 Found 890 hits with Last Name = 'day' and Initial = 't'
Found 890 hits with Last Name = 'day' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330427
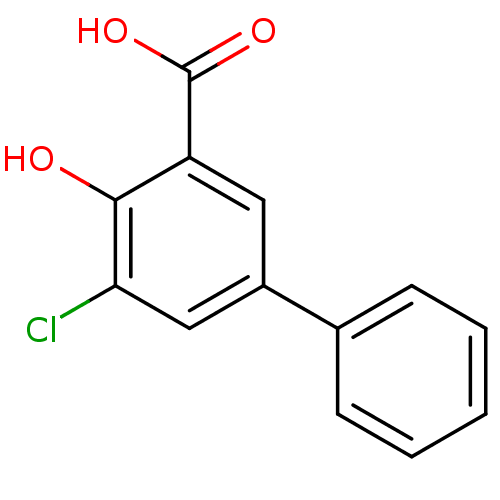
(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330426
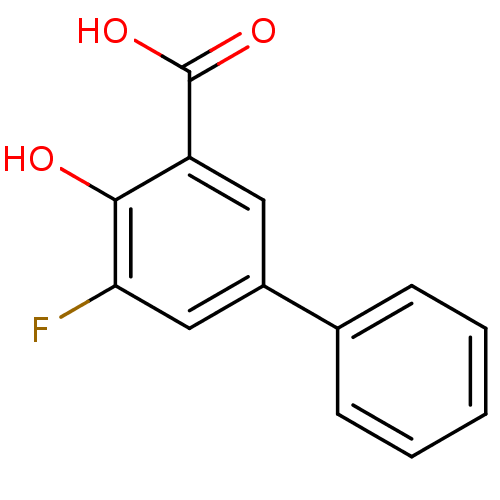
(5-Fluoro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9FO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330428
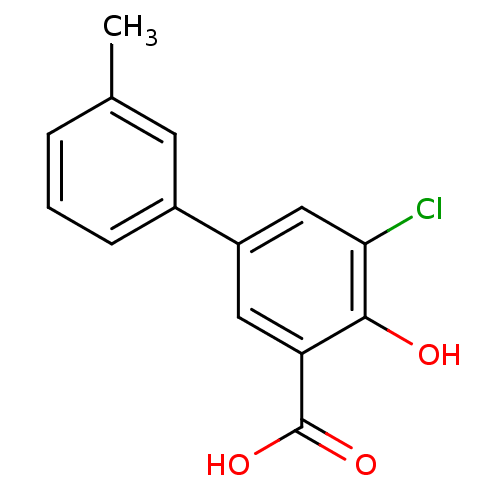
(5-Chloro-4-hydroxy-3'-methylbiphenyl-3-carboxylic ...)Show InChI InChI=1S/C14H11ClO3/c1-8-3-2-4-9(5-8)10-6-11(14(17)18)13(16)12(15)7-10/h2-7,16H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330426

(5-Fluoro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9FO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330432
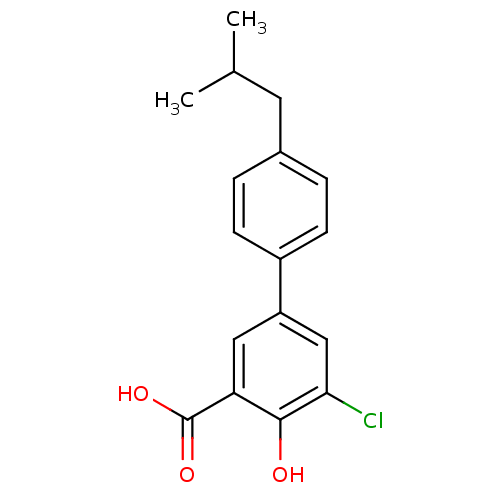
(5-Chloro-4-hydroxy-4'-isobutylbiphenyl-3-carboxyli...)Show InChI InChI=1S/C17H17ClO3/c1-10(2)7-11-3-5-12(6-4-11)13-8-14(17(20)21)16(19)15(18)9-13/h3-6,8-10,19H,7H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330431

(4'-Butyl-5-chloro-4-hydroxybiphenyl-3-carboxylic a...)Show InChI InChI=1S/C17H17ClO3/c1-2-3-4-11-5-7-12(8-6-11)13-9-14(17(20)21)16(19)15(18)10-13/h5-10,19H,2-4H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330429

(5-Chloro-4-hydroxy-4'-methylbiphenyl-3-carboxylic ...)Show InChI InChI=1S/C14H11ClO3/c1-8-2-4-9(5-3-8)10-6-11(14(17)18)13(16)12(15)7-10/h2-7,16H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50219490
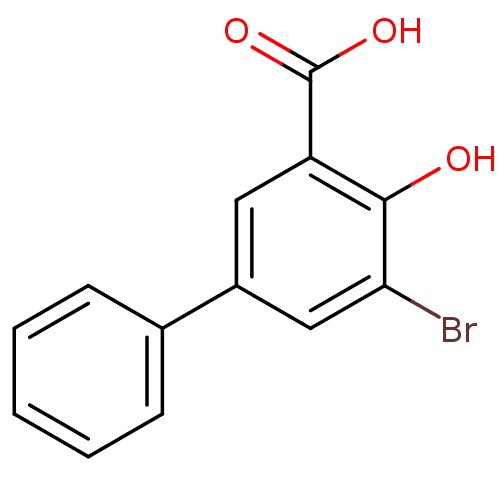
(3-Bromo-5-phenylsalicylc acid | 5-bromo-4-hydroxyb...)Show InChI InChI=1S/C13H9BrO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM26269
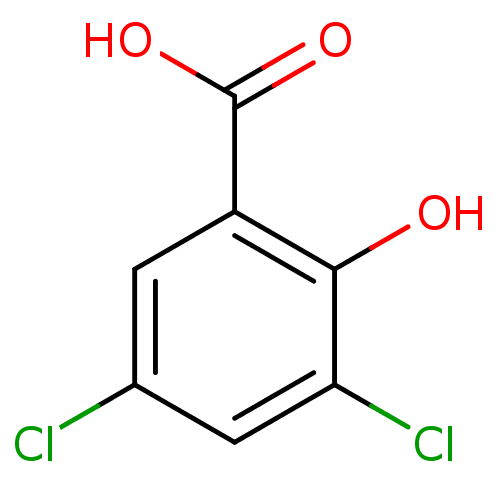
(3,5-dichloro-2-hydroxybenzoic acid | 3,5-dichloros...)Show InChI InChI=1S/C7H4Cl2O3/c8-3-1-4(7(11)12)6(10)5(9)2-3/h1-2,10H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330431

(4'-Butyl-5-chloro-4-hydroxybiphenyl-3-carboxylic a...)Show InChI InChI=1S/C17H17ClO3/c1-2-3-4-11-5-7-12(8-6-11)13-9-14(17(20)21)16(19)15(18)10-13/h5-10,19H,2-4H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330428

(5-Chloro-4-hydroxy-3'-methylbiphenyl-3-carboxylic ...)Show InChI InChI=1S/C14H11ClO3/c1-8-3-2-4-9(5-8)10-6-11(14(17)18)13(16)12(15)7-10/h2-7,16H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330427

(5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...)Show InChI InChI=1S/C13H9ClO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330424
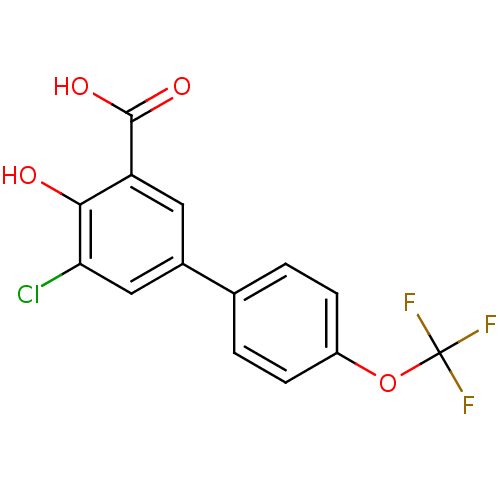
(5-Chloro-4-hydroxy-4'-(trifluoromethoxy)biphenyl-3...)Show InChI InChI=1S/C14H8ClF3O4/c15-11-6-8(5-10(12(11)19)13(20)21)7-1-3-9(4-2-7)22-14(16,17)18/h1-6,19H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330432

(5-Chloro-4-hydroxy-4'-isobutylbiphenyl-3-carboxyli...)Show InChI InChI=1S/C17H17ClO3/c1-10(2)7-11-3-5-12(6-4-11)13-8-14(17(20)21)16(19)15(18)9-13/h3-6,8-10,19H,7H2,1-2H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330424

(5-Chloro-4-hydroxy-4'-(trifluoromethoxy)biphenyl-3...)Show InChI InChI=1S/C14H8ClF3O4/c15-11-6-8(5-10(12(11)19)13(20)21)7-1-3-9(4-2-7)22-14(16,17)18/h1-6,19H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330429

(5-Chloro-4-hydroxy-4'-methylbiphenyl-3-carboxylic ...)Show InChI InChI=1S/C14H11ClO3/c1-8-2-4-9(5-3-8)10-6-11(14(17)18)13(16)12(15)7-10/h2-7,16H,1H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330425
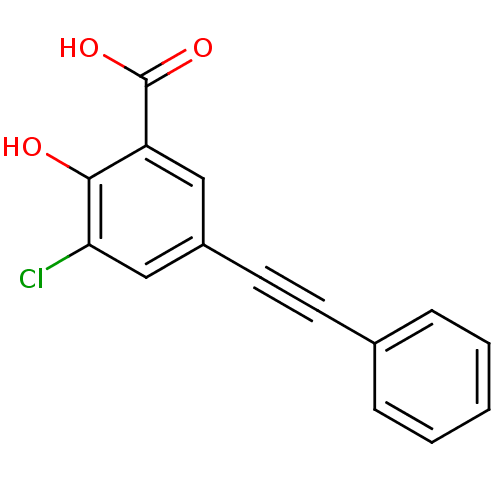
(3-chloro-2-hydroxy-5-(phenylethynyl)benzoic acid |...)Show InChI InChI=1S/C15H9ClO3/c16-13-9-11(8-12(14(13)17)15(18)19)7-6-10-4-2-1-3-5-10/h1-5,8-9,17H,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM26269

(3,5-dichloro-2-hydroxybenzoic acid | 3,5-dichloros...)Show InChI InChI=1S/C7H4Cl2O3/c8-3-1-4(7(11)12)6(10)5(9)2-3/h1-2,10H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50219490

(3-Bromo-5-phenylsalicylc acid | 5-bromo-4-hydroxyb...)Show InChI InChI=1S/C13H9BrO3/c14-11-7-9(8-4-2-1-3-5-8)6-10(12(11)15)13(16)17/h1-7,15H,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330423
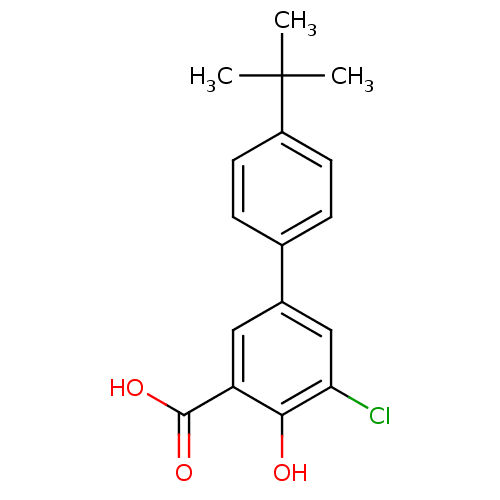
(4'-tert-Butyl-5-chloro-4-hydroxybiphenyl-3-carboxy...)Show InChI InChI=1S/C17H17ClO3/c1-17(2,3)12-6-4-10(5-7-12)11-8-13(16(20)21)15(19)14(18)9-11/h4-9,19H,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330425

(3-chloro-2-hydroxy-5-(phenylethynyl)benzoic acid |...)Show InChI InChI=1S/C15H9ClO3/c16-13-9-11(8-12(14(13)17)15(18)19)7-6-10-4-2-1-3-5-10/h1-5,8-9,17H,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 168 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50330430
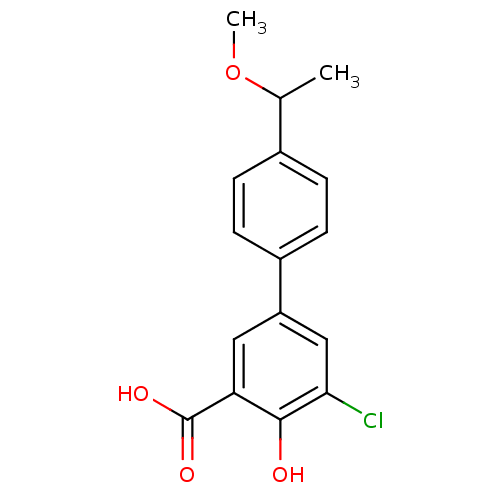
(5-Chloro-4-hydroxy-4'-(1-methoxyethyl)biphenyl-3-c...)Show InChI InChI=1S/C16H15ClO4/c1-9(21-2)10-3-5-11(6-4-10)12-7-13(16(19)20)15(18)14(17)8-12/h3-9,18H,1-2H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human wild type AKR1C1 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330423

(4'-tert-Butyl-5-chloro-4-hydroxybiphenyl-3-carboxy...)Show InChI InChI=1S/C17H17ClO3/c1-17(2,3)12-6-4-10(5-7-12)11-8-13(16(20)21)15(19)14(18)9-11/h4-9,19H,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50330430

(5-Chloro-4-hydroxy-4'-(1-methoxyethyl)biphenyl-3-c...)Show InChI InChI=1S/C16H15ClO4/c1-9(21-2)10-3-5-11(6-4-10)12-7-13(16(19)20)15(18)14(17)8-12/h3-9,18H,1-2H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus)
Curated by ChEMBL
| Assay Description
Inhibition of human AKR1C2 dehydrogenase activity by fluorometric assay |
Eur J Med Chem 45: 5309-17 (2010)
Article DOI: 10.1016/j.ejmech.2010.08.052
BindingDB Entry DOI: 10.7270/Q2R78FF3 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-6
(Homo sapiens (Human)) | BDBM286091
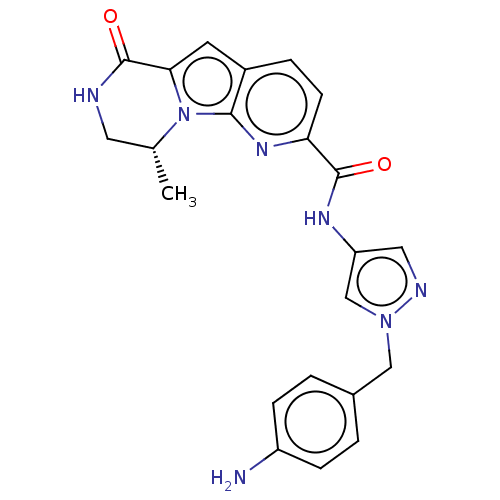
((R)�N-(1-(4-aminobenzyl)-1H-pyrazol-4-yl)-9-methyl...)Show SMILES C[C@@H]1CNC(=O)c2cc3ccc(nc3n12)C(=O)Nc1cnn(Cc2ccc(N)cc2)c1 |r| Show InChI InChI=1S/C27H29N7O4/c1-16-12-28-25(36)22-11-18-7-10-21(32-23(18)34(16)22)24(35)30-20-13-29-33(15-20)14-17-5-8-19(9-6-17)31-26(37)38-27(2,3)4/h5-11,13,15-16H,12,14H2,1-4H3,(H,28,36)(H,30,35)(H,31,37)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGN
US Patent
| Assay Description
Radioisotope assays (SignalChem) were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radio... |
US Patent US9771366 (2017)
BindingDB Entry DOI: 10.7270/Q2862JK5 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-6
(Homo sapiens (Human)) | BDBM286091

((R)�N-(1-(4-aminobenzyl)-1H-pyrazol-4-yl)-9-methyl...)Show SMILES C[C@@H]1CNC(=O)c2cc3ccc(nc3n12)C(=O)Nc1cnn(Cc2ccc(N)cc2)c1 |r| Show InChI InChI=1S/C27H29N7O4/c1-16-12-28-25(36)22-11-18-7-10-21(32-23(18)34(16)22)24(35)30-20-13-29-33(15-20)14-17-5-8-19(9-6-17)31-26(37)38-27(2,3)4/h5-11,13,15-16H,12,14H2,1-4H3,(H,28,36)(H,30,35)(H,31,37)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGNS
US Patent
| Assay Description
Radioisotope assays were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radioactive workin... |
US Patent US10758530 (2020)
BindingDB Entry DOI: 10.7270/Q20R9SGD |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM286091

((R)�N-(1-(4-aminobenzyl)-1H-pyrazol-4-yl)-9-methyl...)Show SMILES C[C@@H]1CNC(=O)c2cc3ccc(nc3n12)C(=O)Nc1cnn(Cc2ccc(N)cc2)c1 |r| Show InChI InChI=1S/C27H29N7O4/c1-16-12-28-25(36)22-11-18-7-10-21(32-23(18)34(16)22)24(35)30-20-13-29-33(15-20)14-17-5-8-19(9-6-17)31-26(37)38-27(2,3)4/h5-11,13,15-16H,12,14H2,1-4H3,(H,28,36)(H,30,35)(H,31,37)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGN
US Patent
| Assay Description
Radioisotope assays (SignalChem) were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radio... |
US Patent US9771366 (2017)
BindingDB Entry DOI: 10.7270/Q2862JK5 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM286091

((R)�N-(1-(4-aminobenzyl)-1H-pyrazol-4-yl)-9-methyl...)Show SMILES C[C@@H]1CNC(=O)c2cc3ccc(nc3n12)C(=O)Nc1cnn(Cc2ccc(N)cc2)c1 |r| Show InChI InChI=1S/C27H29N7O4/c1-16-12-28-25(36)22-11-18-7-10-21(32-23(18)34(16)22)24(35)30-20-13-29-33(15-20)14-17-5-8-19(9-6-17)31-26(37)38-27(2,3)4/h5-11,13,15-16H,12,14H2,1-4H3,(H,28,36)(H,30,35)(H,31,37)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGNS
US Patent
| Assay Description
Radioisotope assays were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radioactive workin... |
US Patent US10758530 (2020)
BindingDB Entry DOI: 10.7270/Q20R9SGD |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM286091

((R)�N-(1-(4-aminobenzyl)-1H-pyrazol-4-yl)-9-methyl...)Show SMILES C[C@@H]1CNC(=O)c2cc3ccc(nc3n12)C(=O)Nc1cnn(Cc2ccc(N)cc2)c1 |r| Show InChI InChI=1S/C27H29N7O4/c1-16-12-28-25(36)22-11-18-7-10-21(32-23(18)34(16)22)24(35)30-20-13-29-33(15-20)14-17-5-8-19(9-6-17)31-26(37)38-27(2,3)4/h5-11,13,15-16H,12,14H2,1-4H3,(H,28,36)(H,30,35)(H,31,37)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGN
US Patent
| Assay Description
Radioisotope assays (SignalChem) were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radio... |
US Patent US9771366 (2017)
BindingDB Entry DOI: 10.7270/Q2862JK5 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM286091

((R)�N-(1-(4-aminobenzyl)-1H-pyrazol-4-yl)-9-methyl...)Show SMILES C[C@@H]1CNC(=O)c2cc3ccc(nc3n12)C(=O)Nc1cnn(Cc2ccc(N)cc2)c1 |r| Show InChI InChI=1S/C27H29N7O4/c1-16-12-28-25(36)22-11-18-7-10-21(32-23(18)34(16)22)24(35)30-20-13-29-33(15-20)14-17-5-8-19(9-6-17)31-26(37)38-27(2,3)4/h5-11,13,15-16H,12,14H2,1-4H3,(H,28,36)(H,30,35)(H,31,37)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGNS
US Patent
| Assay Description
Radioisotope assays were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radioactive workin... |
US Patent US10758530 (2020)
BindingDB Entry DOI: 10.7270/Q20R9SGD |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM581306

((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...)Show SMILES Nc1nc(N)c(NC(=O)Nc2cnc(C(=O)N[C@@H](CCc3nnn[nH]3)C(O)=O)c(F)c2)c(=O)[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2P84GRR |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM581300

((2S)-2-[(6-cyclopropoxy-5-{[(2,4- diamino-6-oxo-1,...)Show SMILES Nc1nc(N)c(NC(=O)Nc2ccc(nc2OC2CC2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(=O)[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2P84GRR |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM27533
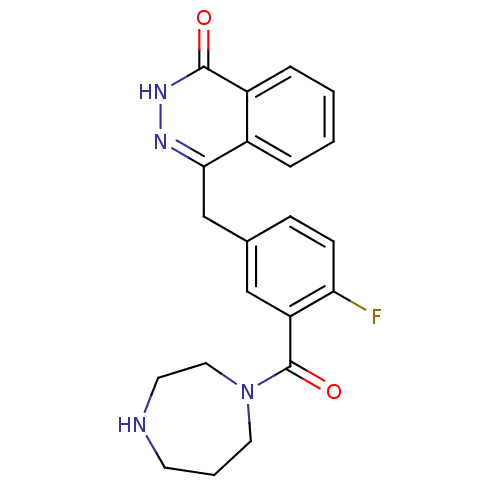
(4-(3-(1,4-diazepane-1-carbonyl)-4-fluorobenzyl)pht...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCCNCC1 Show InChI InChI=1S/C21H21FN4O2/c22-18-7-6-14(12-17(18)21(28)26-10-3-8-23-9-11-26)13-19-15-4-1-2-5-16(15)20(27)25-24-19/h1-2,4-7,12,23H,3,8-11,13H2,(H,25,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 |
J Med Chem 52: 3108-11 (2009)
Article DOI: 10.1021/jm900052j
BindingDB Entry DOI: 10.7270/Q2B56JNV |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM581317

((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...)Show SMILES Nc1nc(N)c(NC(=O)Nc2cnc(C(=O)N[C@@H](Cc3ccccc3)C(O)=O)c(F)c2)c(=O)[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2P84GRR |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM458758
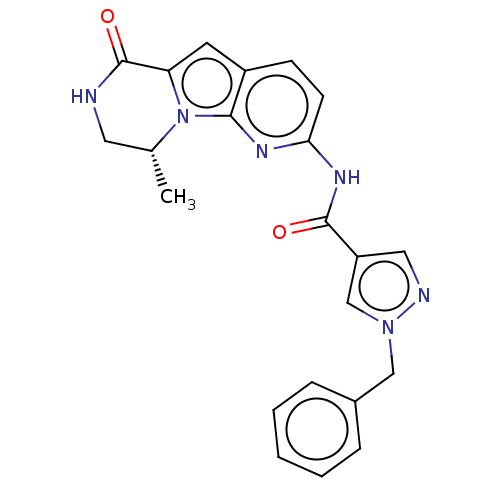
((R)-1-benzyl-N-(9-methyl-6-oxo-6,7,8,9-tetrahydrop...)Show SMILES C[C@@H]1CNC(=O)c2cc3ccc(NC(=O)c4cnn(Cc5ccccc5)c4)nc3n12 |r| Show InChI InChI=1S/C22H20N6O2/c1-14-10-23-22(30)18-9-16-7-8-19(25-20(16)28(14)18)26-21(29)17-11-24-27(13-17)12-15-5-3-2-4-6-15/h2-9,11,13-14H,10,12H2,1H3,(H,23,30)(H,25,26,29)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGNS
US Patent
| Assay Description
Radioisotope assays were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radioactive workin... |
US Patent US10758530 (2020)
BindingDB Entry DOI: 10.7270/Q20R9SGD |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM342166
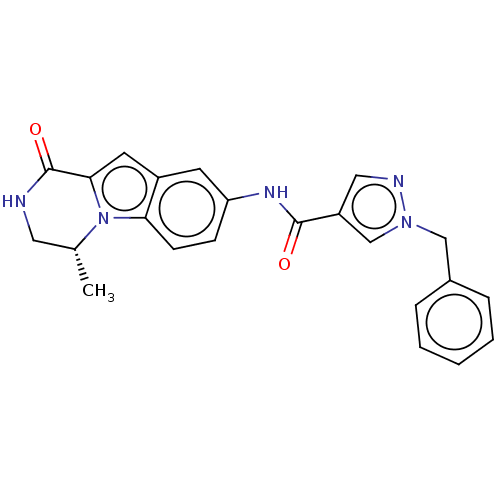
((R)-1-benzyl-N-(9-methyl-6-oxo-6,7,8,9-tetrahydrop...)Show SMILES C[C@@H]1CNC(=O)c2cc3cc(NC(=O)c4cnn(Cc5ccccc5)c4)ccc3n12 |r| Show InChI InChI=1S/C23H21N5O2/c1-15-11-24-23(30)21-10-17-9-19(7-8-20(17)28(15)21)26-22(29)18-12-25-27(14-18)13-16-5-3-2-4-6-16/h2-10,12,14-15H,11,13H2,1H3,(H,24,30)(H,26,29)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGN
US Patent
| Assay Description
Radioisotope assays (SignalChem) were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radio... |
US Patent US9771366 (2017)
BindingDB Entry DOI: 10.7270/Q2862JK5 |
More data for this
Ligand-Target Pair | |
Integrin-linked protein kinase
(Homo sapiens (Human)) | BDBM123945
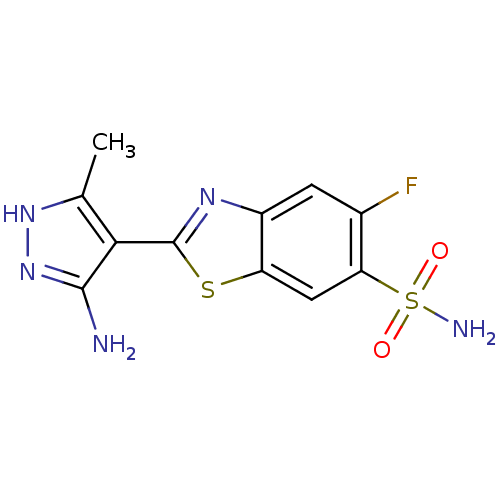
(US8754233, 2-(5-Amino-3-methyl-1H-pyrazol-4-yl)-5-...)Show SMILES Cc1[nH]nc(N)c1-c1nc2cc(F)c(cc2s1)S(N)(=O)=O Show InChI InChI=1S/C11H10FN5O2S2/c1-4-9(10(13)17-16-4)11-15-6-2-5(12)8(21(14,18)19)3-7(6)20-11/h2-3H,1H3,(H3,13,16,17)(H2,14,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | 40 |
Dermira (Canada), Inc.
US Patent
| Assay Description
5 uL of each compound dilution were robotically pipetted to Costar serocluster plates maintaining the same plate layout. All assay mixtures consisted... |
US Patent US8754233 (2014)
BindingDB Entry DOI: 10.7270/Q23N2231 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-6
(Homo sapiens (Human)) | BDBM458758

((R)-1-benzyl-N-(9-methyl-6-oxo-6,7,8,9-tetrahydrop...)Show SMILES C[C@@H]1CNC(=O)c2cc3ccc(NC(=O)c4cnn(Cc5ccccc5)c4)nc3n12 |r| Show InChI InChI=1S/C22H20N6O2/c1-14-10-23-22(30)18-9-16-7-8-19(25-20(16)28(14)18)26-21(29)17-11-24-27(13-17)12-15-5-3-2-4-6-15/h2-9,11,13-14H,10,12H2,1H3,(H,23,30)(H,25,26,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGNS
US Patent
| Assay Description
Radioisotope assays were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radioactive workin... |
US Patent US10758530 (2020)
BindingDB Entry DOI: 10.7270/Q20R9SGD |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM581313
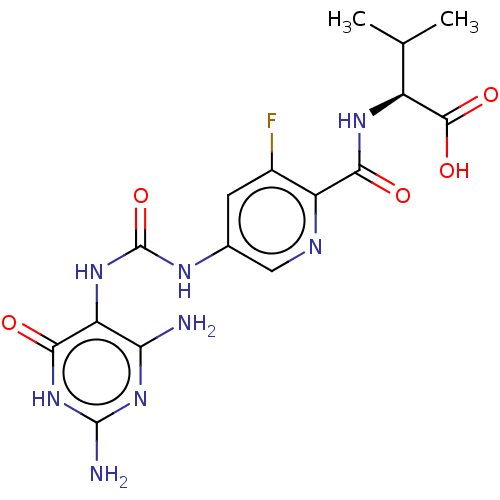
((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...)Show SMILES CC(C)[C@H](NC(=O)c1ncc(NC(=O)Nc2c(N)nc(N)[nH]c2=O)cc1F)C(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2P84GRR |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605601
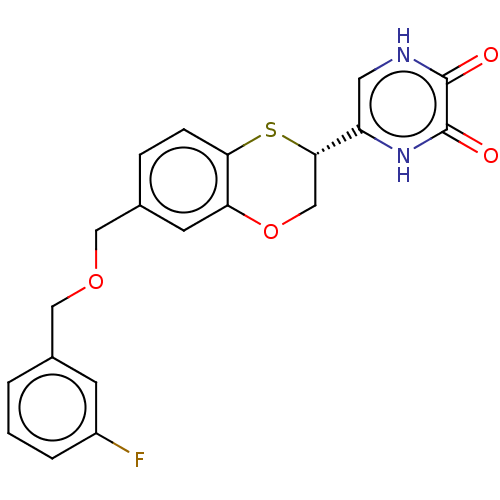
(CHEMBL5184138)Show SMILES Fc1cccc(COCc2ccc3S[C@@H](COc3c2)c2c[nH]c(=O)c(=O)[nH]2)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-6
(Homo sapiens (Human)) | BDBM342166

((R)-1-benzyl-N-(9-methyl-6-oxo-6,7,8,9-tetrahydrop...)Show SMILES C[C@@H]1CNC(=O)c2cc3cc(NC(=O)c4cnn(Cc5ccccc5)c4)ccc3n12 |r| Show InChI InChI=1S/C23H21N5O2/c1-15-11-24-23(30)21-10-17-9-19(7-8-20(17)28(15)21)26-22(29)18-12-25-27(14-18)13-16-5-3-2-4-6-16/h2-10,12,14-15H,11,13H2,1H3,(H,24,30)(H,26,29)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGN
US Patent
| Assay Description
Radioisotope assays (SignalChem) were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radio... |
US Patent US9771366 (2017)
BindingDB Entry DOI: 10.7270/Q2862JK5 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605597
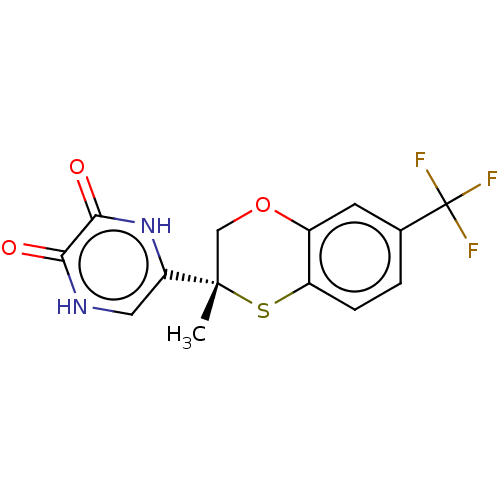
(CHEMBL5204161)Show SMILES C[C@]1(COc2cc(ccc2S1)C(F)(F)F)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605598

(CHEMBL5188193)Show SMILES C[C@]1(COc2cc(Cl)ccc2S1)c1c[nH]c(=O)c(=O)[nH]1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM581288
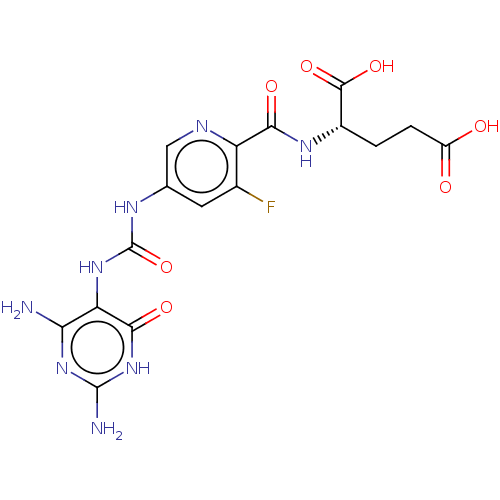
((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...)Show SMILES Nc1nc(N)c(NC(=O)Nc2cnc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(F)c2)c(=O)[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2P84GRR |
More data for this
Ligand-Target Pair | |
Integrin-linked protein kinase
(Homo sapiens (Human)) | BDBM123951
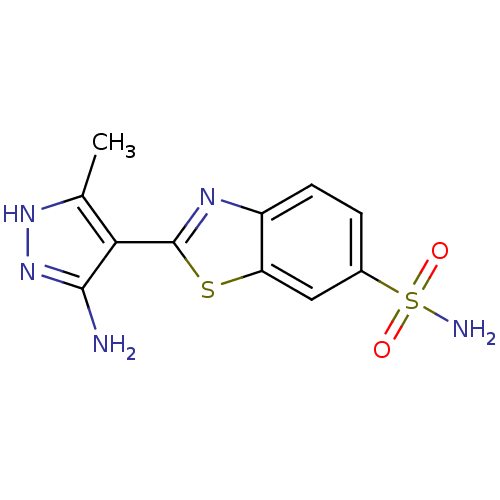
(US8754233, 2-(5-Amino-3-methyl-1H-pyrazol-4-yl)-be...)Show InChI InChI=1S/C11H11N5O2S2/c1-5-9(10(12)16-15-5)11-14-7-3-2-6(20(13,17)18)4-8(7)19-11/h2-4H,1H3,(H3,12,15,16)(H2,13,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 40 |
Dermira (Canada), Inc.
US Patent
| Assay Description
5 uL of each compound dilution were robotically pipetted to Costar serocluster plates maintaining the same plate layout. All assay mixtures consisted... |
US Patent US8754233 (2014)
BindingDB Entry DOI: 10.7270/Q23N2231 |
More data for this
Ligand-Target Pair | |
Integrin-linked protein kinase
(Homo sapiens (Human)) | BDBM124052
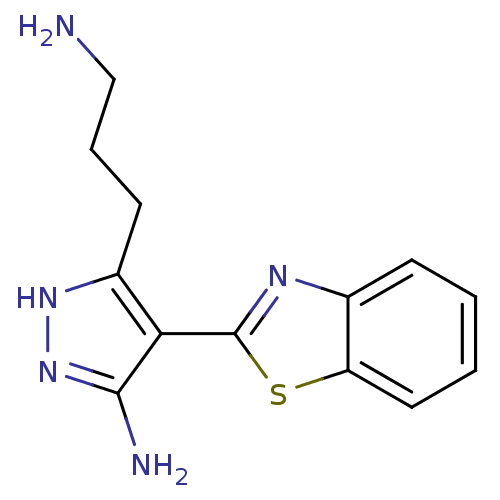
(US8754233, 5-(3-Amino-propyl)-4-benzothiazol-2-yl-...)Show InChI InChI=1S/C13H15N5S/c14-7-3-5-9-11(12(15)18-17-9)13-16-8-4-1-2-6-10(8)19-13/h1-2,4,6H,3,5,7,14H2,(H3,15,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 40 |
Dermira (Canada), Inc.
US Patent
| Assay Description
5 uL of each compound dilution were robotically pipetted to Costar serocluster plates maintaining the same plate layout. All assay mixtures consisted... |
US Patent US8754233 (2014)
BindingDB Entry DOI: 10.7270/Q23N2231 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50605555
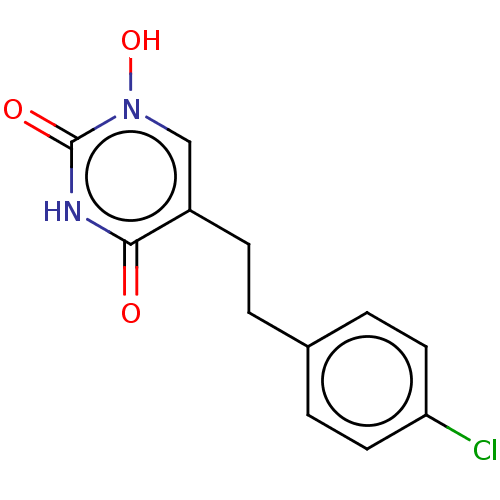
(CHEMBL5173250) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00118
BindingDB Entry DOI: 10.7270/Q2MG7TK7 |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM581298

((2S)-4- [(benzenesulfonyl)carbamoyl]-2- [(5-{[(2,4...)Show SMILES Nc1nc(N)c(NC(=O)Nc2ccc(nc2)C(=O)N[C@@H](CCC(=O)NS(=O)(=O)c2ccccc2)C(O)=O)c(=O)[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2P84GRR |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-2
(Homo sapiens (Human)) | BDBM286091

((R)�N-(1-(4-aminobenzyl)-1H-pyrazol-4-yl)-9-methyl...)Show SMILES C[C@@H]1CNC(=O)c2cc3ccc(nc3n12)C(=O)Nc1cnn(Cc2ccc(N)cc2)c1 |r| Show InChI InChI=1S/C27H29N7O4/c1-16-12-28-25(36)22-11-18-7-10-21(32-23(18)34(16)22)24(35)30-20-13-29-33(15-20)14-17-5-8-19(9-6-17)31-26(37)38-27(2,3)4/h5-11,13,15-16H,12,14H2,1-4H3,(H,28,36)(H,30,35)(H,31,37)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
PHOENIX MOLECULAR DESIGNS
US Patent
| Assay Description
Radioisotope assays were performed for the evaluation of the kinase target profiling and all assays were performed in a designated radioactive workin... |
US Patent US10758530 (2020)
BindingDB Entry DOI: 10.7270/Q20R9SGD |
More data for this
Ligand-Target Pair | |
Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial
(Homo sapiens (Human)) | BDBM581286

((2S)-2-[(5-{[(2,4-diamino-6-oxo- 1,6-dihydropyrimi...)Show SMILES Nc1nc(N)c(NC(=O)Nc2ccc(nc2)C(=O)N[C@@H](CCc2nnn[nH]2)C(O)=O)c(=O)[nH]1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
To determine the IC50 value of a compound, an 11-concentration dose-response curve with 3-fold difference in concentration between assay points was g... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2P84GRR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data