 Found 339 hits with Last Name = 'discenza' and Initial = 'ln'
Found 339 hits with Last Name = 'discenza' and Initial = 'ln' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM230106

(US10106559, Example 31 | US10435415, Example 31 | ...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(Cl)cccn2c1=O)-c1c(F)cc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(3.03,,;1.7,-.77,;.37,,;-.97,-.77,;-.97,-2.31,;.37,-3.08,;1.7,-2.31,;3.03,-3.08,;3.03,-4.62,;1.7,-5.39,;4.37,-5.39,;5.7,-4.62,;7.03,-5.39,;7.03,-6.93,;8.37,-4.62,;8.37,-3.08,;7.03,-2.31,;5.7,-3.08,;4.37,-2.31,;4.37,-.77,;.37,1.54,;1.7,2.31,;3.03,1.54,;1.7,3.85,;.37,4.62,;.37,6.16,;1.7,6.93,;-.97,6.93,;-.97,3.85,;-2.43,4.33,;-3.34,3.08,;-4.87,2.92,;-5.49,1.51,;-4.59,.27,;-3.06,.43,;-2.43,1.83,;-.97,2.31,;-7.03,1.51,;-7.03,-.03,;-8.37,.74,;-7.8,2.85,)| Show InChI InChI=1S/C31H24ClFN4O4/c1-15-17(6-4-8-23(15)37-25(38)14-24-20(32)7-5-11-36(24)30(37)40)26-21(33)13-19(29(34)39)28-27(26)18-10-9-16(31(2,3)41)12-22(18)35-28/h4-14,35,41H,1-3H3,(H2,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194718
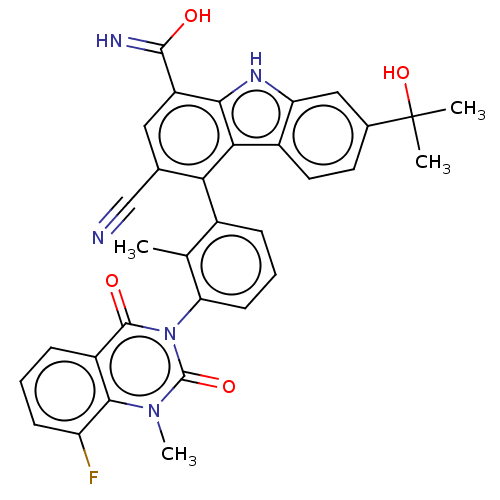
(CHEMBL3899411)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(cc(C(O)=N)c2[nH]c3cc(ccc3c12)C(C)(C)O)C#N |(-2.03,-1.34,;-1.6,.13,;-.1,.5,;.33,1.98,;-.74,3.09,;-2.23,2.73,;-2.66,1.25,;-4.16,.88,;-4.59,-.6,;-3.52,-1.71,;-6.08,-.97,;-6.51,-2.44,;-7.15,.15,;-8.65,-.22,;-9.08,-1.7,;-9.71,.89,;-9.28,2.37,;-7.78,2.74,;-6.72,1.63,;-5.22,1.99,;-4.79,3.47,;.96,-.61,;.53,-2.09,;1.6,-3.2,;3.09,-2.84,;4.16,-3.95,;5.66,-3.58,;3.73,-5.43,;3.52,-1.36,;5.06,-1.52,;6.34,-.67,;7.62,.19,;7.51,1.73,;6.13,2.41,;4.85,1.55,;4.95,.01,;2.46,-.24,;8.79,2.58,;7.94,3.86,;9.65,1.3,;10.07,3.44,;-.96,-2.46,;-2.46,-2.82,)| Show InChI InChI=1S/C33H26FN5O4/c1-16-19(7-6-10-25(16)39-31(41)21-8-5-9-23(34)29(21)38(4)32(39)42)26-17(15-35)13-22(30(36)40)28-27(26)20-12-11-18(33(2,3)43)14-24(20)37-28/h5-14,37,43H,1-4H3,(H2,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252094
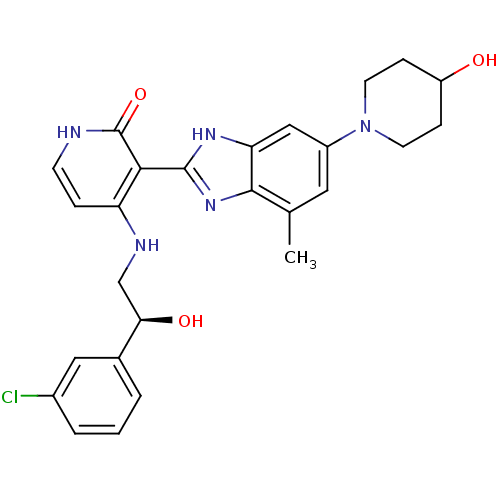
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(O)CC1 |r| Show InChI InChI=1S/C26H28ClN5O3/c1-15-11-18(32-9-6-19(33)7-10-32)13-21-24(15)31-25(30-21)23-20(5-8-28-26(23)35)29-14-22(34)16-3-2-4-17(27)12-16/h2-5,8,11-13,19,22,33-34H,6-7,9-10,14H2,1H3,(H,30,31)(H2,28,29,35)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194722
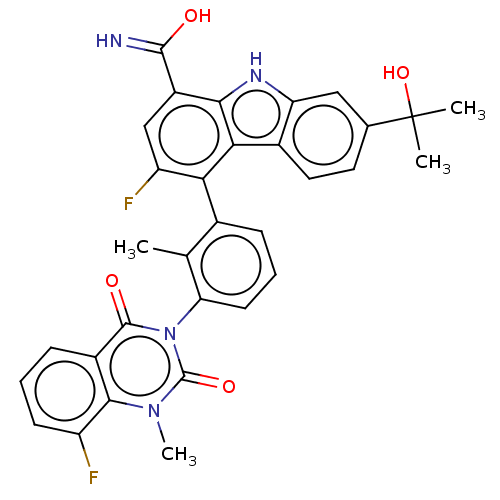
(CHEMBL3896019)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(F)cc(C(O)=N)c2[nH]c3cc(ccc3c12)C(C)(C)O |(-2.05,-1.45,;-1.63,.03,;-.14,.41,;.29,1.89,;-.78,2.99,;-2.28,2.62,;-2.7,1.14,;-4.2,.77,;-4.62,-.71,;-3.55,-1.82,;-6.11,-1.09,;-6.54,-2.57,;-7.18,.02,;-8.68,-.35,;-9.1,-1.84,;-9.75,.75,;-9.32,2.23,;-7.83,2.61,;-6.76,1.5,;-5.27,1.87,;-4.84,3.35,;.93,-.7,;.51,-2.18,;-.98,-2.55,;1.58,-3.29,;3.08,-2.91,;4.15,-4.02,;5.64,-3.65,;3.72,-5.5,;3.5,-1.43,;5.03,-1.59,;6.31,-.73,;7.58,.13,;7.47,1.67,;6.09,2.34,;4.81,1.48,;4.92,-.06,;2.43,-.33,;8.75,2.53,;7.89,3.81,;9.61,1.25,;10.03,3.39,)| Show InChI InChI=1S/C32H26F2N4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-21(33)28(19)37(4)31(38)41)25-22(34)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-14,36,42H,1-4H3,(H2,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50547850

(CHEMBL4741099)Show SMILES Cc1c(cccc1-n1c(=O)cc2cc(Cl)ccn2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(14.13,-49.58,;12.8,-50.36,;11.47,-49.59,;10.14,-50.36,;10.13,-51.9,;11.47,-52.67,;12.8,-51.89,;14.14,-52.65,;14.15,-54.2,;12.81,-54.97,;15.48,-54.96,;16.82,-54.19,;18.15,-54.95,;19.49,-54.17,;20.82,-54.93,;19.47,-52.62,;18.13,-51.87,;16.81,-52.64,;15.47,-51.88,;15.47,-50.34,;11.47,-48.05,;12.8,-47.28,;12.8,-45.73,;11.47,-44.96,;11.46,-43.42,;12.79,-42.65,;10.13,-42.66,;10.13,-45.74,;8.66,-45.28,;7.76,-46.52,;6.24,-46.7,;5.62,-48.09,;6.54,-49.34,;8.06,-49.16,;8.67,-47.76,;10.14,-47.28,;4.09,-48.27,;3.18,-47.03,;3.47,-49.68,;2.59,-48.67,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252295

(3-(6-(4-((1R,4S)-5-oxa-2-aza-bicyclo[2.2.1]heptan-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1C[C@@H]2C[C@@H]1CO2 |r,THB:31:34:39.40:37| Show InChI InChI=1S/C31H35ClN6O3/c1-18-11-22(37-9-6-21(7-10-37)38-16-24-13-23(38)17-41-24)14-26-29(18)36-30(35-26)28-25(5-8-33-31(28)40)34-15-27(39)19-3-2-4-20(32)12-19/h2-5,8,11-12,14,21,23-24,27,39H,6-7,9-10,13,15-17H2,1H3,(H,35,36)(H2,33,34,40)/t23-,24+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194720

(CHEMBL3900554)Show SMILES [H][C@@]1(CCc2c(C1)[nH]c1c(cc(F)c(-c3cccc(c3C)-n3c(=O)n(C)c4c(F)cccc4c3=O)c21)C(O)=N)C(C)(C)O |r,wU:1.0,(7.64,3.3,;7.24,1.81,;5.84,2.47,;4.58,1.59,;4.71,.05,;6.1,-.6,;7.37,.28,;4.84,-1.48,;3.3,-1.34,;2.9,-2.83,;1.41,-3.22,;.33,-2.13,;-1.16,-2.53,;.73,-.64,;-.36,.45,;.05,1.93,;-1.04,3.03,;-2.53,2.63,;-2.93,1.15,;-1.85,.05,;-2.25,-1.43,;-4.42,.75,;-4.82,-.73,;-3.74,-1.83,;-6.31,-1.13,;-6.71,-2.62,;-7.4,-.04,;-8.89,-.43,;-9.29,-1.92,;-9.97,.66,;-9.57,2.15,;-8.08,2.54,;-6.99,1.45,;-5.51,1.84,;-5.1,3.33,;2.22,-.25,;3.99,-3.92,;5.48,-3.53,;3.58,-5.41,;8.77,1.67,;8.91,3.21,;8.63,.14,;10.3,1.53,)| Show InChI InChI=1S/C32H30F2N4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-21(33)28(19)37(4)31(38)41)25-22(34)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-10,14,16,36,42H,11-13H2,1-4H3,(H2,35,39)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM27879
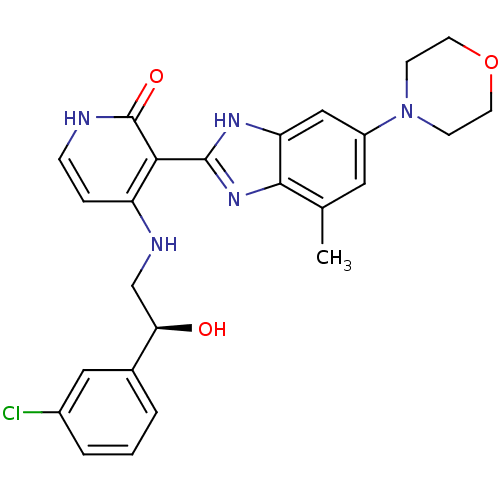
(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194720

(CHEMBL3900554)Show SMILES [H][C@@]1(CCc2c(C1)[nH]c1c(cc(F)c(-c3cccc(c3C)-n3c(=O)n(C)c4c(F)cccc4c3=O)c21)C(O)=N)C(C)(C)O |r,wU:1.0,(7.64,3.3,;7.24,1.81,;5.84,2.47,;4.58,1.59,;4.71,.05,;6.1,-.6,;7.37,.28,;4.84,-1.48,;3.3,-1.34,;2.9,-2.83,;1.41,-3.22,;.33,-2.13,;-1.16,-2.53,;.73,-.64,;-.36,.45,;.05,1.93,;-1.04,3.03,;-2.53,2.63,;-2.93,1.15,;-1.85,.05,;-2.25,-1.43,;-4.42,.75,;-4.82,-.73,;-3.74,-1.83,;-6.31,-1.13,;-6.71,-2.62,;-7.4,-.04,;-8.89,-.43,;-9.29,-1.92,;-9.97,.66,;-9.57,2.15,;-8.08,2.54,;-6.99,1.45,;-5.51,1.84,;-5.1,3.33,;2.22,-.25,;3.99,-3.92,;5.48,-3.53,;3.58,-5.41,;8.77,1.67,;8.91,3.21,;8.63,.14,;10.3,1.53,)| Show InChI InChI=1S/C32H30F2N4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-21(33)28(19)37(4)31(38)41)25-22(34)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-10,14,16,36,42H,11-13H2,1-4H3,(H2,35,39)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252237

((S)-methyl 1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...)Show SMILES COC(=O)NC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H31ClN6O4/c1-16-12-20(35-10-7-19(8-11-35)32-28(38)39-2)14-22-25(16)34-26(33-22)24-21(6-9-30-27(24)37)31-15-23(36)17-4-3-5-18(29)13-17/h3-6,9,12-14,19,23,36H,7-8,10-11,15H2,1-2H3,(H,32,38)(H,33,34)(H2,30,31,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM264137
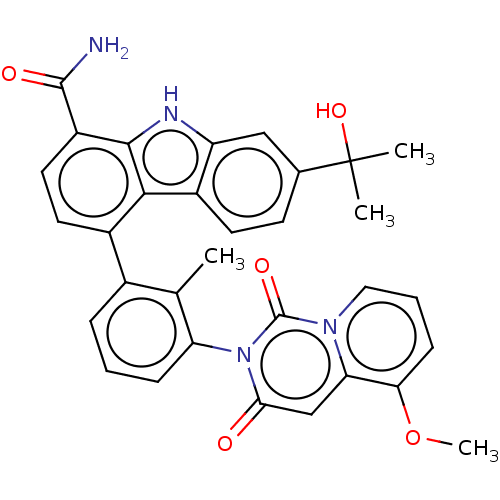
(7-(2-Hydroxypropan-2-yl)-4-(3-(5-methoxy-1,3-dioxo...)Show SMILES COc1cccn2c1cc(=O)n(-c1cccc(c1C)-c1ccc(C(N)=O)c3[nH]c4cc(ccc4c13)C(C)(C)O)c2=O |(8.37,-7.31,;7.03,-6.54,;7.03,-5,;8.37,-4.23,;8.37,-2.69,;7.03,-1.92,;5.7,-2.69,;5.7,-4.23,;4.37,-5,;3.03,-4.23,;1.7,-5,;3.03,-2.69,;1.7,-1.93,;.37,-2.69,;-.97,-1.93,;-.97,-.38,;.37,.38,;1.7,-.38,;2.47,.95,;.37,1.92,;1.7,2.69,;1.7,4.23,;.37,5,;.37,6.54,;1.7,7.31,;-.97,7.31,;-.97,4.23,;-2.43,4.71,;-3.34,3.47,;-4.87,3.3,;-5.49,1.9,;-4.59,.65,;-3.06,.81,;-2.43,2.22,;-.97,2.69,;-7.03,1.9,;-8.37,1.13,;-6.64,.41,;-7.8,3.23,;4.37,-1.93,;4.37,-.38,)| Show InChI InChI=1S/C32H28N4O5/c1-17-19(7-5-8-24(17)36-27(37)16-25-26(41-4)9-6-14-35(25)31(36)39)20-12-13-22(30(33)38)29-28(20)21-11-10-18(32(2,3)40)15-23(21)34-29/h5-16,34,40H,1-4H3,(H2,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM230107
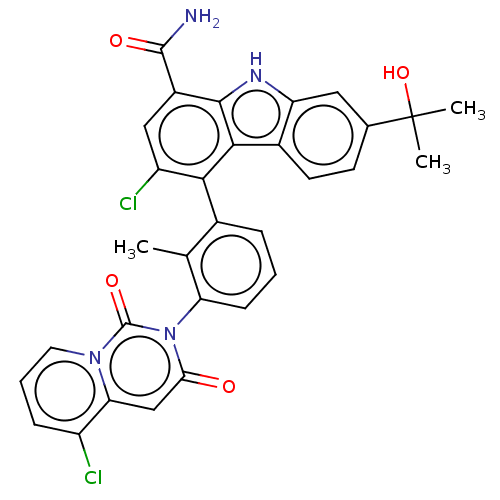
(US10106559, Example 33 | US10435415, Example 33 | ...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(Cl)cccn2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(3.03,8.9,;1.7,8.13,;.37,8.9,;-.97,8.13,;-.97,6.59,;.37,5.82,;1.7,6.59,;3.03,5.82,;3.03,4.28,;1.7,3.51,;4.37,3.51,;5.7,4.28,;7.03,3.51,;7.03,1.97,;8.37,4.28,;8.37,5.82,;7.03,6.59,;5.7,5.82,;4.37,6.59,;4.37,8.13,;.37,10.44,;1.7,11.21,;3.03,10.44,;1.7,12.75,;.37,13.52,;.37,15.06,;1.7,15.83,;-.97,15.83,;-.97,12.75,;-2.43,13.22,;-3.34,11.98,;-4.87,11.82,;-5.49,10.41,;-4.59,9.16,;-3.06,9.32,;-2.43,10.73,;-.97,11.21,;-7.03,10.41,;-6.64,8.92,;-8.37,9.64,;-7.8,11.74,)| Show InChI InChI=1S/C31H24Cl2N4O4/c1-15-17(6-4-8-23(15)37-25(38)14-24-20(32)7-5-11-36(24)30(37)40)26-21(33)13-19(29(34)39)28-27(26)18-10-9-16(31(2,3)41)12-22(18)35-28/h4-14,35,41H,1-3H3,(H2,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50547849

(CHEMBL4789404)Show SMILES COc1ccn2c(c1)cc(=O)n(-c1cccc(c1C)-c1ccc(C(N)=O)c3[nH]c4cc(ccc4c13)C(C)(C)O)c2=O |(66.87,-34.3,;66.86,-32.77,;65.53,-32.02,;65.52,-30.47,;64.18,-29.71,;62.85,-30.49,;62.86,-32.03,;64.19,-32.8,;61.52,-32.81,;60.19,-32.04,;58.85,-32.82,;60.18,-30.5,;58.84,-29.74,;57.51,-30.51,;56.18,-29.74,;56.18,-28.2,;57.52,-27.43,;58.84,-28.2,;60.17,-27.42,;57.51,-25.89,;58.84,-25.13,;58.84,-23.57,;57.51,-22.81,;57.5,-21.27,;58.83,-20.49,;56.17,-20.5,;56.17,-23.59,;54.7,-23.12,;53.8,-24.36,;52.28,-24.54,;51.66,-25.94,;52.58,-27.18,;54.1,-27.01,;54.71,-25.6,;56.18,-25.13,;50.13,-26.11,;49.22,-24.87,;49.51,-27.52,;48.63,-26.51,;61.51,-29.73,;61.51,-28.19,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194717
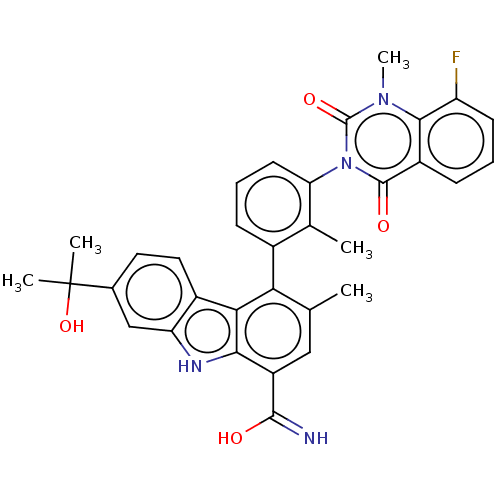
(CHEMBL3931086)Show SMILES Cc1cc(C(O)=N)c2[nH]c3cc(ccc3c2c1-c1cccc(c1C)-n1c(=O)n(C)c2c(F)cccc2c1=O)C(C)(C)O |(-.98,-2.55,;.51,-2.18,;1.58,-3.29,;3.08,-2.91,;4.15,-4.02,;5.64,-3.65,;3.72,-5.5,;3.5,-1.43,;5.03,-1.59,;6.31,-.73,;7.58,.13,;7.47,1.67,;6.09,2.34,;4.81,1.48,;4.92,-.06,;2.43,-.33,;.93,-.7,;-.14,.41,;.29,1.89,;-.78,2.99,;-2.28,2.62,;-2.7,1.14,;-1.63,.03,;-2.05,-1.45,;-4.2,.77,;-4.62,-.71,;-3.55,-1.82,;-6.11,-1.09,;-6.54,-2.57,;-7.18,.02,;-8.68,-.35,;-9.1,-1.84,;-9.75,.75,;-9.32,2.23,;-7.83,2.61,;-6.76,1.5,;-5.27,1.87,;-4.84,3.35,;8.75,2.53,;7.89,3.81,;9.61,1.25,;10.03,3.39,)| Show InChI InChI=1S/C33H29FN4O4/c1-16-14-22(30(35)39)28-27(20-13-12-18(33(3,4)42)15-24(20)36-28)26(16)19-8-7-11-25(17(19)2)38-31(40)21-9-6-10-23(34)29(21)37(5)32(38)41/h6-15,36,42H,1-5H3,(H2,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252193
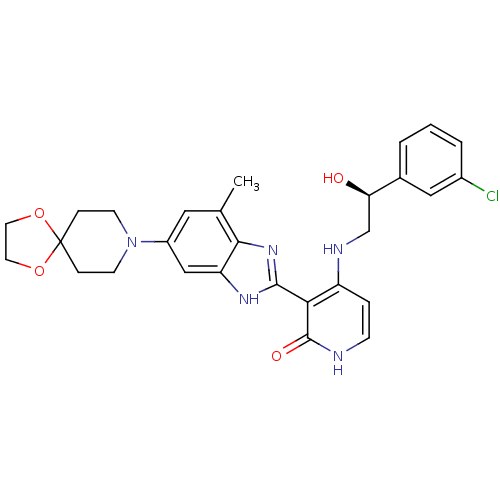
(4-((S)-2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC2(CC1)OCCO2 |r| Show InChI InChI=1S/C28H30ClN5O4/c1-17-13-20(34-9-6-28(7-10-34)37-11-12-38-28)15-22-25(17)33-26(32-22)24-21(5-8-30-27(24)36)31-16-23(35)18-3-2-4-19(29)14-18/h2-5,8,13-15,23,35H,6-7,9-12,16H2,1H3,(H,32,33)(H2,30,31,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252236
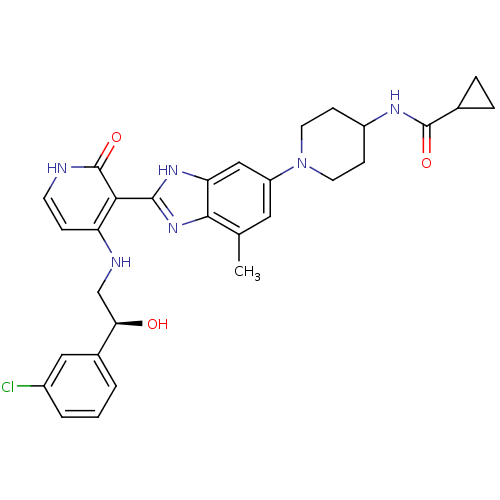
((S)-N-(1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)NC(=O)C1CC1 |r| Show InChI InChI=1S/C30H33ClN6O3/c1-17-13-22(37-11-8-21(9-12-37)34-29(39)18-5-6-18)15-24-27(17)36-28(35-24)26-23(7-10-32-30(26)40)33-16-25(38)19-3-2-4-20(31)14-19/h2-4,7,10,13-15,18,21,25,38H,5-6,8-9,11-12,16H2,1H3,(H,34,39)(H,35,36)(H2,32,33,40)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252297
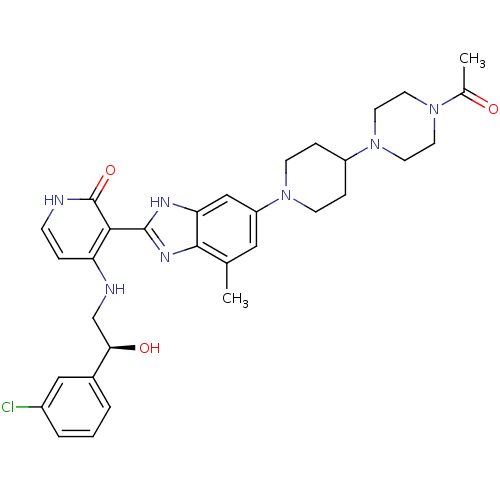
((S)-3-(6-(4-(4-acetylpiperazin-1-yl)piperidin-1-yl...)Show SMILES CC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H38ClN7O3/c1-20-16-25(39-10-7-24(8-11-39)40-14-12-38(13-15-40)21(2)41)18-27-30(20)37-31(36-27)29-26(6-9-34-32(29)43)35-19-28(42)22-4-3-5-23(33)17-22/h3-6,9,16-18,24,28,42H,7-8,10-15,19H2,1-2H3,(H,36,37)(H2,34,35,43)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM264146
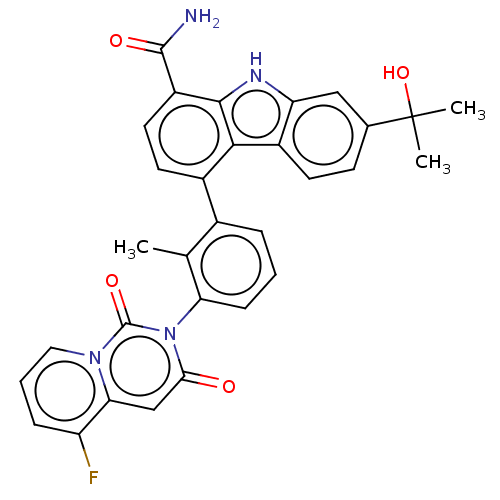
(4-(3-(5-Fluoro-1,3-dioxo-1H-pyrido[1,2-c]pyrimidin...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(F)cccn2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(3.08,,;1.75,-.77,;.42,,;-.92,-.77,;-.92,-2.31,;.42,-3.08,;1.75,-2.31,;3.08,-3.08,;3.08,-4.62,;1.75,-5.39,;4.42,-5.39,;5.75,-4.62,;7.09,-5.39,;7.09,-6.93,;8.42,-4.62,;8.42,-3.08,;7.09,-2.31,;5.75,-3.08,;4.42,-2.31,;4.42,-.77,;.42,1.54,;1.75,2.31,;1.75,3.85,;.42,4.62,;.42,6.16,;1.75,6.93,;-.92,6.93,;-.92,3.85,;-2.38,4.33,;-3.29,3.08,;-4.82,2.92,;-5.44,1.51,;-4.54,.27,;-3.01,.43,;-2.38,1.83,;-.92,2.31,;-6.93,1.11,;-7.33,-.37,;-8.42,.71,;-8.02,2.2,)| Show InChI InChI=1S/C31H25FN4O4/c1-16-18(6-4-8-24(16)36-26(37)15-25-22(32)7-5-13-35(25)30(36)39)19-11-12-21(29(33)38)28-27(19)20-10-9-17(31(2,3)40)14-23(20)34-28/h4-15,34,40H,1-3H3,(H2,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM264146

(4-(3-(5-Fluoro-1,3-dioxo-1H-pyrido[1,2-c]pyrimidin...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(F)cccn2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(3.08,,;1.75,-.77,;.42,,;-.92,-.77,;-.92,-2.31,;.42,-3.08,;1.75,-2.31,;3.08,-3.08,;3.08,-4.62,;1.75,-5.39,;4.42,-5.39,;5.75,-4.62,;7.09,-5.39,;7.09,-6.93,;8.42,-4.62,;8.42,-3.08,;7.09,-2.31,;5.75,-3.08,;4.42,-2.31,;4.42,-.77,;.42,1.54,;1.75,2.31,;1.75,3.85,;.42,4.62,;.42,6.16,;1.75,6.93,;-.92,6.93,;-.92,3.85,;-2.38,4.33,;-3.29,3.08,;-4.82,2.92,;-5.44,1.51,;-4.54,.27,;-3.01,.43,;-2.38,1.83,;-.92,2.31,;-6.93,1.11,;-7.33,-.37,;-8.42,.71,;-8.02,2.2,)| Show InChI InChI=1S/C31H25FN4O4/c1-16-18(6-4-8-24(16)36-26(37)15-25-22(32)7-5-13-35(25)30(36)39)19-11-12-21(29(33)38)28-27(19)20-10-9-17(31(2,3)40)14-23(20)34-28/h4-15,34,40H,1-3H3,(H2,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252143
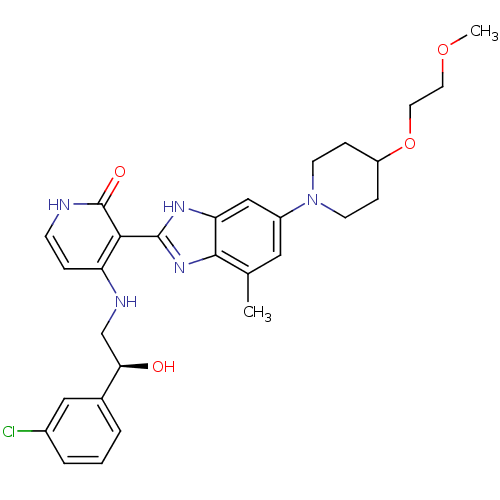
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCOC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C29H34ClN5O4/c1-18-14-21(35-10-7-22(8-11-35)39-13-12-38-2)16-24-27(18)34-28(33-24)26-23(6-9-31-29(26)37)32-17-25(36)19-4-3-5-20(30)15-19/h3-6,9,14-16,22,25,36H,7-8,10-13,17H2,1-2H3,(H,33,34)(H2,31,32,37)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM264139

(4-(3-(R)-(5-Chloro-1,3-dioxo-1H-pyrido[1,2-c]pyrim...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(Cl)cccn2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(2.1,10.61,;1.7,9.12,;.37,9.89,;-.97,9.12,;-.97,7.58,;.37,6.81,;1.7,7.58,;3.03,6.81,;3.03,5.27,;1.7,4.5,;4.37,4.5,;5.7,5.27,;7.03,4.5,;7.03,2.96,;8.37,5.27,;8.37,6.81,;7.03,7.58,;5.7,6.81,;4.37,7.58,;4.37,9.12,;.37,11.43,;1.7,12.2,;1.7,13.74,;.37,14.51,;.37,16.05,;1.7,16.82,;-.97,16.82,;-.97,13.74,;-2.43,14.22,;-3.34,12.97,;-4.87,12.81,;-5.49,11.4,;-4.59,10.16,;-3.06,10.32,;-2.43,11.73,;-.97,12.2,;-7.03,11.4,;-8.37,10.63,;-6.64,9.92,;-7.8,12.74,)| Show InChI InChI=1S/C31H25ClN4O4/c1-16-18(6-4-8-24(16)36-26(37)15-25-22(32)7-5-13-35(25)30(36)39)19-11-12-21(29(33)38)28-27(19)20-10-9-17(31(2,3)40)14-23(20)34-28/h4-15,34,40H,1-3H3,(H2,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM230107

(US10106559, Example 33 | US10435415, Example 33 | ...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(Cl)cccn2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(3.03,8.9,;1.7,8.13,;.37,8.9,;-.97,8.13,;-.97,6.59,;.37,5.82,;1.7,6.59,;3.03,5.82,;3.03,4.28,;1.7,3.51,;4.37,3.51,;5.7,4.28,;7.03,3.51,;7.03,1.97,;8.37,4.28,;8.37,5.82,;7.03,6.59,;5.7,5.82,;4.37,6.59,;4.37,8.13,;.37,10.44,;1.7,11.21,;3.03,10.44,;1.7,12.75,;.37,13.52,;.37,15.06,;1.7,15.83,;-.97,15.83,;-.97,12.75,;-2.43,13.22,;-3.34,11.98,;-4.87,11.82,;-5.49,10.41,;-4.59,9.16,;-3.06,9.32,;-2.43,10.73,;-.97,11.21,;-7.03,10.41,;-6.64,8.92,;-8.37,9.64,;-7.8,11.74,)| Show InChI InChI=1S/C31H24Cl2N4O4/c1-15-17(6-4-8-23(15)37-25(38)14-24-20(32)7-5-11-36(24)30(37)40)26-21(33)13-19(29(34)39)28-27(26)18-10-9-16(31(2,3)41)12-22(18)35-28/h4-14,35,41H,1-3H3,(H2,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human peripheral B cells assessed as reduction in anti-IgM/IgG-induced CD86 surface expression |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252142

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H30ClN5O3/c1-16-12-19(33-10-7-20(36-2)8-11-33)14-22-25(16)32-26(31-22)24-21(6-9-29-27(24)35)30-15-23(34)17-4-3-5-18(28)13-17/h3-6,9,12-14,20,23,34H,7-8,10-11,15H2,1-2H3,(H,31,32)(H2,29,30,35)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM230081
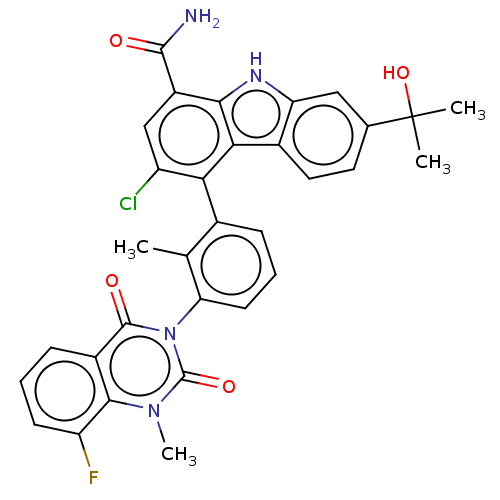
(US9334290, 1 | US9334290, 2)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(2.75,9.19,;1.42,8.42,;.08,9.19,;-1.25,8.42,;-1.25,6.88,;.08,6.11,;1.42,6.88,;2.75,6.11,;2.75,4.57,;1.42,3.8,;4.09,3.8,;4.09,2.26,;5.42,4.57,;6.75,3.8,;6.75,2.26,;8.09,4.57,;8.09,6.11,;6.75,6.88,;5.42,6.11,;4.09,6.88,;4.09,8.42,;.08,10.73,;1.42,11.5,;2.96,11.5,;1.42,13.04,;.08,13.81,;.08,15.35,;1.42,16.12,;-1.25,16.12,;-1.25,13.04,;-2.71,13.51,;-3.62,12.27,;-5.15,12.1,;-5.78,10.7,;-4.87,9.45,;-3.34,9.61,;-2.71,11.02,;-1.25,11.5,;-7.32,10.7,;-8.09,9.36,;-6.55,9.36,;-8.09,12.03,)| Show InChI InChI=1S/C32H26ClFN4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-22(34)28(19)37(4)31(38)41)25-21(33)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-14,36,42H,1-4H3,(H2,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM230081

(US9334290, 1 | US9334290, 2)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(2.75,9.19,;1.42,8.42,;.08,9.19,;-1.25,8.42,;-1.25,6.88,;.08,6.11,;1.42,6.88,;2.75,6.11,;2.75,4.57,;1.42,3.8,;4.09,3.8,;4.09,2.26,;5.42,4.57,;6.75,3.8,;6.75,2.26,;8.09,4.57,;8.09,6.11,;6.75,6.88,;5.42,6.11,;4.09,6.88,;4.09,8.42,;.08,10.73,;1.42,11.5,;2.96,11.5,;1.42,13.04,;.08,13.81,;.08,15.35,;1.42,16.12,;-1.25,16.12,;-1.25,13.04,;-2.71,13.51,;-3.62,12.27,;-5.15,12.1,;-5.78,10.7,;-4.87,9.45,;-3.34,9.61,;-2.71,11.02,;-1.25,11.5,;-7.32,10.7,;-8.09,9.36,;-6.55,9.36,;-8.09,12.03,)| Show InChI InChI=1S/C32H26ClFN4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-22(34)28(19)37(4)31(38)41)25-21(33)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-14,36,42H,1-4H3,(H2,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252238

((S)-methyl 1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...)Show SMILES COCCN(CCOC)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H41ClN6O4/c1-21-17-25(38-11-8-24(9-12-38)39(13-15-42-2)14-16-43-3)19-27-30(21)37-31(36-27)29-26(7-10-34-32(29)41)35-20-28(40)22-5-4-6-23(33)18-22/h4-7,10,17-19,24,28,40H,8-9,11-16,20H2,1-3H3,(H,36,37)(H2,34,35,41)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM264139

(4-(3-(R)-(5-Chloro-1,3-dioxo-1H-pyrido[1,2-c]pyrim...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(Cl)cccn2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(2.1,10.61,;1.7,9.12,;.37,9.89,;-.97,9.12,;-.97,7.58,;.37,6.81,;1.7,7.58,;3.03,6.81,;3.03,5.27,;1.7,4.5,;4.37,4.5,;5.7,5.27,;7.03,4.5,;7.03,2.96,;8.37,5.27,;8.37,6.81,;7.03,7.58,;5.7,6.81,;4.37,7.58,;4.37,9.12,;.37,11.43,;1.7,12.2,;1.7,13.74,;.37,14.51,;.37,16.05,;1.7,16.82,;-.97,16.82,;-.97,13.74,;-2.43,14.22,;-3.34,12.97,;-4.87,12.81,;-5.49,11.4,;-4.59,10.16,;-3.06,10.32,;-2.43,11.73,;-.97,12.2,;-7.03,11.4,;-8.37,10.63,;-6.64,9.92,;-7.8,12.74,)| Show InChI InChI=1S/C31H25ClN4O4/c1-16-18(6-4-8-24(16)36-26(37)15-25-22(32)7-5-13-35(25)30(36)39)19-11-12-21(29(33)38)28-27(19)20-10-9-17(31(2,3)40)14-23(20)34-28/h4-15,34,40H,1-3H3,(H2,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM264139

(4-(3-(R)-(5-Chloro-1,3-dioxo-1H-pyrido[1,2-c]pyrim...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(Cl)cccn2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(2.1,10.61,;1.7,9.12,;.37,9.89,;-.97,9.12,;-.97,7.58,;.37,6.81,;1.7,7.58,;3.03,6.81,;3.03,5.27,;1.7,4.5,;4.37,4.5,;5.7,5.27,;7.03,4.5,;7.03,2.96,;8.37,5.27,;8.37,6.81,;7.03,7.58,;5.7,6.81,;4.37,7.58,;4.37,9.12,;.37,11.43,;1.7,12.2,;1.7,13.74,;.37,14.51,;.37,16.05,;1.7,16.82,;-.97,16.82,;-.97,13.74,;-2.43,14.22,;-3.34,12.97,;-4.87,12.81,;-5.49,11.4,;-4.59,10.16,;-3.06,10.32,;-2.43,11.73,;-.97,12.2,;-7.03,11.4,;-8.37,10.63,;-6.64,9.92,;-7.8,12.74,)| Show InChI InChI=1S/C31H25ClN4O4/c1-16-18(6-4-8-24(16)36-26(37)15-25-22(32)7-5-13-35(25)30(36)39)19-11-12-21(29(33)38)28-27(19)20-10-9-17(31(2,3)40)14-23(20)34-28/h4-15,34,40H,1-3H3,(H2,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252144

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)OCCO |r| Show InChI InChI=1S/C28H32ClN5O4/c1-17-13-20(34-9-6-21(7-10-34)38-12-11-35)15-23-26(17)33-27(32-23)25-22(5-8-30-28(25)37)31-16-24(36)18-3-2-4-19(29)14-18/h2-5,8,13-15,21,24,35-36H,6-7,9-12,16H2,1H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM230101
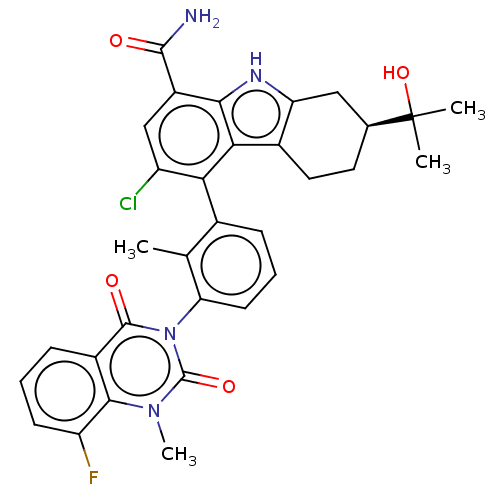
(US10106559, Example 58 | US10435415, Example 26 | ...)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3C[C@H](CCc3c12)C(C)(C)O |r,wD:33.43,(2.75,,;1.42,-.77,;.08,,;-1.25,-.77,;-1.25,-2.31,;.08,-3.08,;1.42,-2.31,;2.75,-3.08,;2.75,-4.62,;1.42,-5.39,;4.09,-5.39,;4.09,-6.93,;5.42,-4.62,;6.75,-5.39,;6.75,-6.93,;8.09,-4.62,;8.09,-3.08,;6.75,-2.31,;5.42,-3.08,;4.09,-2.31,;4.09,-.77,;.08,1.54,;1.42,2.31,;2.75,1.54,;1.42,3.85,;.08,4.62,;.08,6.16,;1.42,6.93,;-1.25,6.93,;-1.25,3.85,;-2.71,4.33,;-3.62,3.08,;-5.15,2.92,;-5.78,1.51,;-4.87,.27,;-3.34,.43,;-2.71,1.83,;-1.25,2.31,;-7.32,1.51,;-8.09,.18,;-6.55,.18,;-8.09,2.85,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM230091
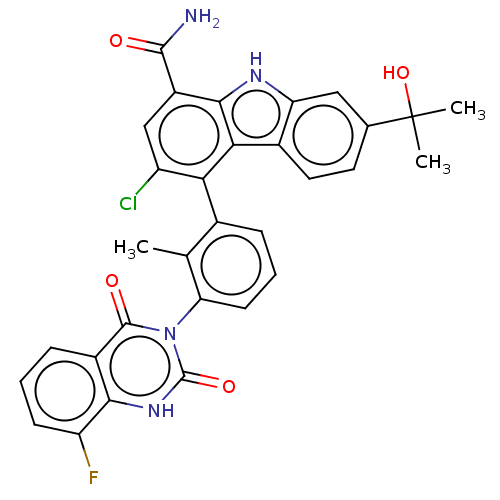
(US10106559, Example 14 | US10435415, Example 14 | ...)Show SMILES Cc1c(cccc1-n1c(=O)[nH]c2c(F)cccc2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(2.75,,;1.42,-.77,;.08,,;-1.25,-.77,;-1.25,-2.31,;.08,-3.08,;1.42,-2.31,;2.75,-3.08,;2.75,-4.62,;1.42,-5.39,;4.09,-5.39,;5.42,-4.62,;6.75,-5.39,;6.75,-6.93,;8.09,-4.62,;8.09,-3.08,;6.75,-2.31,;5.42,-3.08,;4.09,-2.31,;4.09,-.77,;.08,1.54,;1.42,2.31,;2.75,1.54,;1.42,3.85,;.08,4.62,;.08,6.16,;1.42,6.93,;-1.25,6.93,;-1.25,3.85,;-2.71,4.33,;-3.62,3.08,;-5.15,2.92,;-5.78,1.51,;-4.87,.27,;-3.34,.43,;-2.71,1.83,;-1.25,2.31,;-7.32,1.51,;-8.09,.18,;-6.55,.18,;-8.09,2.85,)| Show InChI InChI=1S/C31H24ClFN4O4/c1-14-16(6-5-9-23(14)37-29(39)18-7-4-8-21(33)26(18)36-30(37)40)24-20(32)13-19(28(34)38)27-25(24)17-11-10-15(31(2,3)41)12-22(17)35-27/h4-13,35,41H,1-3H3,(H2,34,38)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM230091

(US10106559, Example 14 | US10435415, Example 14 | ...)Show SMILES Cc1c(cccc1-n1c(=O)[nH]c2c(F)cccc2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(2.75,,;1.42,-.77,;.08,,;-1.25,-.77,;-1.25,-2.31,;.08,-3.08,;1.42,-2.31,;2.75,-3.08,;2.75,-4.62,;1.42,-5.39,;4.09,-5.39,;5.42,-4.62,;6.75,-5.39,;6.75,-6.93,;8.09,-4.62,;8.09,-3.08,;6.75,-2.31,;5.42,-3.08,;4.09,-2.31,;4.09,-.77,;.08,1.54,;1.42,2.31,;2.75,1.54,;1.42,3.85,;.08,4.62,;.08,6.16,;1.42,6.93,;-1.25,6.93,;-1.25,3.85,;-2.71,4.33,;-3.62,3.08,;-5.15,2.92,;-5.78,1.51,;-4.87,.27,;-3.34,.43,;-2.71,1.83,;-1.25,2.31,;-7.32,1.51,;-8.09,.18,;-6.55,.18,;-8.09,2.85,)| Show InChI InChI=1S/C31H24ClFN4O4/c1-14-16(6-5-9-23(14)37-29(39)18-7-4-8-21(33)26(18)36-30(37)40)24-20(32)13-19(28(34)38)27-25(24)17-11-10-15(31(2,3)41)12-22(17)35-27/h4-13,35,41H,1-3H3,(H2,34,38)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50547848

(CHEMBL4741884)Show SMILES Cc1c(cccc1-n1c(=O)cc2ccccn2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(36.26,-27.65,;34.93,-28.42,;33.61,-27.65,;32.27,-28.43,;32.27,-29.97,;33.6,-30.73,;34.94,-29.96,;36.27,-30.72,;36.28,-32.26,;34.95,-33.04,;37.62,-33.03,;38.95,-32.25,;40.29,-33.02,;41.62,-32.24,;41.61,-30.69,;40.27,-29.94,;38.94,-30.71,;37.61,-29.95,;37.6,-28.41,;33.6,-26.12,;34.94,-25.35,;34.93,-23.8,;33.6,-23.03,;33.6,-21.49,;34.92,-20.72,;32.26,-20.73,;32.26,-23.81,;30.8,-23.34,;29.9,-24.59,;28.37,-24.76,;27.76,-26.16,;28.67,-27.4,;30.19,-27.23,;30.81,-25.83,;32.27,-25.35,;26.23,-26.34,;25.31,-25.1,;25.6,-27.74,;24.73,-26.73,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM264146

(4-(3-(5-Fluoro-1,3-dioxo-1H-pyrido[1,2-c]pyrimidin...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(F)cccn2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(3.08,,;1.75,-.77,;.42,,;-.92,-.77,;-.92,-2.31,;.42,-3.08,;1.75,-2.31,;3.08,-3.08,;3.08,-4.62,;1.75,-5.39,;4.42,-5.39,;5.75,-4.62,;7.09,-5.39,;7.09,-6.93,;8.42,-4.62,;8.42,-3.08,;7.09,-2.31,;5.75,-3.08,;4.42,-2.31,;4.42,-.77,;.42,1.54,;1.75,2.31,;1.75,3.85,;.42,4.62,;.42,6.16,;1.75,6.93,;-.92,6.93,;-.92,3.85,;-2.38,4.33,;-3.29,3.08,;-4.82,2.92,;-5.44,1.51,;-4.54,.27,;-3.01,.43,;-2.38,1.83,;-.92,2.31,;-6.93,1.11,;-7.33,-.37,;-8.42,.71,;-8.02,2.2,)| Show InChI InChI=1S/C31H25FN4O4/c1-16-18(6-4-8-24(16)36-26(37)15-25-22(32)7-5-13-35(25)30(36)39)19-11-12-21(29(33)38)28-27(19)20-10-9-17(31(2,3)40)14-23(20)34-28/h4-15,34,40H,1-3H3,(H2,33,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252300
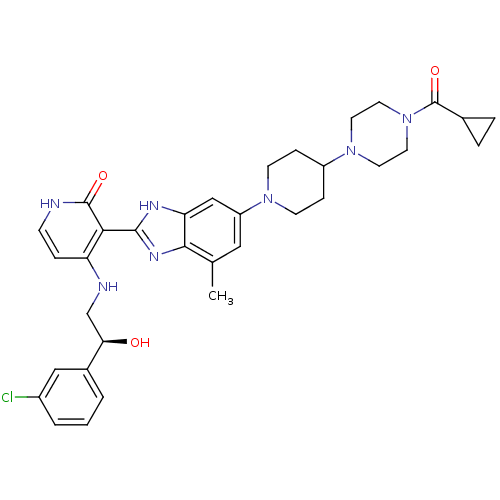
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1CCN(CC1)C(=O)C1CC1 |r| Show InChI InChI=1S/C34H40ClN7O3/c1-21-17-26(40-11-8-25(9-12-40)41-13-15-42(16-14-41)34(45)22-5-6-22)19-28-31(21)39-32(38-28)30-27(7-10-36-33(30)44)37-20-29(43)23-3-2-4-24(35)18-23/h2-4,7,10,17-19,22,25,29,43H,5-6,8-9,11-16,20H2,1H3,(H,38,39)(H2,36,37,44)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252303
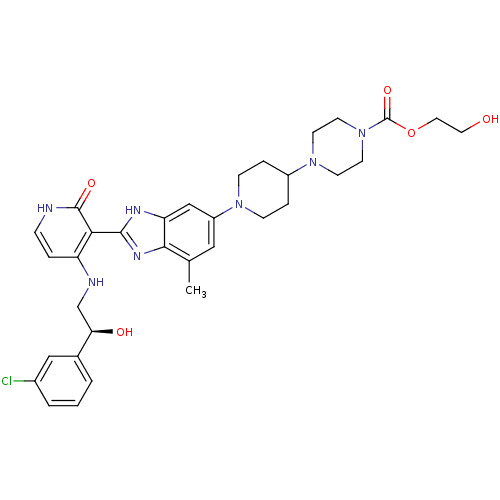
((S)-2-hydroxyethyl 4-(1-(2-(4-(2-(3-chlorophenyl)-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1CCN(CC1)C(=O)OCCO |r| Show InChI InChI=1S/C33H40ClN7O5/c1-21-17-25(39-9-6-24(7-10-39)40-11-13-41(14-12-40)33(45)46-16-15-42)19-27-30(21)38-31(37-27)29-26(5-8-35-32(29)44)36-20-28(43)22-3-2-4-23(34)18-22/h2-5,8,17-19,24,28,42-43H,6-7,9-16,20H2,1H3,(H,37,38)(H2,35,36,44)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50547848

(CHEMBL4741884)Show SMILES Cc1c(cccc1-n1c(=O)cc2ccccn2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(36.26,-27.65,;34.93,-28.42,;33.61,-27.65,;32.27,-28.43,;32.27,-29.97,;33.6,-30.73,;34.94,-29.96,;36.27,-30.72,;36.28,-32.26,;34.95,-33.04,;37.62,-33.03,;38.95,-32.25,;40.29,-33.02,;41.62,-32.24,;41.61,-30.69,;40.27,-29.94,;38.94,-30.71,;37.61,-29.95,;37.6,-28.41,;33.6,-26.12,;34.94,-25.35,;34.93,-23.8,;33.6,-23.03,;33.6,-21.49,;34.92,-20.72,;32.26,-20.73,;32.26,-23.81,;30.8,-23.34,;29.9,-24.59,;28.37,-24.76,;27.76,-26.16,;28.67,-27.4,;30.19,-27.23,;30.81,-25.83,;32.27,-25.35,;26.23,-26.34,;25.31,-25.1,;25.6,-27.74,;24.73,-26.73,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant full-length His-tagged human BTK expressed in baculovirus expression system using fluoresceinated peptide as substrate incu... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM230107

(US10106559, Example 33 | US10435415, Example 33 | ...)Show SMILES Cc1c(cccc1-n1c(=O)cc2c(Cl)cccn2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(3.03,8.9,;1.7,8.13,;.37,8.9,;-.97,8.13,;-.97,6.59,;.37,5.82,;1.7,6.59,;3.03,5.82,;3.03,4.28,;1.7,3.51,;4.37,3.51,;5.7,4.28,;7.03,3.51,;7.03,1.97,;8.37,4.28,;8.37,5.82,;7.03,6.59,;5.7,5.82,;4.37,6.59,;4.37,8.13,;.37,10.44,;1.7,11.21,;3.03,10.44,;1.7,12.75,;.37,13.52,;.37,15.06,;1.7,15.83,;-.97,15.83,;-.97,12.75,;-2.43,13.22,;-3.34,11.98,;-4.87,11.82,;-5.49,10.41,;-4.59,9.16,;-3.06,9.32,;-2.43,10.73,;-.97,11.21,;-7.03,10.41,;-6.64,8.92,;-8.37,9.64,;-7.8,11.74,)| Show InChI InChI=1S/C31H24Cl2N4O4/c1-15-17(6-4-8-23(15)37-25(38)14-24-20(32)7-5-11-36(24)30(37)40)26-21(33)13-19(29(34)39)28-27(26)18-10-9-16(31(2,3)41)12-22(18)35-28/h4-14,35,41H,1-3H3,(H2,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BTK in human PBMC cells assessed as inhibition of FCepsilonR1/immune complex-induced TNFalpha production |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00335
BindingDB Entry DOI: 10.7270/Q2PN9966 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50571879
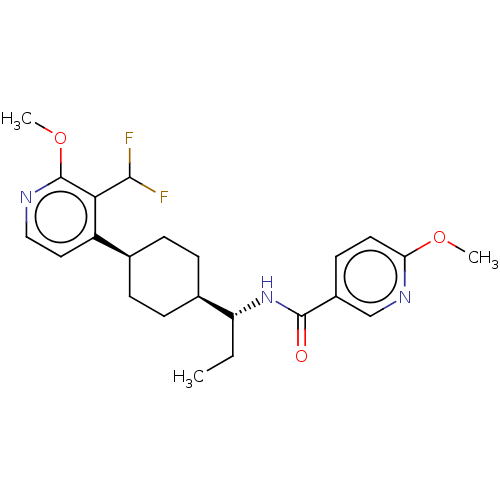
(CHEMBL4848094)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc(OC)c1C(F)F)[C@@H](CC)NC(=O)c1ccc(OC)nc1 |r,wU:4.7,18.20,wD:1.0,(34.27,-3.48,;34.68,-4.96,;33.36,-4.19,;32.03,-4.96,;32.03,-6.5,;33.36,-7.27,;34.68,-6.5,;30.7,-7.28,;29.36,-6.51,;28.03,-7.28,;28.03,-8.82,;29.37,-9.58,;29.38,-11.12,;28.06,-11.89,;30.7,-8.81,;32.03,-9.57,;33.36,-8.8,;32.04,-11.11,;36.02,-4.2,;36.03,-2.66,;37.36,-1.9,;37.35,-4.97,;37.35,-6.51,;36.01,-7.28,;38.68,-7.29,;40.01,-6.52,;41.34,-7.29,;41.34,-8.84,;42.68,-9.61,;42.68,-11.14,;40,-9.6,;38.67,-8.83,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00236
BindingDB Entry DOI: 10.7270/Q2KS6W9W |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50571880
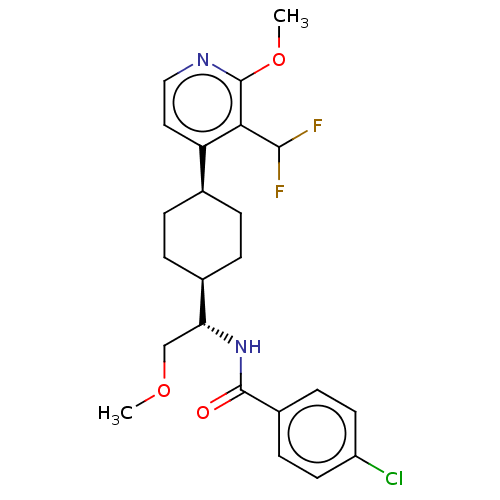
(CHEMBL4851220)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc(OC)c1C(F)F)[C@@H](COC)NC(=O)c1ccc(Cl)cc1 |r,wU:4.7,18.20,wD:1.0,(10.11,-4.67,;10.52,-6.16,;9.2,-5.38,;7.87,-6.16,;7.87,-7.7,;9.2,-8.46,;10.52,-7.7,;6.54,-8.47,;5.2,-7.7,;3.86,-8.47,;3.87,-10.02,;5.21,-10.77,;5.22,-12.31,;3.9,-13.08,;6.54,-10,;7.87,-10.77,;9.2,-10,;7.88,-12.3,;11.86,-5.39,;11.86,-3.85,;13.2,-3.09,;13.2,-1.56,;13.19,-6.16,;13.19,-7.71,;11.85,-8.48,;14.52,-8.48,;14.51,-10.02,;15.84,-10.79,;17.18,-10.03,;18.52,-10.8,;17.18,-8.48,;15.85,-7.71,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00236
BindingDB Entry DOI: 10.7270/Q2KS6W9W |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50571881
(CHEMBL4845988)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc(OC)c1C(F)F)[C@@H](CC)NC(=O)c1ccc(Cl)cc1 |r,wU:4.7,18.20,wD:1.0,(66.33,-47.56,;66.74,-49.04,;65.42,-48.27,;64.08,-49.04,;64.08,-50.59,;65.42,-51.35,;66.74,-50.59,;62.75,-51.36,;61.41,-50.59,;60.08,-51.36,;60.08,-52.91,;61.43,-53.66,;61.44,-55.2,;60.12,-55.97,;62.76,-52.89,;64.09,-53.65,;65.41,-52.88,;64.09,-55.19,;68.08,-48.28,;68.08,-46.74,;69.41,-45.98,;69.41,-49.05,;69.41,-50.59,;68.07,-51.36,;70.74,-51.37,;70.73,-52.91,;72.06,-53.68,;73.4,-52.92,;74.73,-53.69,;73.4,-51.37,;72.07,-50.6,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00236
BindingDB Entry DOI: 10.7270/Q2KS6W9W |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
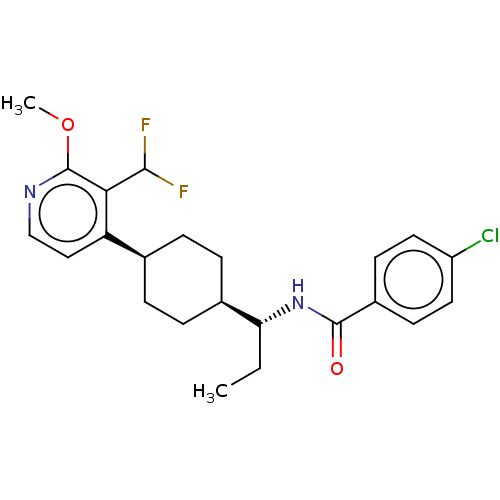
(Homo sapiens (Human)) | BDBM50571883

(CHEMBL4868709)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc(OC)c1C)[C@@H](CC)NC(=O)c1ccc(Cl)cc1 |r,wU:4.7,16.18,wD:1.0,(28.06,-49.01,;28.46,-50.5,;27.14,-49.72,;25.81,-50.5,;25.81,-52.04,;27.14,-52.81,;28.46,-52.04,;24.48,-52.82,;23.14,-52.04,;21.81,-52.82,;21.81,-54.36,;23.16,-55.12,;23.17,-56.65,;21.84,-57.42,;24.48,-54.34,;25.81,-55.11,;29.8,-49.73,;29.81,-48.19,;31.14,-47.43,;31.14,-50.51,;31.13,-52.05,;29.79,-52.82,;32.46,-52.82,;32.45,-54.36,;33.78,-55.13,;35.12,-54.37,;36.46,-55.14,;35.12,-52.82,;33.79,-52.05,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00236
BindingDB Entry DOI: 10.7270/Q2KS6W9W |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
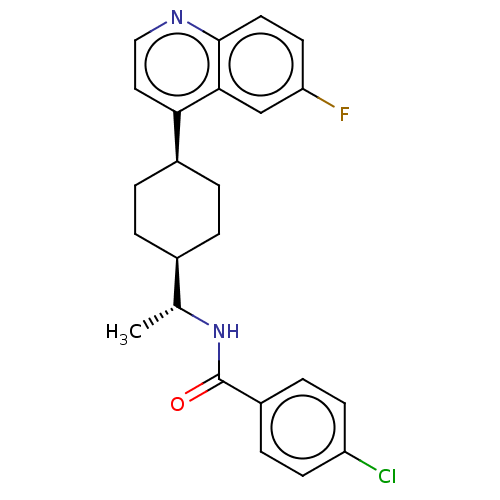
(Mus musculus) | BDBM50550026
(CHEMBL4786690)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)NC(=O)c1ccc(Cl)cc1 |r,wU:4.7,18.21,wD:1.0,(55.56,-25.24,;55.58,-26.78,;54.24,-26.01,;52.9,-26.78,;52.91,-28.32,;54.25,-29.09,;55.57,-28.32,;51.57,-29.08,;50.24,-28.32,;48.91,-29.1,;48.92,-30.63,;50.25,-31.38,;50.25,-32.93,;51.58,-33.7,;52.92,-32.93,;54.25,-33.69,;52.91,-31.38,;51.58,-30.61,;56.91,-26.01,;56.91,-24.47,;58.24,-26.78,;58.24,-28.32,;56.9,-29.08,;59.58,-29.09,;59.57,-30.63,;60.9,-31.4,;62.24,-30.63,;63.57,-31.4,;62.23,-29.09,;60.9,-28.32,)| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated mouse M109 cells preincubated for 2 hrs followed by recombinant murine IFNgamma stimulation and measured af... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00236
BindingDB Entry DOI: 10.7270/Q2KS6W9W |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50550026

(CHEMBL4786690)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)NC(=O)c1ccc(Cl)cc1 |r,wU:4.7,18.21,wD:1.0,(55.56,-25.24,;55.58,-26.78,;54.24,-26.01,;52.9,-26.78,;52.91,-28.32,;54.25,-29.09,;55.57,-28.32,;51.57,-29.08,;50.24,-28.32,;48.91,-29.1,;48.92,-30.63,;50.25,-31.38,;50.25,-32.93,;51.58,-33.7,;52.92,-32.93,;54.25,-33.69,;52.91,-31.38,;51.58,-30.61,;56.91,-26.01,;56.91,-24.47,;58.24,-26.78,;58.24,-28.32,;56.9,-29.08,;59.58,-29.09,;59.57,-30.63,;60.9,-31.4,;62.24,-30.63,;63.57,-31.4,;62.23,-29.09,;60.9,-28.32,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by recombinant human IFNgamma stimulation and measured aft... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00236
BindingDB Entry DOI: 10.7270/Q2KS6W9W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
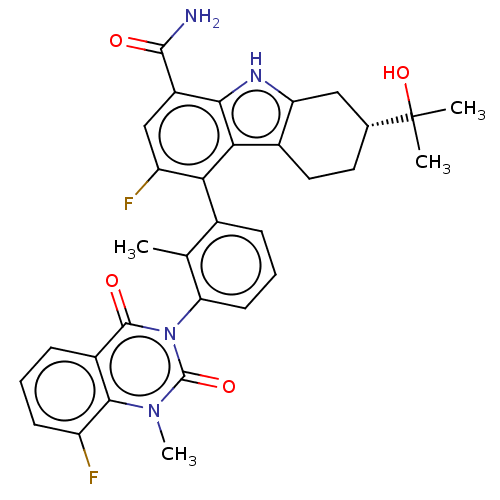
(Homo sapiens (Human)) | BDBM230103
(US10106559, Example 27 | US10435415, Example 27 | ...)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(F)cc(C(N)=O)c2[nH]c3C[C@@H](CCc3c12)C(C)(C)O |r,wU:33.43,(2.75,,;1.42,-.77,;.08,,;-1.25,-.77,;-1.25,-2.31,;.08,-3.08,;1.42,-2.31,;2.75,-3.08,;2.75,-4.62,;1.42,-5.39,;4.09,-5.39,;4.09,-6.93,;5.42,-4.62,;6.75,-5.39,;6.75,-6.93,;8.09,-4.62,;8.09,-3.08,;6.75,-2.31,;5.42,-3.08,;4.09,-2.31,;4.09,-.77,;.08,1.54,;1.42,2.31,;2.75,1.54,;1.42,3.85,;.08,4.62,;.08,6.16,;1.42,6.93,;-1.25,6.93,;-1.25,3.85,;-2.71,4.33,;-3.62,3.08,;-5.15,2.92,;-5.78,1.51,;-4.87,.27,;-3.34,.43,;-2.71,1.83,;-1.25,2.31,;-7.32,1.51,;-8.09,.18,;-6.55,.18,;-8.09,2.85,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50571879

(CHEMBL4848094)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc(OC)c1C(F)F)[C@@H](CC)NC(=O)c1ccc(OC)nc1 |r,wU:4.7,18.20,wD:1.0,(34.27,-3.48,;34.68,-4.96,;33.36,-4.19,;32.03,-4.96,;32.03,-6.5,;33.36,-7.27,;34.68,-6.5,;30.7,-7.28,;29.36,-6.51,;28.03,-7.28,;28.03,-8.82,;29.37,-9.58,;29.38,-11.12,;28.06,-11.89,;30.7,-8.81,;32.03,-9.57,;33.36,-8.8,;32.04,-11.11,;36.02,-4.2,;36.03,-2.66,;37.36,-1.9,;37.35,-4.97,;37.35,-6.51,;36.01,-7.28,;38.68,-7.29,;40.01,-6.52,;41.34,-7.29,;41.34,-8.84,;42.68,-9.61,;42.68,-11.14,;40,-9.6,;38.67,-8.83,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma/LPS-stimulated human whole blood preincubated for 4 hrs followed by IFNgamma/LPS stimulation and incubated for 18 hrs ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00236
BindingDB Entry DOI: 10.7270/Q2KS6W9W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
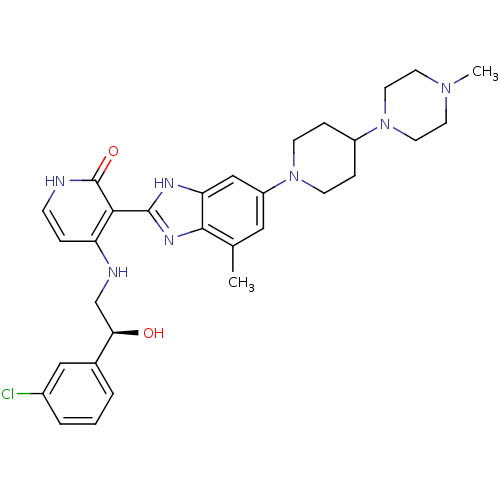
(Homo sapiens (Human)) | BDBM50252296
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CN1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C31H38ClN7O2/c1-20-16-24(38-10-7-23(8-11-38)39-14-12-37(2)13-15-39)18-26-29(20)36-30(35-26)28-25(6-9-33-31(28)41)34-19-27(40)21-4-3-5-22(32)17-21/h3-6,9,16-18,23,27,40H,7-8,10-15,19H2,1-2H3,(H,35,36)(H2,33,34,41)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252302

((S)-2-methoxyethyl 4-(1-(2-(4-(2-(3-chlorophenyl)-...)Show SMILES COCCOC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C34H42ClN7O5/c1-22-18-26(40-10-7-25(8-11-40)41-12-14-42(15-13-41)34(45)47-17-16-46-2)20-28-31(22)39-32(38-28)30-27(6-9-36-33(30)44)37-21-29(43)23-4-3-5-24(35)19-23/h3-6,9,18-20,25,29,43H,7-8,10-17,21H2,1-2H3,(H,38,39)(H2,36,37,44)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
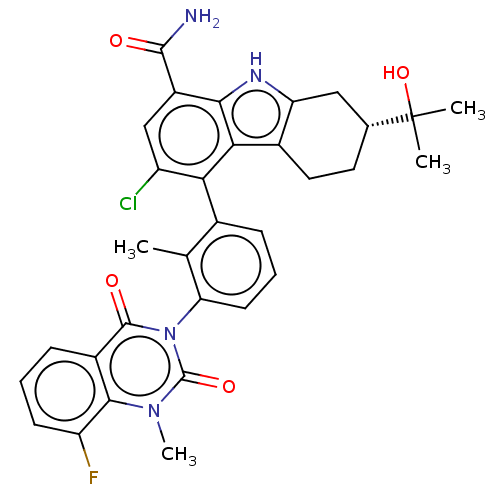
(Homo sapiens (Human)) | BDBM50194723
(CHEMBL3944049)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3C[C@@H](CCc3c12)C(C)(C)O |r,wU:33.43,(73.77,-22.36,;72.44,-23.13,;71.11,-22.37,;69.77,-23.14,;69.77,-24.68,;71.11,-25.45,;72.45,-24.67,;73.78,-25.43,;73.78,-26.97,;72.44,-27.74,;75.1,-27.74,;75.11,-29.28,;76.44,-26.97,;77.77,-27.73,;77.77,-29.27,;79.1,-26.96,;79.09,-25.42,;77.76,-24.66,;76.44,-25.43,;75.1,-24.66,;75.09,-23.12,;71.11,-20.83,;72.45,-20.06,;73.78,-20.83,;72.44,-18.51,;71.1,-17.75,;71.1,-16.21,;72.43,-15.44,;69.77,-15.44,;69.78,-18.52,;68.31,-18.04,;67.4,-19.28,;65.87,-19.44,;65.23,-20.84,;66.14,-22.1,;67.67,-21.94,;68.3,-20.54,;69.77,-20.06,;63.73,-20.44,;62.63,-19.34,;64.13,-18.94,;62.64,-21.53,)| Show InChI InChI=1S/C32H30ClFN4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-22(34)28(19)37(4)31(38)41)25-21(33)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-10,14,16,36,42H,11-13H2,1-4H3,(H2,35,39)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194724
(CHEMBL3941224)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2ccccc2c1=O)-c1ccc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(15.19,-30.14,;13.85,-30.89,;12.53,-30.1,;11.19,-30.85,;11.16,-32.39,;12.48,-33.18,;13.83,-32.42,;15.15,-33.2,;15.13,-34.74,;13.78,-35.5,;16.45,-35.53,;16.43,-37.08,;17.79,-34.77,;19.11,-35.56,;20.45,-34.81,;20.47,-33.27,;19.15,-32.49,;17.82,-33.24,;16.49,-32.44,;16.51,-30.91,;12.56,-28.57,;13.91,-27.82,;13.92,-26.27,;12.6,-25.48,;12.62,-23.95,;13.96,-23.19,;11.29,-23.16,;11.26,-26.24,;9.79,-25.73,;8.86,-26.97,;7.33,-27.11,;6.68,-28.5,;7.55,-29.76,;9.09,-29.64,;9.75,-28.24,;11.23,-27.78,;5.14,-28.62,;3.74,-27.97,;5.01,-27.08,;4.49,-30.01,)| Show InChI InChI=1S/C32H28N4O4/c1-17-19(9-7-11-25(17)36-30(38)22-8-5-6-10-26(22)35(4)31(36)39)20-14-15-23(29(33)37)28-27(20)21-13-12-18(32(2,3)40)16-24(21)34-28/h5-16,34,40H,1-4H3,(H2,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data