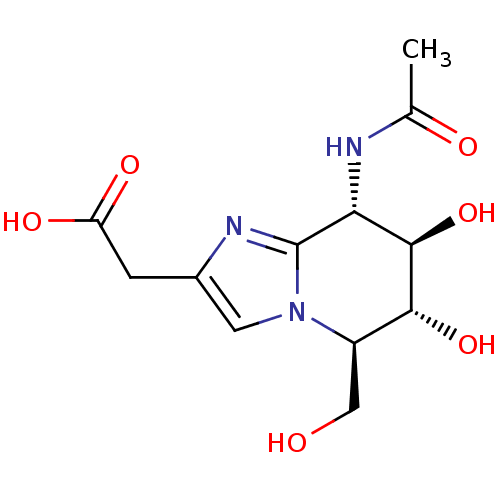
(2-((5R,6R,7R,8S)-8-acetamido-6,7-dihydroxy-5-(hydr...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)n2cc(CC(O)=O)nc12 Show InChI InChI=1S/C12H17N3O6/c1-5(17)13-9-11(21)10(20)7(4-16)15-3-6(2-8(18)19)14-12(9)15/h3,7,9-11,16,20-21H,2,4H2,1H3,(H,13,17)(H,18,19)/t7-,9-,10-,11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human O-GlcNAcase |
Nat Chem Biol 4: 483-90 (2008)
Article DOI: 10.1038/nchembio.96
BindingDB Entry DOI: 10.7270/Q28C9WGB |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236515
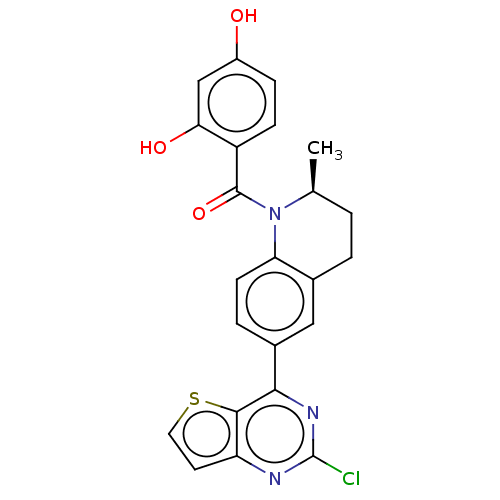
(CHEMBL3717621)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc(Cl)nc2ccsc12 |r| Show InChI InChI=1S/C23H18ClN3O3S/c1-12-2-3-13-10-14(20-21-17(8-9-31-21)25-23(24)26-20)4-7-18(13)27(12)22(30)16-6-5-15(28)11-19(16)29/h4-12,28-29H,2-3H2,1H3/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 3, mitochondrial
(Homo sapiens (Human)) | BDBM50236522
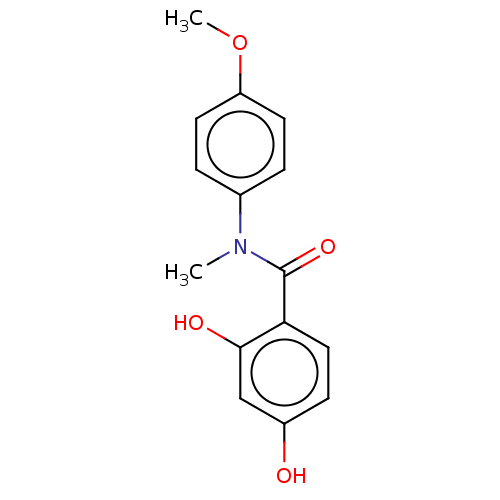
(CHEMBL4061698)Show InChI InChI=1S/C15H15NO4/c1-16(10-3-6-12(20-2)7-4-10)15(19)13-8-5-11(17)9-14(13)18/h3-9,17-18H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK3 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Homo sapiens (Human)) | BDBM50323696

((3AR,5R,6S,7R,7AR)-5-(HYDROXYMETHYL)-2-PROPYL-5,6,...)Show SMILES CCCC1=N[C@H]2[C@H](O[C@H](CO)[C@@H](O)[C@@H]2O)S1 |r,t:3| Show InChI InChI=1S/C10H17NO4S/c1-2-3-6-11-7-9(14)8(13)5(4-12)15-10(7)16-6/h5,7-10,12-14H,2-4H2,1H3/t5-,7-,8-,9-,10-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Simon Fraser University
Curated by ChEMBL
| Assay Description
Inhibition of human O-GlcNAcase |
Nat Chem Biol 4: 483-90 (2008)
Article DOI: 10.1038/nchembio.96
BindingDB Entry DOI: 10.7270/Q28C9WGB |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394069
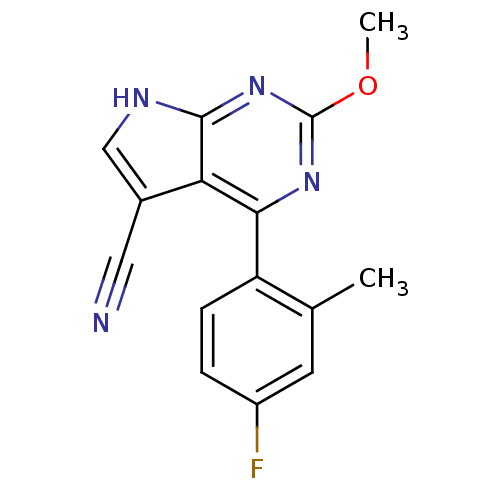
(CHEMBL2158622)Show InChI InChI=1S/C15H11FN4O/c1-8-5-10(16)3-4-11(8)13-12-9(6-17)7-18-14(12)20-15(19-13)21-2/h3-5,7H,1-2H3,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 628 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236513
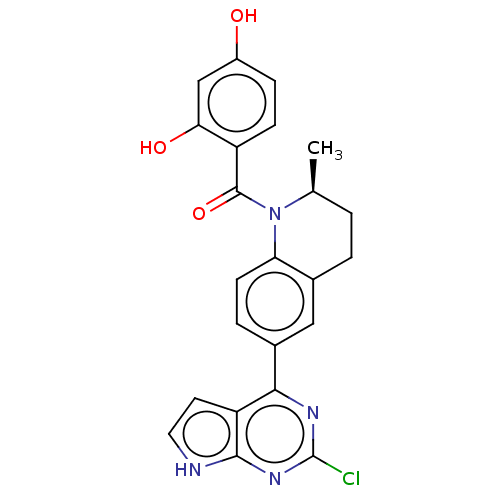
(CHEMBL3715902)Show SMILES C[C@H]1CCc2cc(ccc2N1C(=O)c1ccc(O)cc1O)-c1nc(Cl)nc2[nH]ccc12 |r| Show InChI InChI=1S/C23H19ClN4O3/c1-12-2-3-13-10-14(20-17-8-9-25-21(17)27-23(24)26-20)4-7-18(13)28(12)22(31)16-6-5-15(29)11-19(16)30/h4-12,29-30H,2-3H2,1H3,(H,25,26,27)/t12-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labelled VER160364 binding to PDHK1 (unknown origin) after 90 mins by fluorescence polarization assay |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50394085
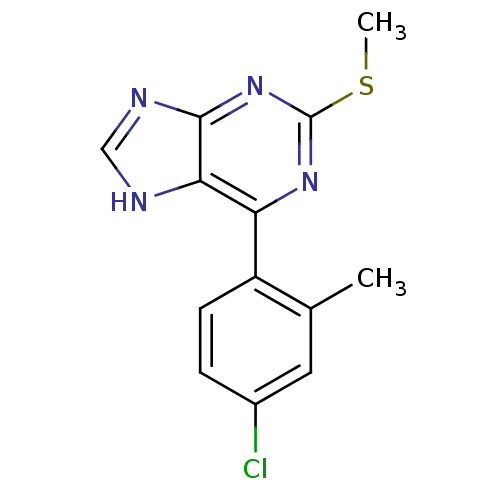
(CHEMBL2158635)Show InChI InChI=1S/C13H11ClN4S/c1-7-5-8(14)3-4-9(7)10-11-12(16-6-15-11)18-13(17-10)19-2/h3-6H,1-2H3,(H,15,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of fluorescently labeled VER51001 binding to full length human HSP90beta after 30 mins by fluorescence polarization assay |
Bioorg Med Chem 20: 6770-89 (2012)
Article DOI: 10.1016/j.bmc.2012.08.050
BindingDB Entry DOI: 10.7270/Q2KS6SN4 |
More data for this
Ligand-Target Pair | |
Beta-mannosidase
(Bacteroides thetaiotaomicron) | BDBM36384
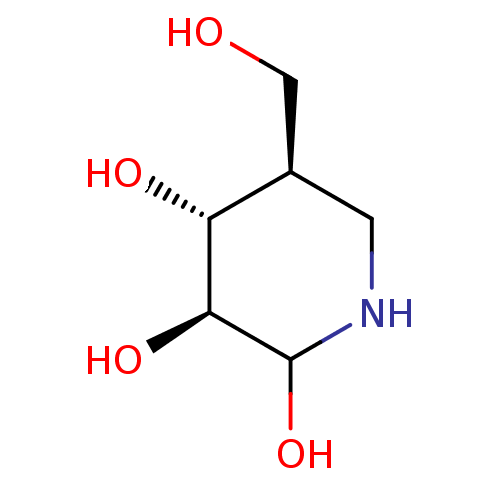
((2R/S,3S,4R,5R)-5-(Hydroxymethyl)piperidine-2,3,4-...)Show InChI InChI=1S/C6H13NO4/c8-2-3-1-7-6(11)5(10)4(3)9/h3-11H,1-2H2/t3-,4-,5+,6?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 975 | -35.7 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Medical School, Newcastle University
| Assay Description
Putative mannosidase assay using wild-type protein Btman2A. Enzyme inhibition assay. |
Nat Chem Biol 4: 306-12 (2008)
Article DOI: 10.1038/nchembio.81
BindingDB Entry DOI: 10.7270/Q2X34VS6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
[Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 1, mitochondrial
(Homo sapiens (Human)) | BDBM50236529
(CHEMBL3730154)Show InChI InChI=1S/C20H17NO3/c22-17-11-12-18(19(23)13-17)20(24)21(16-9-5-2-6-10-16)14-15-7-3-1-4-8-15/h1-13,22-23H,14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to PDHK1 (unknown origin) by isothermal titration calorimetry |
J Med Chem 60: 2271-2286 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01478
BindingDB Entry DOI: 10.7270/Q2XG9TDJ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50213784
(CHEMBL91840)Show InChI InChI=1S/C21H26N2O/c1-24-19-10-5-3-8-17(19)15-22-20-11-6-14-23-21(20)13-12-16-7-2-4-9-18(16)21/h2-5,7-10,20,22-23H,6,11-15H2,1H3/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity measured by displacement of tritiated radiolabeled substance P from cloned human NK1 receptor expressed in CHO cell membranes |
Bioorg Med Chem Lett 5: 2671-2676 (1995)
Article DOI: 10.1016/0960-894X(95)00481-8
BindingDB Entry DOI: 10.7270/Q2V40V5B |
More data for this
Ligand-Target Pair | |
Alpha-1,2-mannosidase, putative
(Bacteroides thetaiotaomicron ) | BDBM36373

((5R,6R,7S,8R)-5,6,7,8-Tetrahydro-5-(hydroxymethyl)...)Show InChI InChI=1S/C8H12N2O4/c11-3-4-5(12)6(13)7(14)8-9-1-2-10(4)8/h1-2,4-7,11-14H,3H2/t4-,5-,6+,7+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of Bacteroides thetaiotaomicron GH92 alpha-mannosidase 3130 assessed as reduction of mannose release using 4NP-mannopyranoside substrate |
Nat Chem Biol 6: 125-32 (2010)
Article DOI: 10.1038/nchembio.278
BindingDB Entry DOI: 10.7270/Q2ZW1PQB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data