 Found 99 hits with Last Name = 'dinkelborg' and Initial = 'l'
Found 99 hits with Last Name = 'dinkelborg' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899
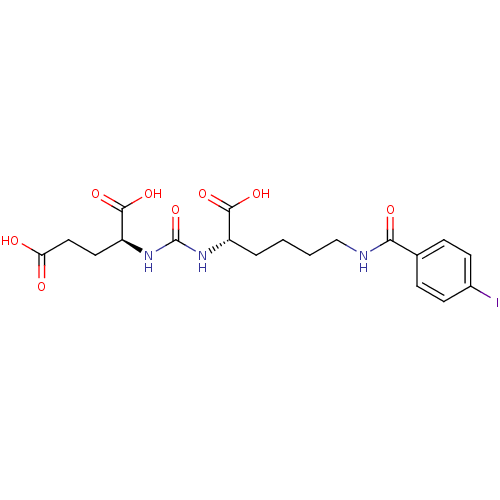
((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17759
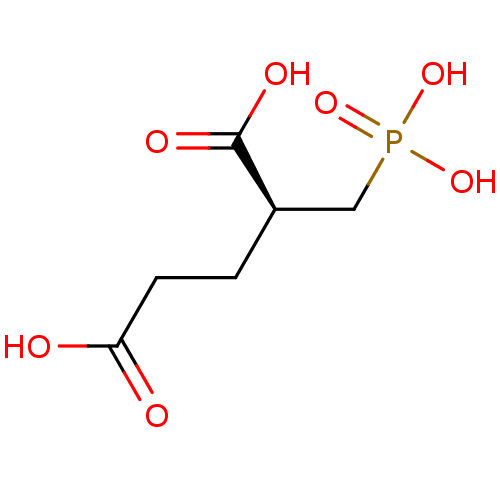
((2S)-2-(phosphonomethyl)pentanedioic acid | (S)-2-...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13)/t4-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17659
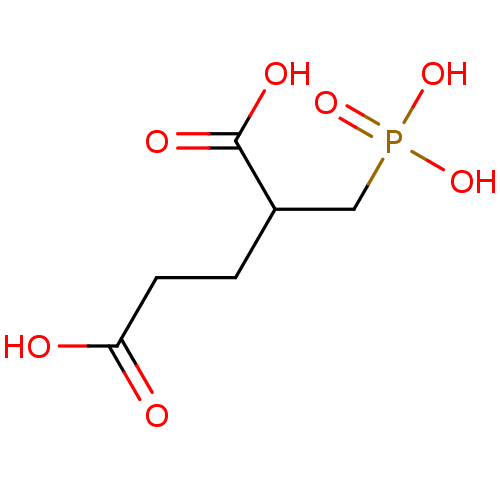
((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17659

((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Inhibition of NAALADase activity in human LNCaP cell membranes assessed as inhibition of [3H]NAG conversion to [3H]glutamate after 30 mins by liquid ... |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50398811
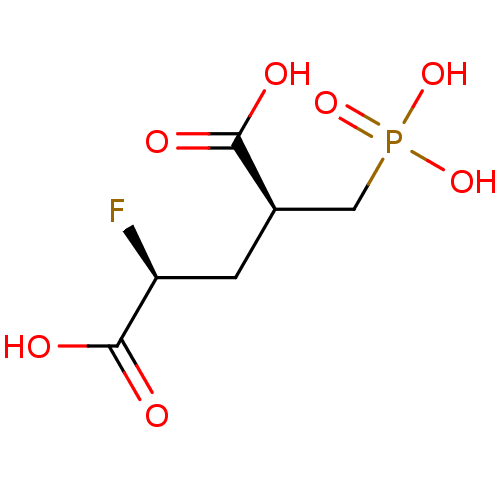
(CHEMBL2177507)Show SMILES OC(=O)[C@@H](F)C[C@H](CP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C6H10FO7P/c7-4(6(10)11)1-3(5(8)9)2-15(12,13)14/h3-4H,1-2H2,(H,8,9)(H,10,11)(H2,12,13,14)/t3-,4+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Inhibition of NAALADase activity in human LNCaP cell membranes assessed as inhibition of [3H]NAG conversion to [3H]glutamate after 30 mins by liquid ... |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50398808
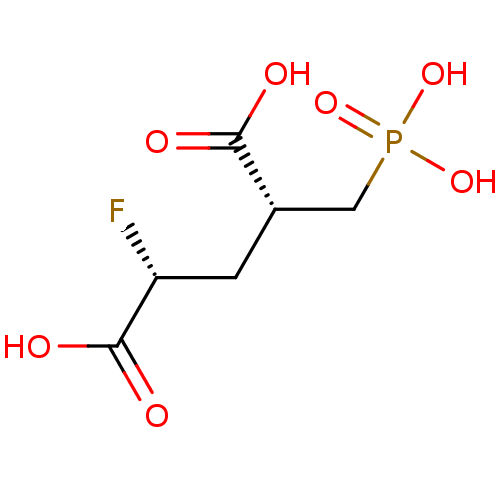
(CHEMBL2177506)Show SMILES OC(=O)[C@H](F)C[C@@H](CP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C6H10FO7P/c7-4(6(10)11)1-3(5(8)9)2-15(12,13)14/h3-4H,1-2H2,(H,8,9)(H,10,11)(H2,12,13,14)/t3-,4+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Inhibition of NAALADase activity in human LNCaP cell membranes assessed as inhibition of [3H]NAG conversion to [3H]glutamate after 30 mins by liquid ... |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50398810
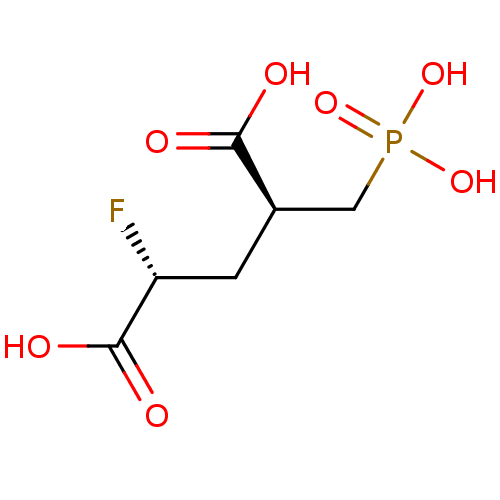
(CHEMBL2177509)Show InChI InChI=1S/C6H10FO7P/c7-4(6(10)11)1-3(5(8)9)2-15(12,13)14/h3-4H,1-2H2,(H,8,9)(H,10,11)(H2,12,13,14)/t3-,4-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Inhibition of NAALADase activity in human LNCaP cell membranes assessed as inhibition of [3H]NAG conversion to [3H]glutamate after 30 mins by liquid ... |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50304731
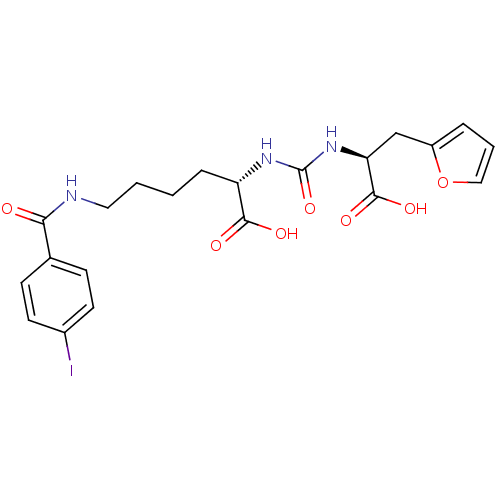
((S)-2-(3-((S)-1-Carboxy-2-(furan-2-yl)ethyl)ureido...)Show SMILES OC(=O)[C@H](CCCCNC(=O)c1ccc(I)cc1)NC(=O)N[C@@H](Cc1ccco1)C(O)=O |r| Show InChI InChI=1S/C21H24IN3O7/c22-14-8-6-13(7-9-14)18(26)23-10-2-1-5-16(19(27)28)24-21(31)25-17(20(29)30)12-15-4-3-11-32-15/h3-4,6-9,11,16-17H,1-2,5,10,12H2,(H,23,26)(H,27,28)(H,29,30)(H2,24,25,31)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50304722
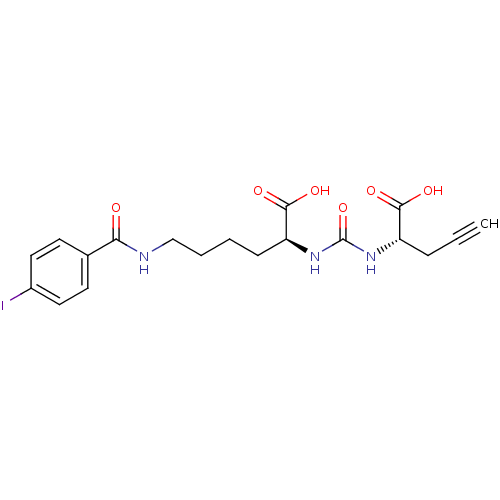
((S)-2-(3-((S)-1-Carboxybut-3-ynyl)ureido)-6-(4-iod...)Show SMILES OC(=O)[C@H](CCCCNC(=O)c1ccc(I)cc1)NC(=O)N[C@@H](CC#C)C(O)=O |r| Show InChI InChI=1S/C19H22IN3O6/c1-2-5-14(17(25)26)22-19(29)23-15(18(27)28)6-3-4-11-21-16(24)12-7-9-13(20)10-8-12/h1,7-10,14-15H,3-6,11H2,(H,21,24)(H,25,26)(H,27,28)(H2,22,23,29)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50102258
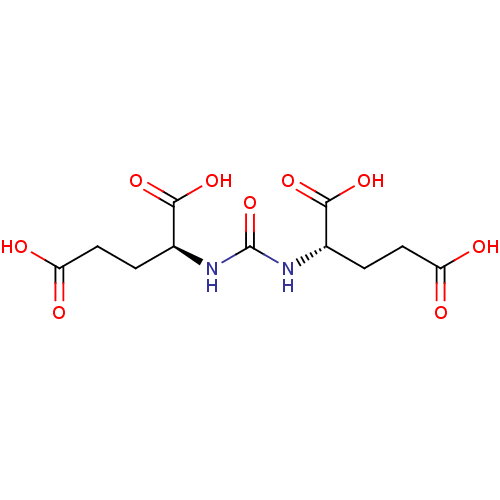
((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O Show InChI InChI=1S/C11H16N2O9/c14-7(15)3-1-5(9(18)19)12-11(22)13-6(10(20)21)2-4-8(16)17/h5-6H,1-4H2,(H,14,15)(H,16,17)(H,18,19)(H,20,21)(H2,12,13,22)/t5-,6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50304751
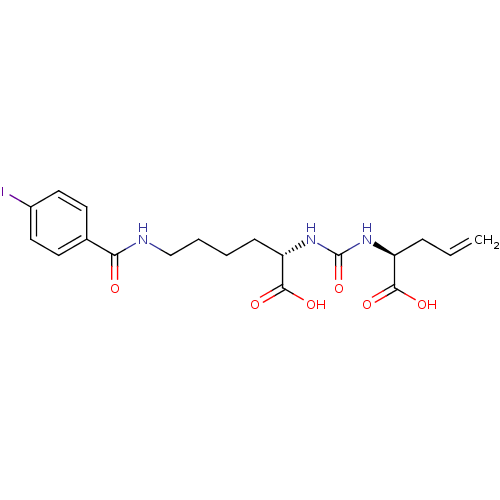
((S)-2-(3-((S)-1-Carboxybut-3-enyl)ureido)-6-(4-iod...)Show SMILES OC(=O)[C@H](CCCCNC(=O)c1ccc(I)cc1)NC(=O)N[C@@H](CC=C)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O6/c1-2-5-14(17(25)26)22-19(29)23-15(18(27)28)6-3-4-11-21-16(24)12-7-9-13(20)10-8-12/h2,7-10,14-15H,1,3-6,11H2,(H,21,24)(H,25,26)(H,27,28)(H2,22,23,29)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50304735
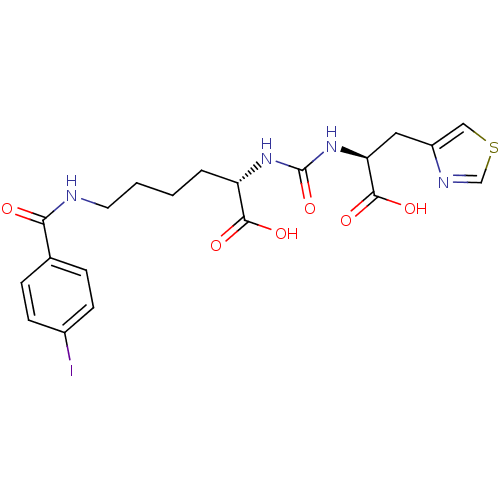
((S)-2-(3-((S)-1-Carboxy-2-(thiazol-4-yl)ethyl)urei...)Show SMILES OC(=O)[C@H](CCCCNC(=O)c1ccc(I)cc1)NC(=O)N[C@@H](Cc1cscn1)C(O)=O |r| Show InChI InChI=1S/C20H23IN4O6S/c21-13-6-4-12(5-7-13)17(26)22-8-2-1-3-15(18(27)28)24-20(31)25-16(19(29)30)9-14-10-32-11-23-14/h4-7,10-11,15-16H,1-3,8-9H2,(H,22,26)(H,27,28)(H,29,30)(H2,24,25,31)/t15-,16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50304732
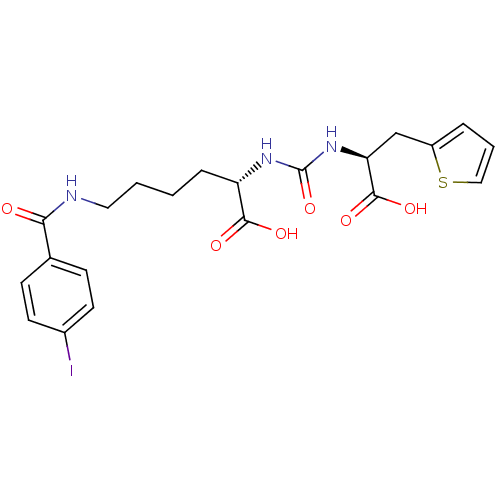
((S)-2-(3-((S)-1-Carboxy-2-(thiophen-2-yl)ethyl)ure...)Show SMILES OC(=O)[C@H](CCCCNC(=O)c1ccc(I)cc1)NC(=O)N[C@@H](Cc1cccs1)C(O)=O |r| Show InChI InChI=1S/C21H24IN3O6S/c22-14-8-6-13(7-9-14)18(26)23-10-2-1-5-16(19(27)28)24-21(31)25-17(20(29)30)12-15-4-3-11-32-15/h3-4,6-9,11,16-17H,1-2,5,10,12H2,(H,23,26)(H,27,28)(H,29,30)(H2,24,25,31)/t16-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50398809
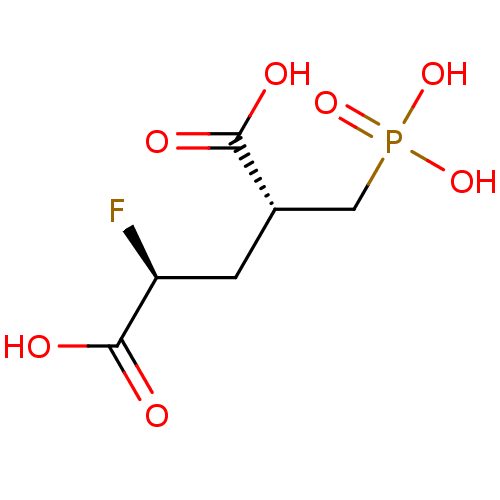
(CHEMBL2177510)Show SMILES OC(=O)[C@@H](F)C[C@@H](CP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C6H10FO7P/c7-4(6(10)11)1-3(5(8)9)2-15(12,13)14/h3-4H,1-2H2,(H,8,9)(H,10,11)(H2,12,13,14)/t3-,4-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Inhibition of NAALADase activity in human LNCaP cell membranes assessed as inhibition of [3H]NAG conversion to [3H]glutamate after 30 mins by liquid ... |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50304727
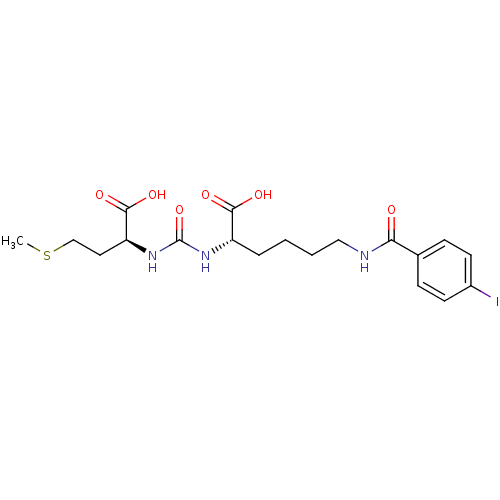
((S)-2-(3-((S)-1-Carboxy-3-(methylthio)propyl)ureid...)Show SMILES CSCC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H26IN3O6S/c1-30-11-9-15(18(27)28)23-19(29)22-14(17(25)26)4-2-3-10-21-16(24)12-5-7-13(20)8-6-12/h5-8,14-15H,2-4,9-11H2,1H3,(H,21,24)(H,25,26)(H,27,28)(H2,22,23,29)/t14-,15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17758
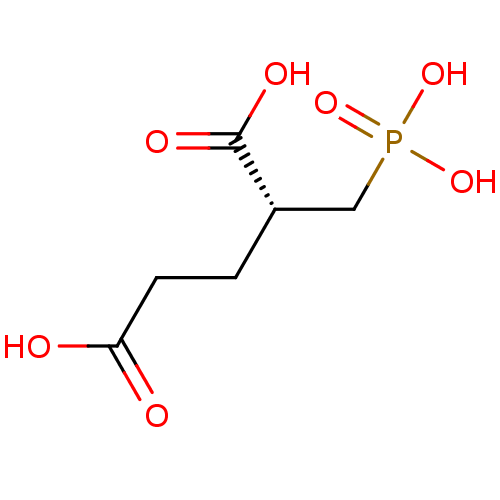
((2R)-2-(phosphonomethyl)pentanedioic acid | (R)-2-...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13)/t4-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50398808

(CHEMBL2177506)Show SMILES OC(=O)[C@H](F)C[C@@H](CP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C6H10FO7P/c7-4(6(10)11)1-3(5(8)9)2-15(12,13)14/h3-4H,1-2H2,(H,8,9)(H,10,11)(H2,12,13,14)/t3-,4+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Inhibition of NAALADase activity in human LNCaP cell membranes assessed as inhibition of [3H]NAG conversion to [3H]glutamate after 30 mins by liquid ... |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50398812
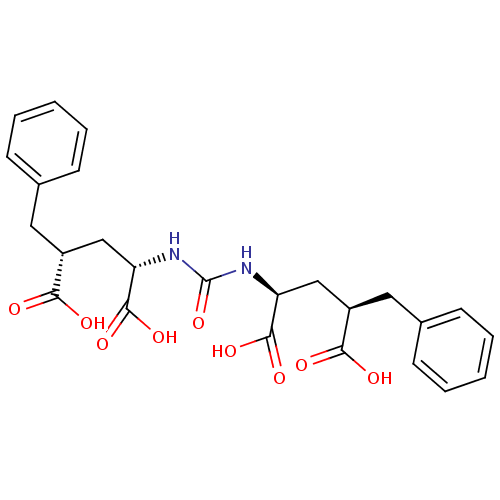
(CHEMBL2177511)Show SMILES OC(=O)[C@@H](C[C@H](NC(=O)N[C@@H](C[C@@H](Cc1ccccc1)C(O)=O)C(O)=O)C(O)=O)Cc1ccccc1 |r| Show InChI InChI=1S/C25H28N2O9/c28-21(29)17(11-15-7-3-1-4-8-15)13-19(23(32)33)26-25(36)27-20(24(34)35)14-18(22(30)31)12-16-9-5-2-6-10-16/h1-10,17-20H,11-14H2,(H,28,29)(H,30,31)(H,32,33)(H,34,35)(H2,26,27,36)/t17-,18-,19+,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 392 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50048693
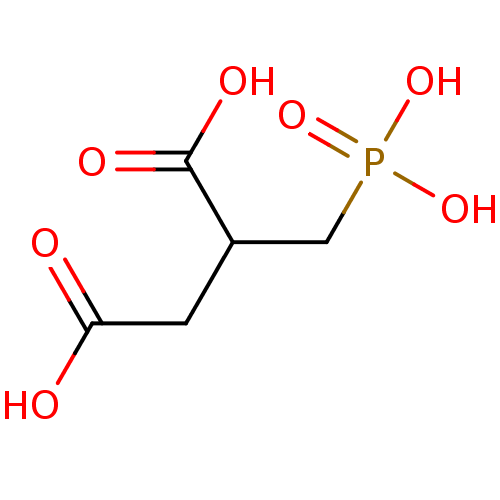
(2-Phosphonomethyl-succinic acid | 2-Phosphonomethy...)Show InChI InChI=1S/C5H9O7P/c6-4(7)1-3(5(8)9)2-13(10,11)12/h3H,1-2H2,(H,6,7)(H,8,9)(H2,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50398813
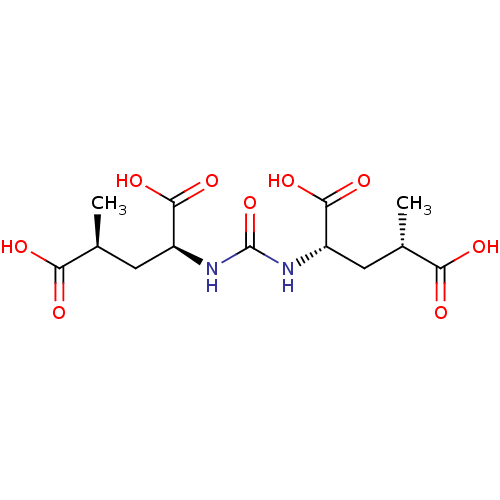
(CHEMBL2114987)Show SMILES C[C@@H](C[C@H](NC(=O)N[C@@H](C[C@H](C)C(O)=O)C(O)=O)C(O)=O)C(O)=O Show InChI InChI=1S/C13H20N2O9/c1-5(9(16)17)3-7(11(20)21)14-13(24)15-8(12(22)23)4-6(2)10(18)19/h5-8H,3-4H2,1-2H3,(H,16,17)(H,18,19)(H,20,21)(H,22,23)(H2,14,15,24)/t5-,6-,7-,8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50398814
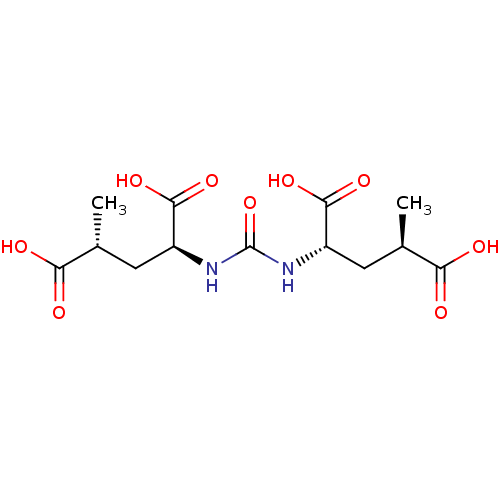
(CHEMBL2115435)Show SMILES C[C@H](C[C@H](NC(=O)N[C@@H](C[C@@H](C)C(O)=O)C(O)=O)C(O)=O)C(O)=O Show InChI InChI=1S/C13H20N2O9/c1-5(9(16)17)3-7(11(20)21)14-13(24)15-8(12(22)23)4-6(2)10(18)19/h5-8H,3-4H2,1-2H3,(H,16,17)(H,18,19)(H,20,21)(H,22,23)(H2,14,15,24)/t5-,6-,7+,8+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Binding affinity to NAALADase |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50476140
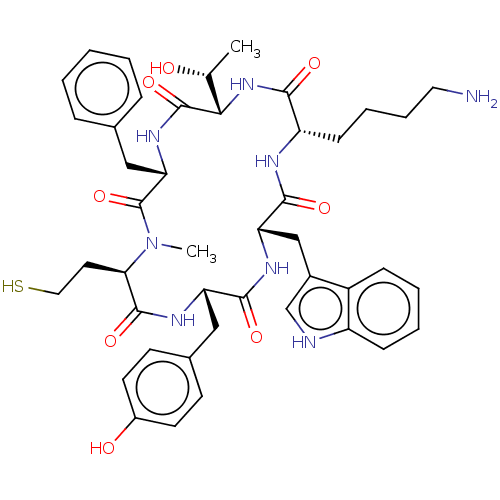
(CHEMBL373632)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCS)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O Show InChI InChI=1S/C44H56N8O8S/c1-26(53)38-43(59)50-36(23-27-10-4-3-5-11-27)44(60)52(2)37(19-21-61)42(58)49-34(22-28-15-17-30(54)18-16-28)40(56)48-35(24-29-25-46-32-13-7-6-12-31(29)32)41(57)47-33(39(55)51-38)14-8-9-20-45/h3-7,10-13,15-18,25-26,33-38,46,53-54,61H,8-9,14,19-24,45H2,1-2H3,(H,47,57)(H,48,56)(H,49,58)(H,50,59)(H,51,55)/t26-,33+,34+,35+,36+,37-,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SRIF-14 from SSTR of rat AR42J cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(Homo sapiens (Human)) | BDBM50476134
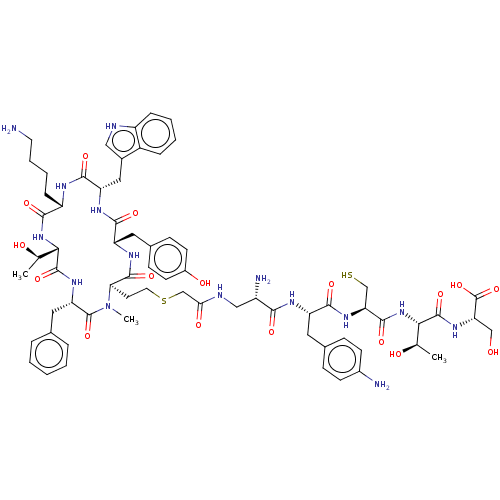
(CHEMBL437296)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCSCC(=O)NC[C@H](N)C(=O)N[C@@H](Cc2ccc(N)cc2)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O Show InChI InChI=1S/C68H91N15O17S2/c1-36(85)56-65(96)78-51(29-38-11-5-4-6-12-38)67(98)83(3)54(64(95)77-49(28-40-18-22-43(87)23-19-40)60(91)76-50(30-41-31-72-46-14-8-7-13-44(41)46)62(93)74-47(59(90)81-56)15-9-10-25-69)24-26-102-35-55(88)73-32-45(71)58(89)75-48(27-39-16-20-42(70)21-17-39)61(92)80-53(34-101)63(94)82-57(37(2)86)66(97)79-52(33-84)68(99)100/h4-8,11-14,16-23,31,36-37,45,47-54,56-57,72,84-87,101H,9-10,15,24-30,32-35,69-71H2,1-3H3,(H,73,88)(H,74,93)(H,75,89)(H,76,91)(H,77,95)(H,78,96)(H,79,97)(H,80,92)(H,81,90)(H,82,94)(H,99,100)/t36-,37-,45+,47+,48+,49+,50+,51+,52+,53+,54-,56+,57+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr-11]-SRIF from SSTR of human NCI-H69 cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(Homo sapiens (Human)) | BDBM50476138
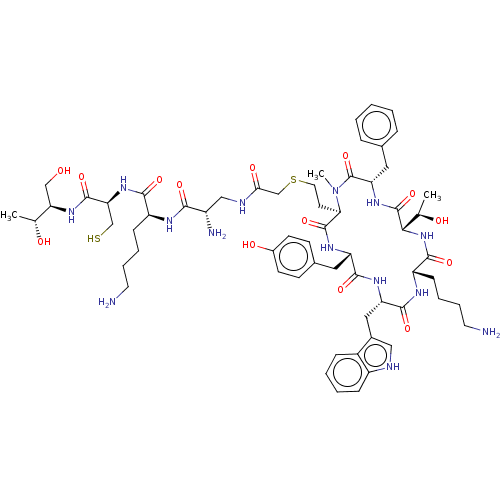
(CHEMBL376485)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCSCC(=O)NC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)N[C@H](CO)[C@@H](C)O)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O Show InChI InChI=1S/C62H90N14O14S2/c1-35(78)49(32-77)73-59(87)50(33-91)74-55(83)44(17-9-11-24-63)68-54(82)42(65)31-67-52(81)34-92-26-23-51-60(88)71-46(27-38-19-21-40(80)22-20-38)57(85)70-47(29-39-30-66-43-16-8-7-15-41(39)43)58(86)69-45(18-10-12-25-64)56(84)75-53(36(2)79)61(89)72-48(62(90)76(51)3)28-37-13-5-4-6-14-37/h4-8,13-16,19-22,30,35-36,42,44-51,53,66,77-80,91H,9-12,17-18,23-29,31-34,63-65H2,1-3H3,(H,67,81)(H,68,82)(H,69,86)(H,70,85)(H,71,88)(H,72,89)(H,73,87)(H,74,83)(H,75,84)/t35-,36-,42+,44+,45+,46+,47+,48+,49-,50+,51-,53+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr-11]-SRIF from SSTR of human NCI-H69 cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50476137

(CHEMBL222751)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2ccccc2)N(C)C(=O)[C@H](CCS)NC1=O)[C@@H](C)O Show InChI InChI=1S/C44H56N8O8S/c1-26(53)38-43(59)48-34(19-21-61)44(60)52(2)37(23-27-10-4-3-5-11-27)42(58)50-35(22-28-15-17-30(54)18-16-28)40(56)49-36(24-29-25-46-32-13-7-6-12-31(29)32)41(57)47-33(39(55)51-38)14-8-9-20-45/h3-7,10-13,15-18,25-26,33-38,46,53-54,61H,8-9,14,19-24,45H2,1-2H3,(H,47,57)(H,48,59)(H,49,56)(H,50,58)(H,51,55)/t26-,33+,34+,35+,36+,37-,38+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SRIF-14 from SSTR of rat AR42J cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
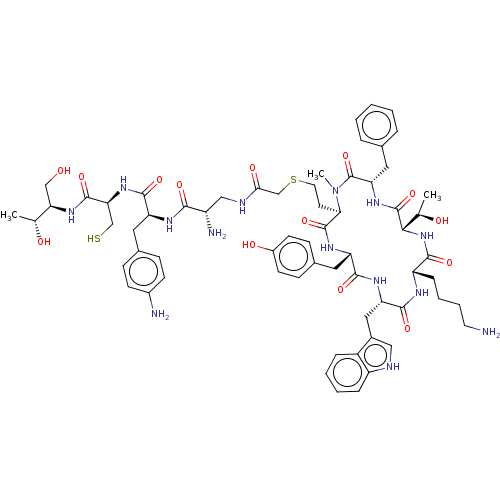
(Homo sapiens (Human)) | BDBM50476142
(CHEMBL376484)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCSCC(=O)NC[C@H](N)C(=O)N[C@@H](Cc2ccc(N)cc2)C(=O)N[C@@H](CS)C(=O)N[C@H](CO)[C@@H](C)O)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O Show InChI InChI=1S/C65H88N14O14S2/c1-36(81)52(33-80)76-62(90)53(34-94)77-60(88)48(27-39-16-20-42(67)21-17-39)72-57(85)45(68)32-70-55(84)35-95-26-24-54-63(91)74-49(28-40-18-22-43(83)23-19-40)59(87)73-50(30-41-31-69-46-14-8-7-13-44(41)46)61(89)71-47(15-9-10-25-66)58(86)78-56(37(2)82)64(92)75-51(65(93)79(54)3)29-38-11-5-4-6-12-38/h4-8,11-14,16-23,31,36-37,45,47-54,56,69,80-83,94H,9-10,15,24-30,32-35,66-68H2,1-3H3,(H,70,84)(H,71,89)(H,72,85)(H,73,87)(H,74,91)(H,75,92)(H,76,90)(H,77,88)(H,78,86)/t36-,37-,45+,47+,48+,49+,50+,51+,52-,53+,54-,56+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr-11]-SRIF from SSTR of human NCI-H69 cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
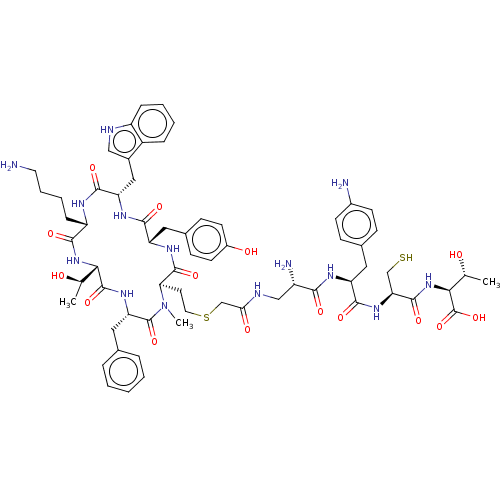
(Homo sapiens (Human)) | BDBM50476141
(CHEMBL426548)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCSCC(=O)NC[C@H](N)C(=O)N[C@@H](Cc2ccc(N)cc2)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O Show InChI InChI=1S/C65H86N14O15S2/c1-35(80)54-63(91)75-50(29-37-11-5-4-6-12-37)64(92)79(3)52(24-26-96-34-53(83)70-32-44(68)56(84)72-47(27-38-16-20-41(67)21-17-38)59(87)76-51(33-95)61(89)78-55(36(2)81)65(93)94)62(90)74-48(28-39-18-22-42(82)23-19-39)58(86)73-49(30-40-31-69-45-14-8-7-13-43(40)45)60(88)71-46(57(85)77-54)15-9-10-25-66/h4-8,11-14,16-23,31,35-36,44,46-52,54-55,69,80-82,95H,9-10,15,24-30,32-34,66-68H2,1-3H3,(H,70,83)(H,71,88)(H,72,84)(H,73,86)(H,74,90)(H,75,91)(H,76,87)(H,77,85)(H,78,89)(H,93,94)/t35-,36-,44+,46+,47+,48+,49+,50+,51+,52-,54+,55+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr-11]-SRIF from SSTR of human NCI-H69 cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50476135

(CHEMBL374316)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCS)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C45H58N8O7S/c1-27(2)39-44(59)51-37(24-28-11-5-4-6-12-28)45(60)53(3)38(20-22-61)43(58)50-35(23-29-16-18-31(54)19-17-29)41(56)49-36(25-30-26-47-33-14-8-7-13-32(30)33)42(57)48-34(40(55)52-39)15-9-10-21-46/h4-8,11-14,16-19,26-27,34-39,47,54,61H,9-10,15,20-25,46H2,1-3H3,(H,48,57)(H,49,56)(H,50,58)(H,51,59)(H,52,55)/t34-,35-,36-,37-,38+,39-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SRIF-14 from SSTR of rat AR42J cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063139
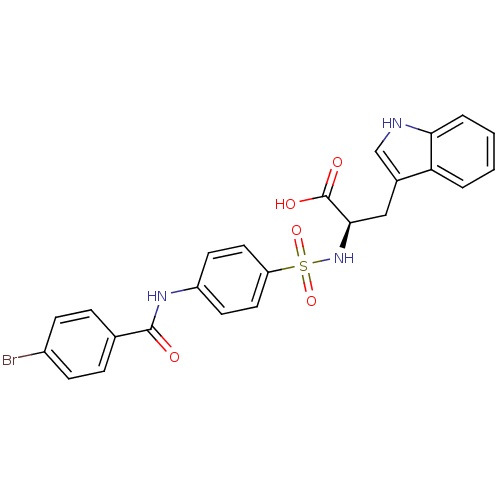
((R)-2-[4-(4-Bromo-benzoylamino)-benzenesulfonylami...)Show SMILES OC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(NC(=O)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C24H20BrN3O5S/c25-17-7-5-15(6-8-17)23(29)27-18-9-11-19(12-10-18)34(32,33)28-22(24(30)31)13-16-14-26-21-4-2-1-3-20(16)21/h1-12,14,22,26,28H,13H2,(H,27,29)(H,30,31)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
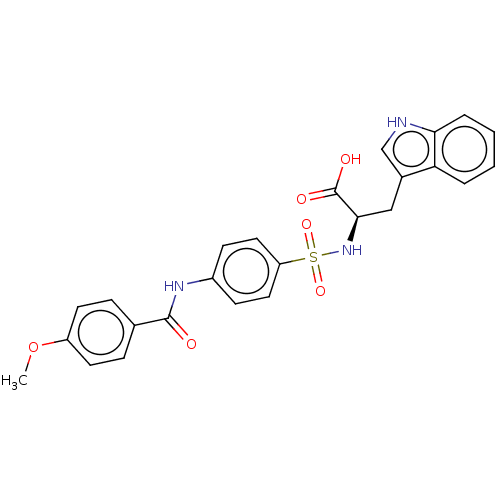
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50491913
(CHEMBL2385443)Show SMILES COc1ccc(cc1)C(=O)Nc1ccc(cc1)S(=O)(=O)N[C@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C25H23N3O6S/c1-34-19-10-6-16(7-11-19)24(29)27-18-8-12-20(13-9-18)35(32,33)28-23(25(30)31)14-17-15-26-22-5-3-2-4-21(17)22/h2-13,15,23,26,28H,14H2,1H3,(H,27,29)(H,30,31)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
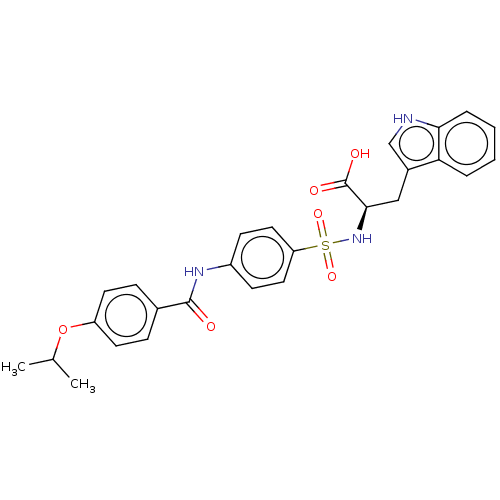
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50491917
(CHEMBL2385444)Show SMILES CC(C)Oc1ccc(cc1)C(=O)Nc1ccc(cc1)S(=O)(=O)N[C@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C27H27N3O6S/c1-17(2)36-21-11-7-18(8-12-21)26(31)29-20-9-13-22(14-10-20)37(34,35)30-25(27(32)33)15-19-16-28-24-6-4-3-5-23(19)24/h3-14,16-17,25,28,30H,15H2,1-2H3,(H,29,31)(H,32,33)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
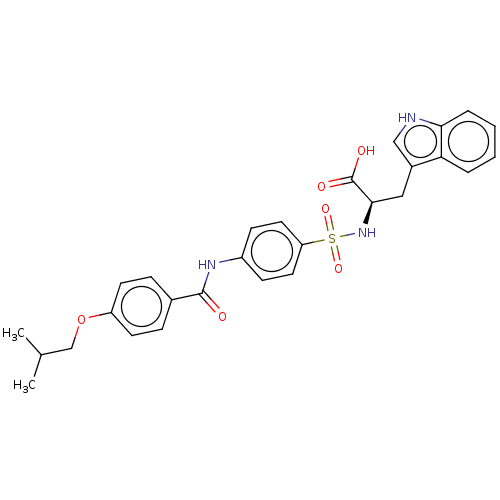
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50491912
(CHEMBL2385445)Show SMILES CC(C)COc1ccc(cc1)C(=O)Nc1ccc(cc1)S(=O)(=O)N[C@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C28H29N3O6S/c1-18(2)17-37-22-11-7-19(8-12-22)27(32)30-21-9-13-23(14-10-21)38(35,36)31-26(28(33)34)15-20-16-29-25-6-4-3-5-24(20)25/h3-14,16,18,26,29,31H,15,17H2,1-2H3,(H,30,32)(H,33,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063139

((R)-2-[4-(4-Bromo-benzoylamino)-benzenesulfonylami...)Show SMILES OC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(NC(=O)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C24H20BrN3O5S/c25-17-7-5-15(6-8-17)23(29)27-18-9-11-19(12-10-18)34(32,33)28-22(24(30)31)13-16-14-26-21-4-2-1-3-20(16)21/h1-12,14,22,26,28H,13H2,(H,27,29)(H,30,31)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP9 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50491906

(CHEMBL2385442)Show SMILES OC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(NC(=O)c2ccc(I)cc2)cc1 |r| Show InChI InChI=1S/C24H20IN3O5S/c25-17-7-5-15(6-8-17)23(29)27-18-9-11-19(12-10-18)34(32,33)28-22(24(30)31)13-16-14-26-21-4-2-1-3-20(16)21/h1-12,14,22,26,28H,13H2,(H,27,29)(H,30,31)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50491906

(CHEMBL2385442)Show SMILES OC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(NC(=O)c2ccc(I)cc2)cc1 |r| Show InChI InChI=1S/C24H20IN3O5S/c25-17-7-5-15(6-8-17)23(29)27-18-9-11-19(12-10-18)34(32,33)28-22(24(30)31)13-16-14-26-21-4-2-1-3-20(16)21/h1-12,14,22,26,28H,13H2,(H,27,29)(H,30,31)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP9 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17659

((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Inhibition of NAALADase activity in human LNCaP cell membranes assessed as inhibition of [3H]NAG conversion to [3H]glutamate after 30 mins by liquid ... |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 1/2/3/4/5
(Homo sapiens (Human)) | BDBM50192018

(CHEMBL3350037)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H](CO)[C@@H](C)O)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O |r| Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29-,34-,36+,37+,38-,39+,40+,41+,42+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr-11]-SRIF from SSTR of human NCI-H69 cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(RAT) | BDBM50476143

(CHEMBL376001)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](Cc2ccccc2)N(C)C(=O)[C@H](CCS)NC1=O Show InChI InChI=1S/C45H58N8O7S/c1-27(2)39-44(59)49-35(20-22-61)45(60)53(3)38(24-28-11-5-4-6-12-28)43(58)51-36(23-29-16-18-31(54)19-17-29)41(56)50-37(25-30-26-47-33-14-8-7-13-32(30)33)42(57)48-34(40(55)52-39)15-9-10-21-46/h4-8,11-14,16-19,26-27,34-39,47,54,61H,9-10,15,20-25,46H2,1-3H3,(H,48,57)(H,49,59)(H,50,56)(H,51,58)(H,52,55)/t34-,35-,36-,37-,38+,39-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SRIF-14 from SSTR of rat AR42J cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(Homo sapiens (Human)) | BDBM50476136
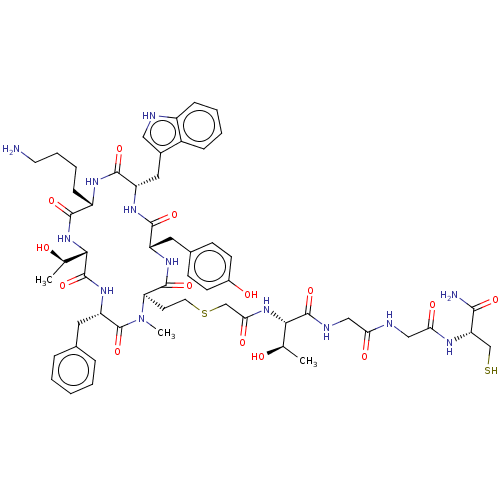
(CHEMBL222699)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCSCC(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)NCC(=O)N[C@@H](CS)C(N)=O)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O Show InChI InChI=1S/C57H77N13O14S2/c1-31(71)48(55(82)62-27-45(74)61-28-46(75)63-43(29-85)50(59)77)68-47(76)30-86-22-20-44-54(81)66-40(23-34-16-18-36(73)19-17-34)52(79)65-41(25-35-26-60-38-14-8-7-13-37(35)38)53(80)64-39(15-9-10-21-58)51(78)69-49(32(2)72)56(83)67-42(57(84)70(44)3)24-33-11-5-4-6-12-33/h4-8,11-14,16-19,26,31-32,39-44,48-49,60,71-73,85H,9-10,15,20-25,27-30,58H2,1-3H3,(H2,59,77)(H,61,74)(H,62,82)(H,63,75)(H,64,80)(H,65,79)(H,66,81)(H,67,83)(H,68,76)(H,69,78)/t31-,32-,39+,40+,41+,42+,43+,44-,48+,49+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr-11]-SRIF from SSTR of human NCI-H69 cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 1/2/3/4/5
(Homo sapiens (Human)) | BDBM50476139
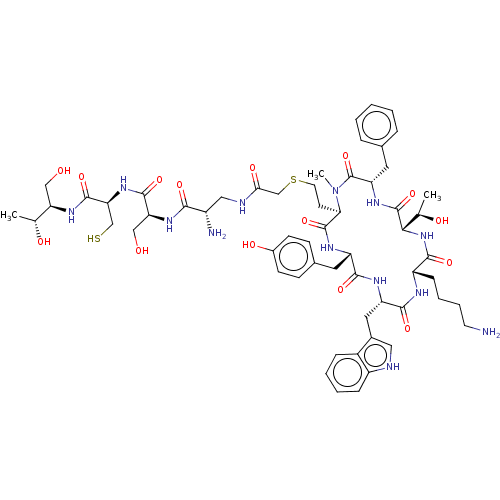
(CHEMBL268060)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CCSCC(=O)NC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)N[C@H](CO)[C@@H](C)O)N(C)C(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O Show InChI InChI=1S/C59H83N13O15S2/c1-32(75)45(28-73)68-56(84)47(30-88)70-55(83)46(29-74)69-51(79)39(61)27-63-49(78)31-89-22-20-48-57(85)66-42(23-35-16-18-37(77)19-17-35)53(81)65-43(25-36-26-62-40-14-8-7-13-38(36)40)54(82)64-41(15-9-10-21-60)52(80)71-50(33(2)76)58(86)67-44(59(87)72(48)3)24-34-11-5-4-6-12-34/h4-8,11-14,16-19,26,32-33,39,41-48,50,62,73-77,88H,9-10,15,20-25,27-31,60-61H2,1-3H3,(H,63,78)(H,64,82)(H,65,81)(H,66,85)(H,67,86)(H,68,84)(H,69,79)(H,70,83)(H,71,80)/t32-,33-,39+,41+,42+,43+,44+,45-,46+,47+,48-,50+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Diatide Research Laboaratories
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr-11]-SRIF from SSTR of human NCI-H69 cell membranes |
J Med Chem 50: 1354-64 (2007)
Article DOI: 10.1021/jm061290i
BindingDB Entry DOI: 10.7270/Q2KK9FK5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50491907
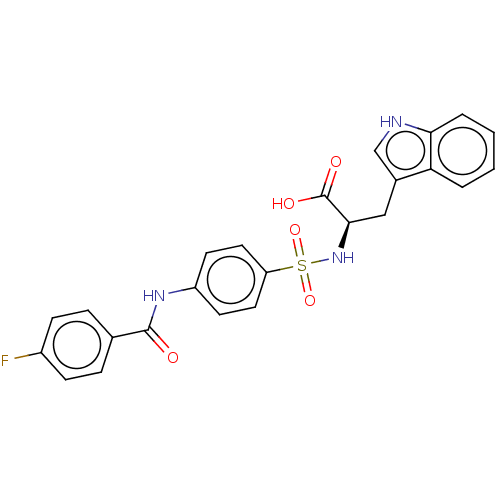
(CHEMBL2385441)Show SMILES OC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(NC(=O)c2ccc(F)cc2)cc1 |r| Show InChI InChI=1S/C24H20FN3O5S/c25-17-7-5-15(6-8-17)23(29)27-18-9-11-19(12-10-18)34(32,33)28-22(24(30)31)13-16-14-26-21-4-2-1-3-20(16)21/h1-12,14,22,26,28H,13H2,(H,27,29)(H,30,31)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50586984

(CHEMBL5084771) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PI-2620 from Tau in human brain homogenate incubated for 60 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116528
BindingDB Entry DOI: 10.7270/Q2PC3698 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50398811

(CHEMBL2177507)Show SMILES OC(=O)[C@@H](F)C[C@H](CP(O)(O)=O)C(O)=O |r| Show InChI InChI=1S/C6H10FO7P/c7-4(6(10)11)1-3(5(8)9)2-15(12,13)14/h3-4H,1-2H2,(H,8,9)(H,10,11)(H2,12,13,14)/t3-,4+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare
Curated by ChEMBL
| Assay Description
Inhibition of NAALADase activity in human LNCaP cell membranes assessed as inhibition of [3H]NAG conversion to [3H]glutamate after 30 mins by liquid ... |
J Med Chem 55: 9510-20 (2012)
Article DOI: 10.1021/jm300710j
BindingDB Entry DOI: 10.7270/Q28053R3 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50586985
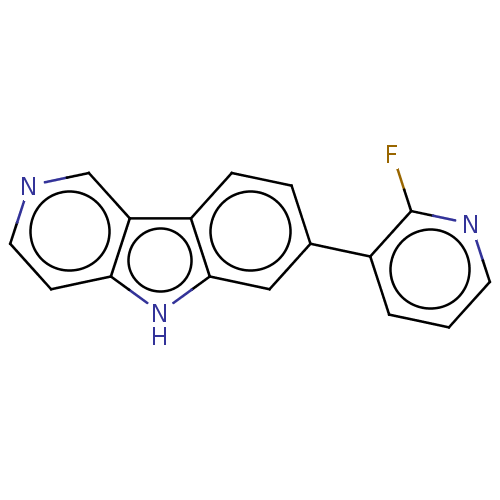
(CHEMBL5082735) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PI-2620 from Tau in human brain homogenate incubated for 60 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116528
BindingDB Entry DOI: 10.7270/Q2PC3698 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50491915
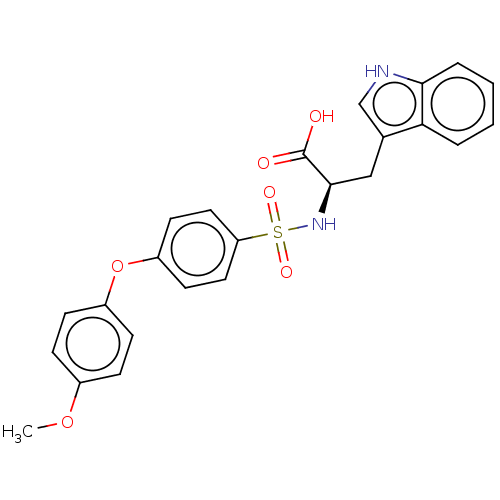
(CHEMBL2385451)Show SMILES COc1ccc(Oc2ccc(cc2)S(=O)(=O)N[C@H](Cc2c[nH]c3ccccc23)C(O)=O)cc1 |r| Show InChI InChI=1S/C24H22N2O6S/c1-31-17-6-8-18(9-7-17)32-19-10-12-20(13-11-19)33(29,30)26-23(24(27)28)14-16-15-25-22-5-3-2-4-21(16)22/h2-13,15,23,25-26H,14H2,1H3,(H,27,28)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063166
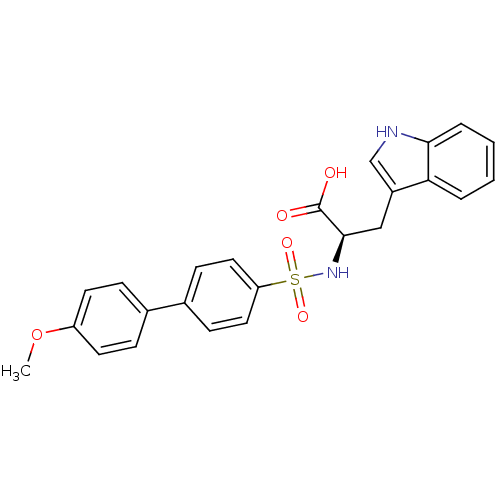
((R)-3-(1H-Indol-3-yl)-2-(4'-methoxy-biphenyl-4-sul...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C24H22N2O5S/c1-31-19-10-6-16(7-11-19)17-8-12-20(13-9-17)32(29,30)26-23(24(27)28)14-18-15-25-22-5-3-2-4-21(18)22/h2-13,15,23,25-26H,14H2,1H3,(H,27,28)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50491914
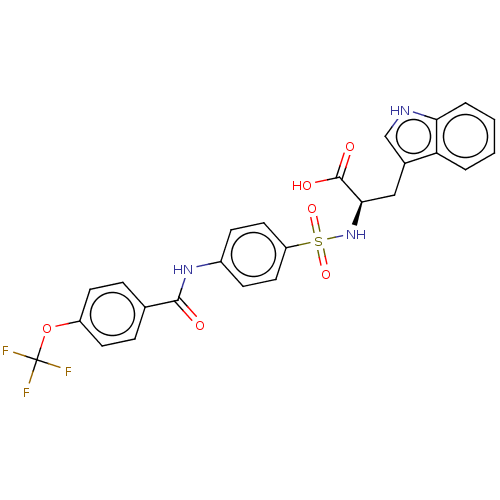
(CHEMBL2385084)Show SMILES OC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(NC(=O)c2ccc(OC(F)(F)F)cc2)cc1 |r| Show InChI InChI=1S/C25H20F3N3O6S/c26-25(27,28)37-18-9-5-15(6-10-18)23(32)30-17-7-11-19(12-8-17)38(35,36)31-22(24(33)34)13-16-14-29-21-4-2-1-3-20(16)21/h1-12,14,22,29,31H,13H2,(H,30,32)(H,33,34)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50586972
(CHEMBL5088921) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]PI-2620 from Tau in human brain homogenate incubated for 60 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116528
BindingDB Entry DOI: 10.7270/Q2PC3698 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50491910
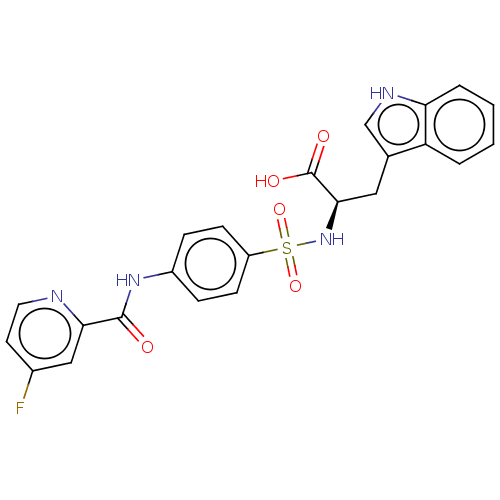
(CHEMBL2385449)Show SMILES OC(=O)[C@@H](Cc1c[nH]c2ccccc12)NS(=O)(=O)c1ccc(NC(=O)c2cc(F)ccn2)cc1 |r| Show InChI InChI=1S/C23H19FN4O5S/c24-15-9-10-25-20(12-15)22(29)27-16-5-7-17(8-6-16)34(32,33)28-21(23(30)31)11-14-13-26-19-4-2-1-3-18(14)19/h1-10,12-13,21,26,28H,11H2,(H,27,29)(H,30,31)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich
Curated by ChEMBL
| Assay Description
Inhibition of human full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa(Dnp)-Ala-Arg-NH2 as substrate measured up to 120 mins by fluorescence assay |
J Med Chem 56: 4912-20 (2013)
Article DOI: 10.1021/jm400156p
BindingDB Entry DOI: 10.7270/Q2959MHQ |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50441006
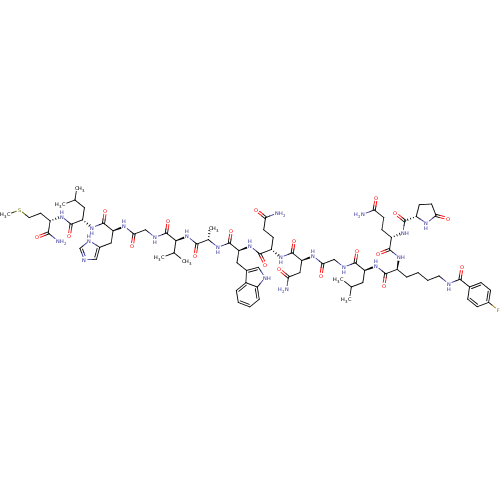
(CHEMBL2429987)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCNC(=O)c1ccc(F)cc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H]1CCC(=O)N1)C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H113FN22O19S/c1-39(2)29-54(98-70(112)50(15-11-12-27-85-68(110)43-16-18-45(79)19-17-43)95-72(114)52(20-23-59(80)102)96-71(113)51-22-25-62(105)91-51)69(111)87-36-63(106)93-58(33-61(82)104)77(119)97-53(21-24-60(81)103)73(115)100-56(31-44-34-86-48-14-10-9-13-47(44)48)74(116)90-42(7)67(109)101-65(41(5)6)78(120)88-37-64(107)92-57(32-46-35-84-38-89-46)76(118)99-55(30-40(3)4)75(117)94-49(66(83)108)26-28-121-8/h9-10,13-14,16-19,34-35,38-42,49-58,65,86H,11-12,15,20-33,36-37H2,1-8H3,(H2,80,102)(H2,81,103)(H2,82,104)(H2,83,108)(H,84,89)(H,85,110)(H,87,111)(H,88,120)(H,90,116)(H,91,105)(H,92,107)(H,93,106)(H,94,117)(H,95,114)(H,96,113)(H,97,119)(H,98,112)(H,99,118)(H,100,115)(H,101,109)/t42-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Technology (ETH) Zurich
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr4-bombesin from GRP receptor in human PC3 cells |
J Med Chem 56: 7552-63 (2013)
Article DOI: 10.1021/jm400857f
BindingDB Entry DOI: 10.7270/Q2VX0HZF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data