 Found 970 hits with Last Name = 'doi' and Initial = 'm'
Found 970 hits with Last Name = 'doi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50076089

((3S,3aR,4R,4aS,8aR,9aS)-3-Methyl-4-[2-((R)-1-methy...)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M2 (brainstem) subtype was evaluated using [3H]quinuclidinyl benzilate |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50076089

((3S,3aR,4R,4aS,8aR,9aS)-3-Methyl-4-[2-((R)-1-methy...)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M2 muscarinic receptor in homogenates of the brainstem of rat using [3H]quinuclidinyl b... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50076089

((3S,3aR,4R,4aS,8aR,9aS)-3-Methyl-4-[2-((R)-1-methy...)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M1 (cortex) subtype was evaluated using [3H]pirenzepine |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50076089

((3S,3aR,4R,4aS,8aR,9aS)-3-Methyl-4-[2-((R)-1-methy...)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M1 muscarinic receptor in homogenates of the cerebral cortex of rat using [3H]pirenzepi... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403996
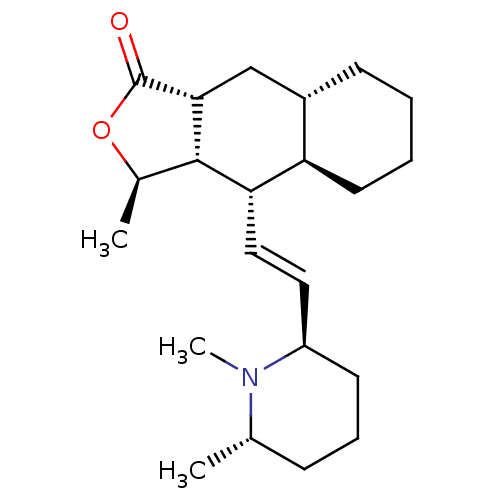
(CHEMBL97765)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15+,16-,17+,18+,19-,20+,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M2 (brainstem) subtype was evaluated using [3H]quinuclidinyl benzilate |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403998
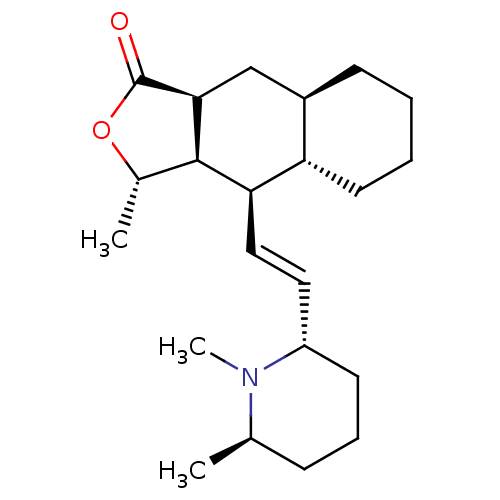
(CHEMBL318849)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@@H]3CCC[C@@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15+,16-,17+,18+,19-,20+,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M2 (brainstem) subtype was evaluated using [3H]quinuclidinyl benzilate |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403998

(CHEMBL318849)Show SMILES C[C@@H]1OC(=O)[C@H]2C[C@H]3CCCC[C@@H]3[C@@H](\C=C\[C@@H]3CCC[C@@H](C)N3C)[C@@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15+,16-,17+,18+,19-,20+,21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M1 (cortex) subtype was evaluated using [3H]pirenzepine |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403996

(CHEMBL97765)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\[C@H]3CCC[C@H](C)N3C)[C@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15+,16-,17+,18+,19-,20+,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M1 (cortex) subtype was evaluated using [3H]pirenzepine |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50404003
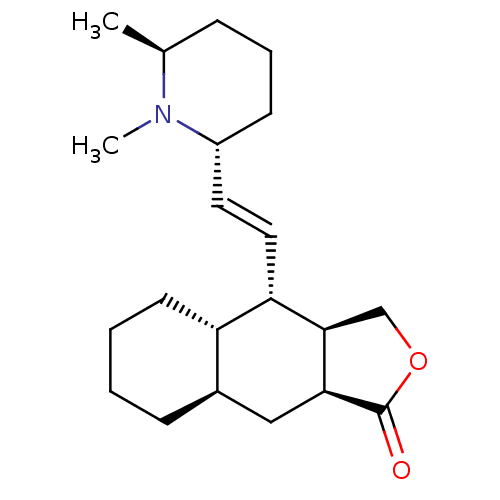
(CHEMBL324725)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18-,19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M2 muscarinic receptor in homogenates of the brainstem of rat using [3H]quinuclidinyl b... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50404003

(CHEMBL324725)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18-,19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M2 muscarinic receptor in homogenates of the brainstem of rat using [3H]quinuclidinyl b... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50404003

(CHEMBL324725)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18-,19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M1 muscarinic receptor in homogenates of the cerebral cortex of rat using [3H]pirenzepi... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50404003

(CHEMBL324725)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18-,19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M1 muscarinic receptor in homogenates of the cerebral cortex of rat using [3H]pirenzepi... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50404004
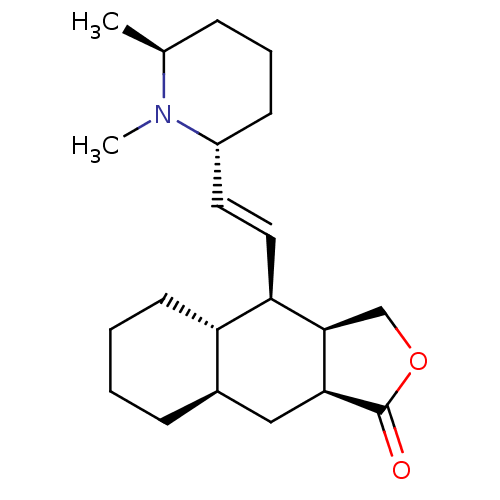
(CHEMBL111224)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18+,19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M2 muscarinic receptor in homogenates of the brainstem of rat using [3H]quinuclidinyl b... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50404004

(CHEMBL111224)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18+,19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M2 muscarinic receptor in homogenates of the brainstem of rat using [3H]quinuclidinyl b... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403997
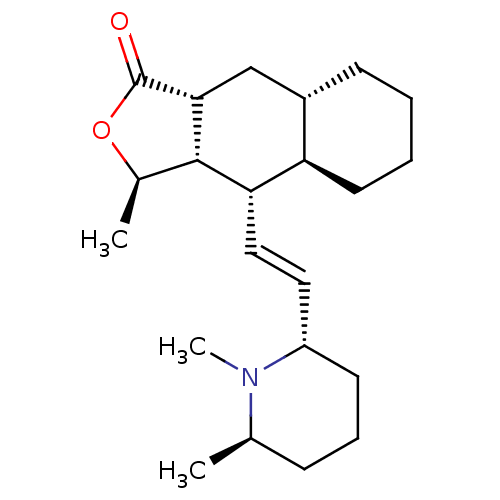
(CHEMBL99618)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\[C@@H]3CCC[C@@H](C)N3C)[C@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M2 (brainstem) subtype was evaluated using [3H]quinuclidinyl benzilate |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50404004

(CHEMBL111224)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18+,19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 999 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M1 muscarinic receptor in homogenates of the cerebral cortex of rat using [3H]pirenzepi... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403997

(CHEMBL99618)Show SMILES C[C@H]1OC(=O)[C@@H]2C[C@@H]3CCCC[C@H]3[C@H](\C=C\[C@@H]3CCC[C@@H](C)N3C)[C@H]12 Show InChI InChI=1S/C22H35NO2/c1-14-7-6-9-17(23(14)3)11-12-19-18-10-5-4-8-16(18)13-20-21(19)15(2)25-22(20)24/h11-12,14-21H,4-10,13H2,1-3H3/b12-11+/t14-,15-,16+,17+,18-,19+,20-,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Company Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity against the muscarinic M1 (cortex) subtype was evaluated using [3H]pirenzepine |
Bioorg Med Chem Lett 12: 2871-3 (2002)
BindingDB Entry DOI: 10.7270/Q2QC04PJ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50404004

(CHEMBL111224)Show SMILES C[C@H]1CCC[C@H](\C=C\[C@H]2[C@H]3COC(=O)[C@H]3C[C@H]3CCCC[C@H]23)N1C Show InChI InChI=1S/C21H33NO2/c1-14-6-5-8-16(22(14)2)10-11-18-17-9-4-3-7-15(17)12-19-20(18)13-24-21(19)23/h10-11,14-20H,3-9,12-13H2,1-2H3/b11-10+/t14-,15+,16+,17-,18+,19-,20+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for binding activity against M1 muscarinic receptor in homogenates of the cerebral cortex of rat using [3H]pirenzepi... |
Bioorg Med Chem Lett 12: 3271-3 (2002)
BindingDB Entry DOI: 10.7270/Q2FT8N6H |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-9
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(BOVINE) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 1
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(BOVINE) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Natriuretic peptides A
(GUINEA PIG) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Voltage-dependent T-type calcium channel subunit alpha-1H
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid type B receptor subunit 1
(Rattus norvegicus (Rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Cavia porcellus (domestic guinea pig)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Insulin receptor substrate 1
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Interleukin-1 beta
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Pro-neuropeptide Y
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data