

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200013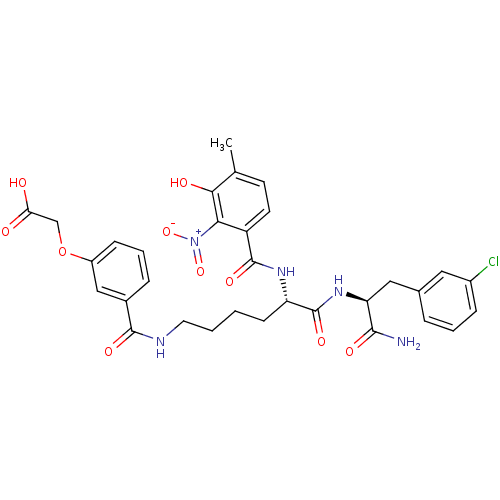 (2-(3-(((S)-6-((S)-1-amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317145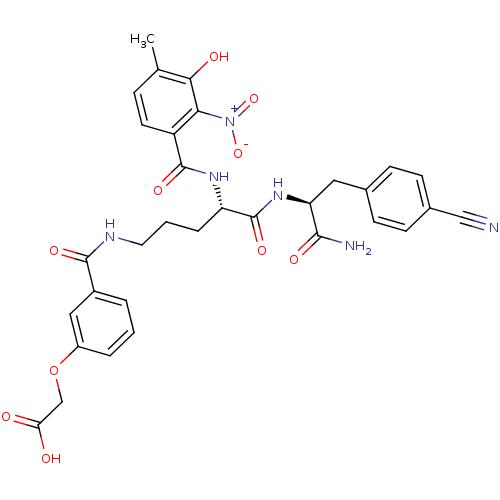 (2-(3-((S)-5-((S)-1-amino-3-(4-cyanophenyl)-1-oxopr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317147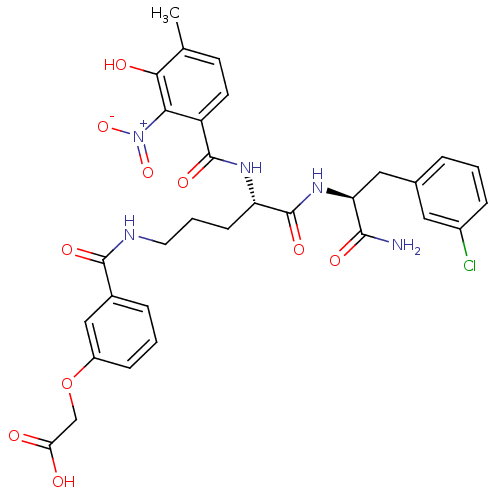 (2-(3-(-(S)-5-((S)-1-Amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200013 (2-(3-(((S)-6-((S)-1-amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317142 (2-(3-((S)-5-((S)-1-amino-3-(3-hydroxy-4-nitropheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317142 (2-(3-((S)-5-((S)-1-amino-3-(3-hydroxy-4-nitropheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317145 (2-(3-((S)-5-((S)-1-amino-3-(4-cyanophenyl)-1-oxopr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317149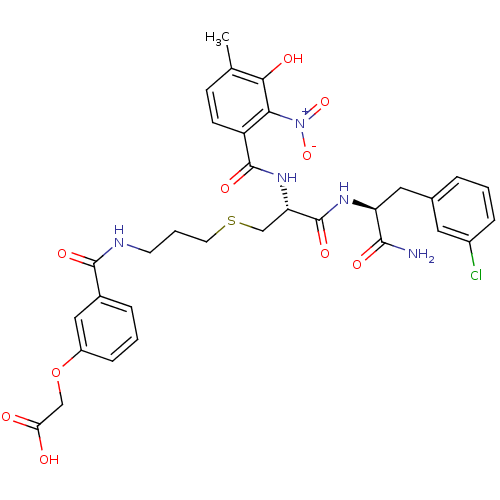 (2-(3-(3-((R)-3-((S)-1-Amino-3-(3-chlorophenyl)-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317141 (2-(3-((4S)-5-(2-(6-(3-amino-3-oxopropyl)dibenzo[b,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317150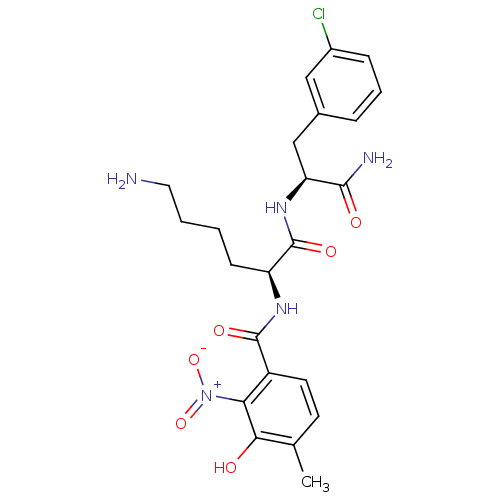 (CHEMBL1088356 | N-((S)-6-Amino-1-((S)-1-amino-3-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317149 (2-(3-(3-((R)-3-((S)-1-Amino-3-(3-chlorophenyl)-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317143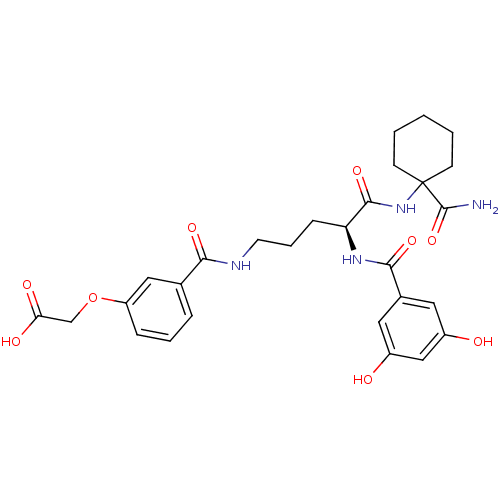 ((S)-2-(3-(5-(1-carbamoylcyclohexylamino)-4-(3,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317144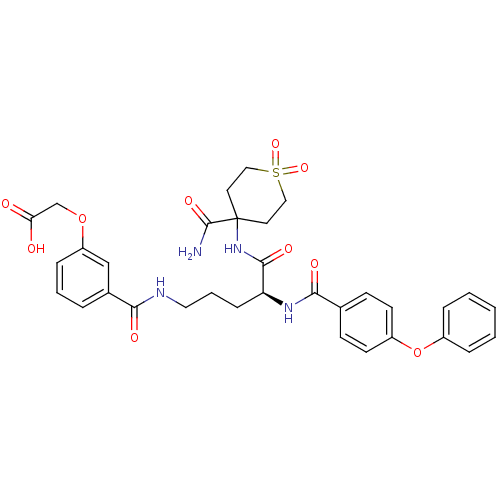 (CHEMBL1094071 | {3-[(S)-4-(4-Carbamoyl-1,1-dioxo-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317141 (2-(3-((4S)-5-(2-(6-(3-amino-3-oxopropyl)dibenzo[b,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317148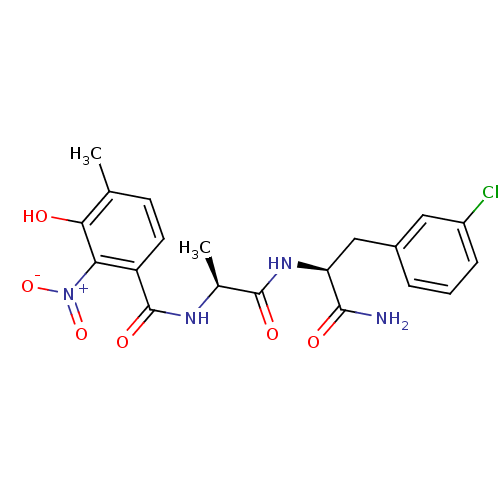 (CHEMBL1094717 | N-((S)-1-((S)-1-Amino-3-(3-chlorop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200003 (3-(carboxymethoxy)benzoic acid | CHEMBL216545) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50200013 (2-(3-(((S)-6-((S)-1-amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317141 (2-(3-((4S)-5-(2-(6-(3-amino-3-oxopropyl)dibenzo[b,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317145 (2-(3-((S)-5-((S)-1-amino-3-(4-cyanophenyl)-1-oxopr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM50185232 ((S)-2-((S)-3-(1H-imidazol-4-yl)-2-(3-phenylpropana...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human serine racemase | J Med Chem 49: 2388-97 (2006) Article DOI: 10.1021/jm050701c BindingDB Entry DOI: 10.7270/Q2V124C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317147 (2-(3-(-(S)-5-((S)-1-Amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317143 ((S)-2-(3-(5-(1-carbamoylcyclohexylamino)-4-(3,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317146 ((S)-2-(3-(4-(3-benzoylpicolinamido)-5-(1-carbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317146 ((S)-2-(3-(4-(3-benzoylpicolinamido)-5-(1-carbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317148 (CHEMBL1094717 | N-((S)-1-((S)-1-Amino-3-(3-chlorop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200003 (3-(carboxymethoxy)benzoic acid | CHEMBL216545) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 5.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317144 (CHEMBL1094071 | {3-[(S)-4-(4-Carbamoyl-1,1-dioxo-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine racemase (Homo sapiens (Human)) | BDBM50185231 ((2S,3R)-2-((S)-3-(1H-imidazol-4-yl)-2-(3-phenylpro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human serine racemase | J Med Chem 49: 2388-97 (2006) Article DOI: 10.1021/jm050701c BindingDB Entry DOI: 10.7270/Q2V124C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200006 (2-(3-carbamoylphenoxy)acetic acid | CHEMBL216601 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 7.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50200013 (2-(3-(((S)-6-((S)-1-amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317150 (CHEMBL1088356 | N-((S)-6-Amino-1-((S)-1-amino-3-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50317147 (2-(3-(-(S)-5-((S)-1-Amino-3-(3-chlorophenyl)-1-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317144 (CHEMBL1094071 | {3-[(S)-4-(4-Carbamoyl-1,1-dioxo-h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50317146 ((S)-2-(3-(4-(3-benzoylpicolinamido)-5-(1-carbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.08E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50317143 ((S)-2-(3-(5-(1-carbamoylcyclohexylamino)-4-(3,5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50200003 (3-(carboxymethoxy)benzoic acid | CHEMBL216545) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50317149 (2-(3-(3-((R)-3-((S)-1-Amino-3-(3-chlorophenyl)-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Anthranilate synthase component 1 (Salmonella typhimurium) | BDBM50200006 (2-(3-carbamoylphenoxy)acetic acid | CHEMBL216601 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 2.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Salmonella typhimurium anthranilate synthase trpE:trpD expressed in Escherichia coli BL21(DE3) coexpressing stop codon in trpD gene for... | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isochorismate synthase EntC (Escherichia coli (strain K12)) | BDBM50200006 (2-(3-carbamoylphenoxy)acetic acid | CHEMBL216601 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 2.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 IS expressed in Escherichia coli BL21(DE3) by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50317150 (CHEMBL1088356 | N-((S)-6-Amino-1-((S)-1-amino-3-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminodeoxychorismate lyase (Escherichia coli (strain K12)) | BDBM50317148 (CHEMBL1094717 | N-((S)-1-((S)-1-Amino-3-(3-chlorop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of Escherichia coli K12 ADCS by spectrophotometry | J Med Chem 53: 3718-29 (2010) Article DOI: 10.1021/jm100158v BindingDB Entry DOI: 10.7270/Q25T3KN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088982 (CHEMBL160253 | CHEMBL367004 | N-[1-(1-Carbamimidoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088978 (CHEMBL176515 | N-[1-(1-Carbamimidoyl-2-hydroxy-pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088984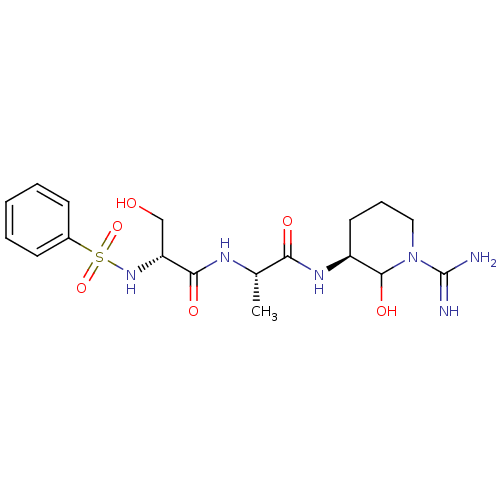 (2-Benzenesulfonylamino-N-[1-(1-carbamimidoyl-2-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088979 (CHEMBL366666 | {(R)-2-[2-((S)-1-Carbamimidoyl-2-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088985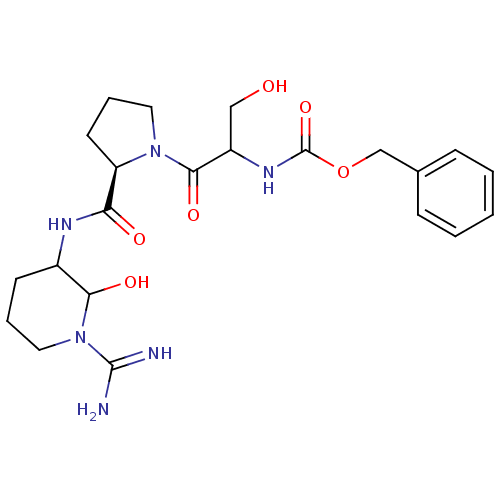 (CHEMBL369042 | {2-[2-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Mus musculus (Mouse)) | BDBM217352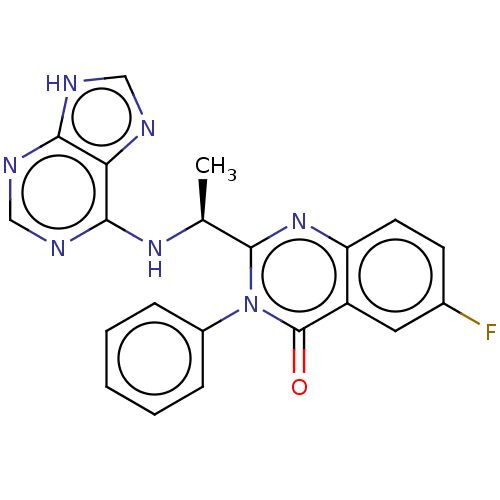 (GS-9820) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Western University | Assay Description Biochemical in vitro lipid kinase assays were performed by the SelectScreenŽ biochemical kinase assay service (Invitrogen Ltd.). A stock solution of ... | J Biol Chem 288: 35346-57 (2013) Article DOI: 10.1074/jbc.M113.507525 BindingDB Entry DOI: 10.7270/Q28051F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088977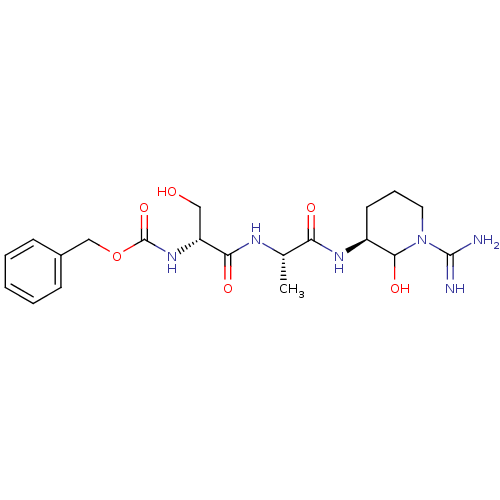 (CHEMBL177557 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088987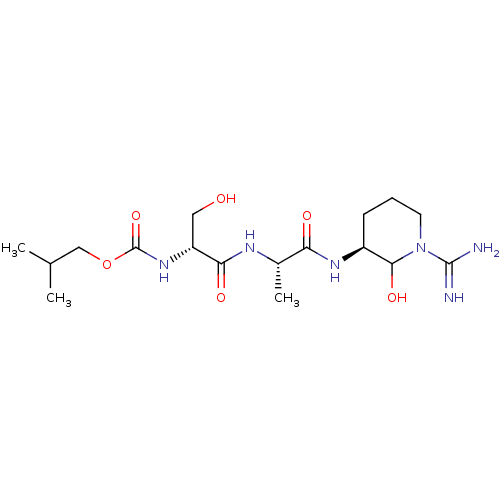 (CHEMBL174813 | {1-[1-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50088986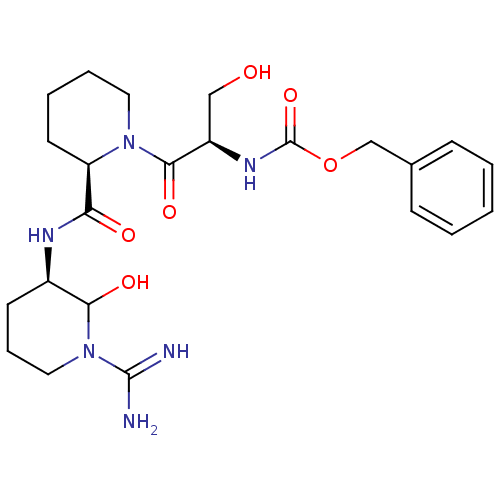 (CHEMBL433809 | {2-[2-(1-Carbamimidoyl-2-hydroxy-pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Corvas International, Inc. Curated by ChEMBL | Assay Description The compound was tested in vitro for its inhibitory activity against human urokinase enzyme, activity expressed as IC50 | Bioorg Med Chem Lett 10: 983-7 (2000) BindingDB Entry DOI: 10.7270/Q2765DKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 190 total ) | Next | Last >> |