

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159843 (BDBM159925 | US9040528, 2 | US9040528, 2(b)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103538 (CHEMBL3357561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103539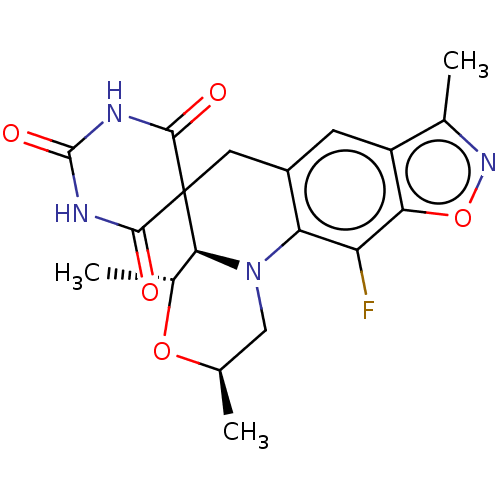 (CHEMBL3357982) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103540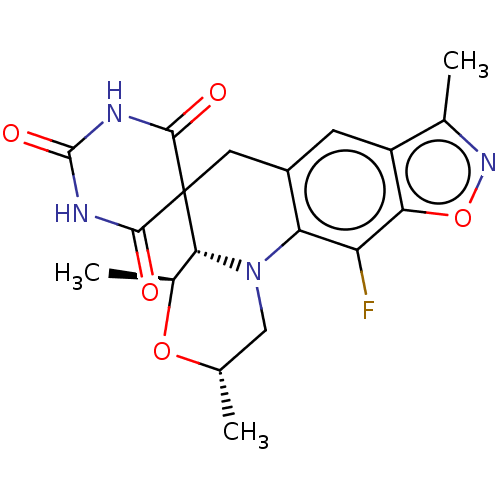 (CHEMBL3357983) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50102663 (CHEMBL3343037) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human AURKB incubated for 20 mins prior to MgCl2 addition measured after 90 mins by mobility shift assay | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102673 (CHEMBL3341771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159845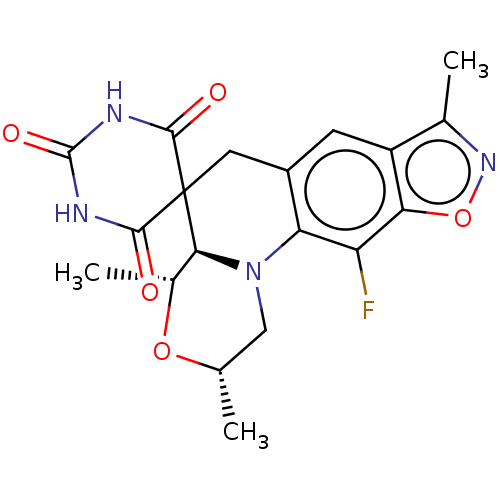 (US9040528, 2(a)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >83 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102677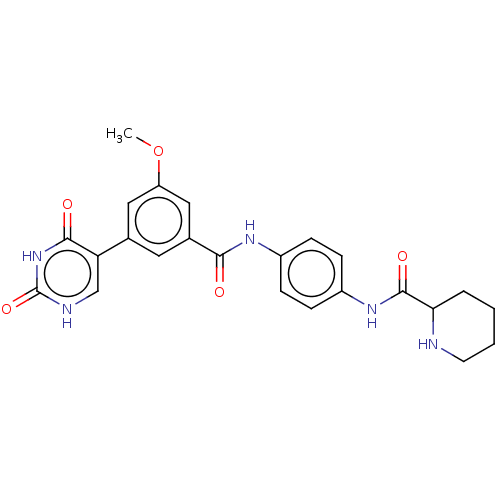 (CHEMBL3343057) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50102665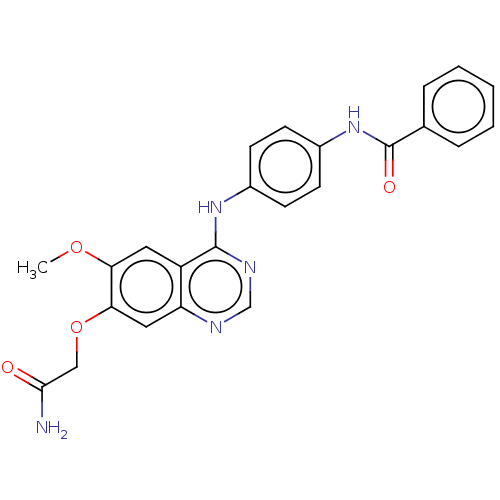 (CHEMBL3343039) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human AURKB incubated for 20 mins prior to MgCl2 addition measured after 90 mins by mobility shift assay | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM160047 (US9040528, 180) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159842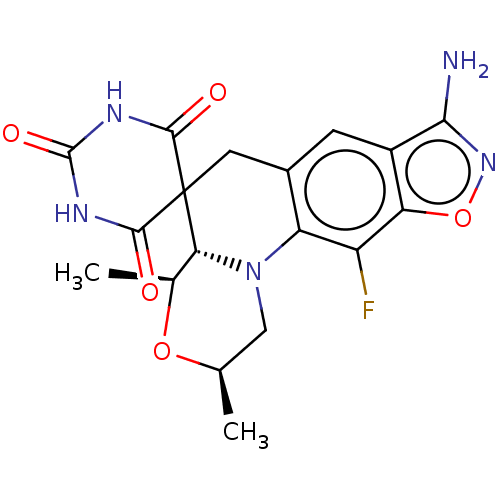 (US9040528, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102714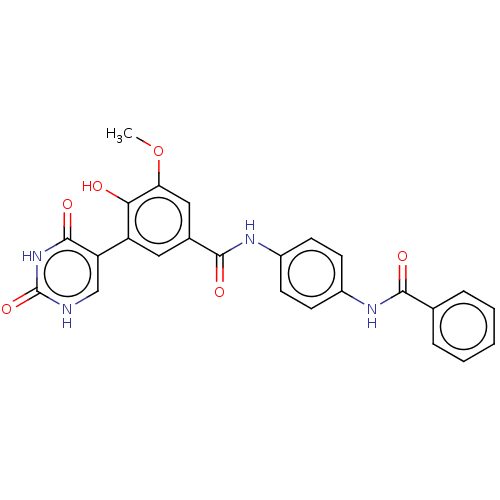 (CHEMBL3343050) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50102661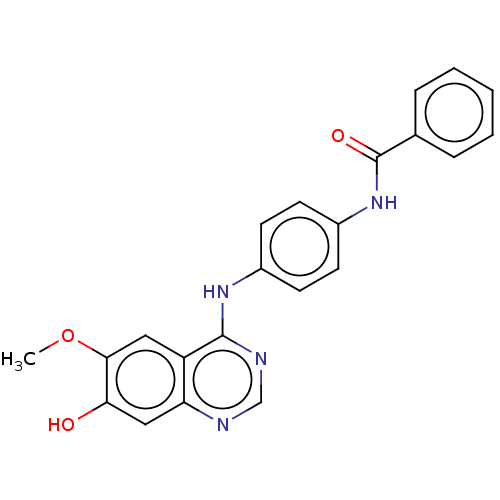 (CHEMBL3343033) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human AURKB incubated for 20 mins prior to MgCl2 addition measured after 90 mins by mobility shift assay | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103535 (CHEMBL3357988) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103541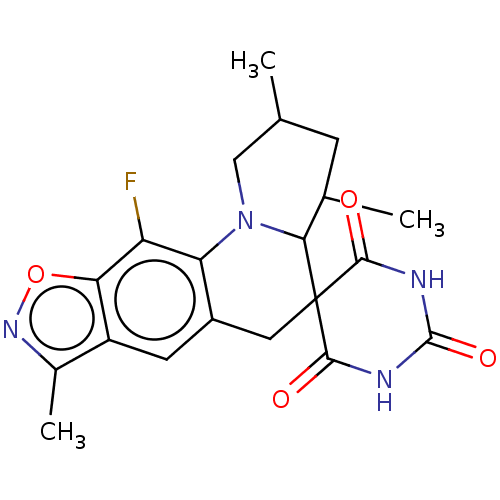 (CHEMBL3357990) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102674 (CHEMBL3343051) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102681 (CHEMBL3343041) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103537 (CHEMBL3357984) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159943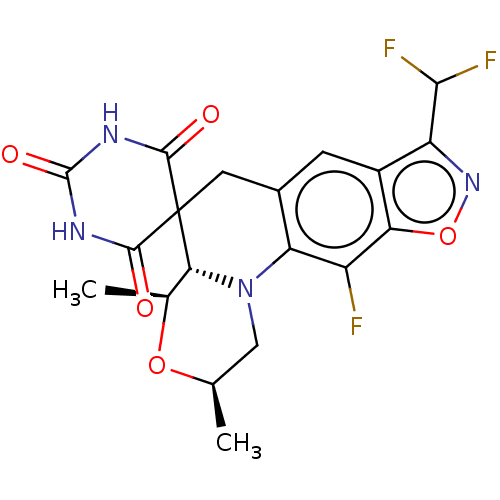 (US9040528, 93 | US9040528, 93(b)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50102667 (CHEMBL3343042) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 579 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human IRAK4 incubated for 20 mins prior to MgCl2 addition measured after 90 mins by mobility shift assay | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159940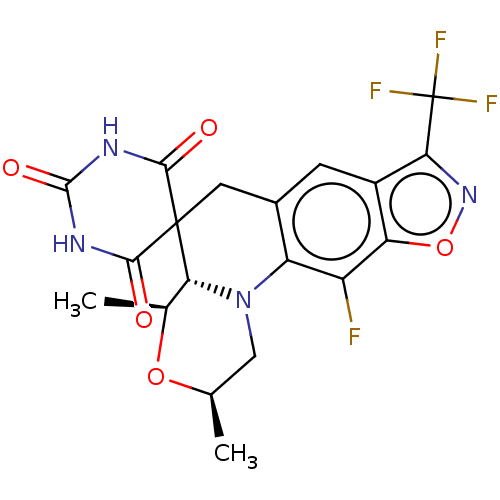 (US9040528, 92 | US9040528, 92(b)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102667 (CHEMBL3343042) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50245790 (4-amino-2-(butylthio)-8-(3,4-dichlorobenzyl)pterid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis murI assessed as effect on conversion of D-glutamate to L-glutamate at pH 8.0 by HPLC | Bioorg Med Chem Lett 18: 6100-3 (2008) Article DOI: 10.1016/j.bmcl.2008.10.022 BindingDB Entry DOI: 10.7270/Q2W66KM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50102664 (CHEMBL3343038) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 689 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human AURKB incubated for 20 mins prior to MgCl2 addition measured after 90 mins by mobility shift assay | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50245948 (2-(butylthio)-8-(4-fluorobenzyl)-4-(methylamino)pt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis murI assessed as effect on conversion of D-glutamate to L-glutamate at pH 8.0 by HPLC | Bioorg Med Chem Lett 18: 6100-3 (2008) Article DOI: 10.1016/j.bmcl.2008.10.022 BindingDB Entry DOI: 10.7270/Q2W66KM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM159895 (US9040528, 51) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102678 (CHEMBL3343058) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50263189 (9-(3-chloro-2,6-difluorobenzyl)-2-(butylthio)-9H-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis MurI | Bioorg Med Chem Lett 18: 4368-72 (2008) Article DOI: 10.1016/j.bmcl.2008.06.068 BindingDB Entry DOI: 10.7270/Q28052D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50245949 (2-(butylthio)-4-(dimethylamino)-8-(4-fluorobenzyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis murI assessed as effect on conversion of D-glutamate to L-glutamate at pH 8.0 by HPLC | Bioorg Med Chem Lett 18: 6100-3 (2008) Article DOI: 10.1016/j.bmcl.2008.10.022 BindingDB Entry DOI: 10.7270/Q2W66KM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102661 (CHEMBL3343033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50263105 (9-(2-methoxy-5-nitrobenzyl)-2-(butylthio)-9H-purin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis MurI | Bioorg Med Chem Lett 18: 4368-72 (2008) Article DOI: 10.1016/j.bmcl.2008.06.068 BindingDB Entry DOI: 10.7270/Q28052D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50245789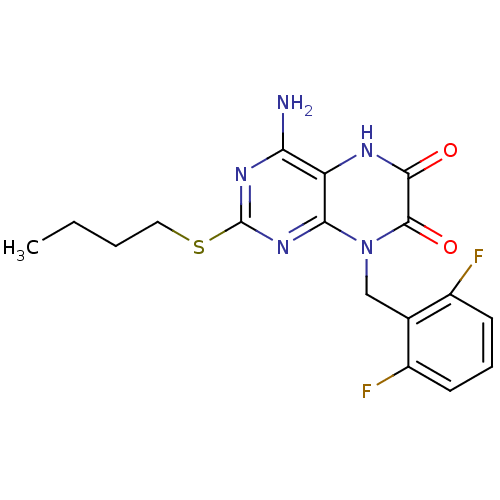 (4-amino-2-(butylthio)-8-(2,6-difluorobenzyl)pterid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis murI assessed as effect on conversion of D-glutamate to L-glutamate at pH 8.0 by HPLC | Bioorg Med Chem Lett 18: 6100-3 (2008) Article DOI: 10.1016/j.bmcl.2008.10.022 BindingDB Entry DOI: 10.7270/Q2W66KM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103536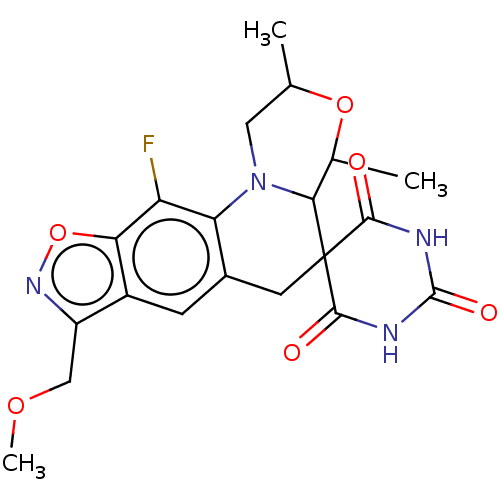 (CHEMBL3357987) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102676 (CHEMBL3343055) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50245788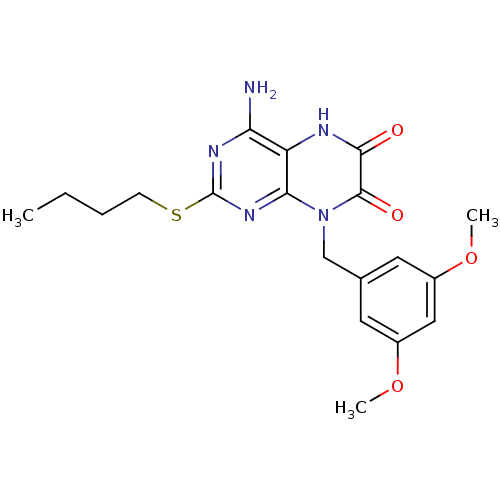 (4-amino-2-(butylthio)-8-(3,5-dimethoxybenzyl)pteri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis murI assessed as effect on conversion of D-glutamate to L-glutamate at pH 8.0 by HPLC | Bioorg Med Chem Lett 18: 6100-3 (2008) Article DOI: 10.1016/j.bmcl.2008.10.022 BindingDB Entry DOI: 10.7270/Q2W66KM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50246530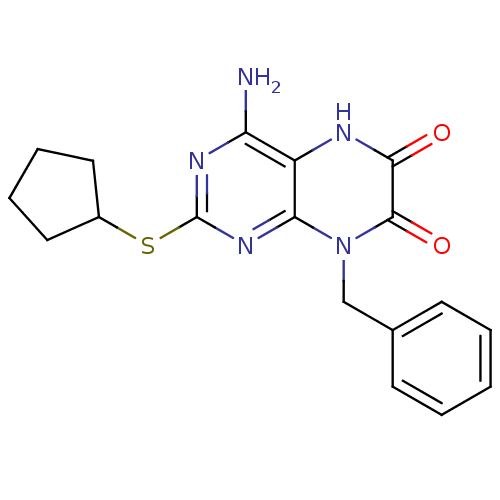 (4-amino-8-benzyl-2-(cyclopentylthio)pteridine-6,7(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis murI assessed as effect on conversion of D-glutamate to L-glutamate at pH 8.0 by HPLC | Bioorg Med Chem Lett 18: 6100-3 (2008) Article DOI: 10.1016/j.bmcl.2008.10.022 BindingDB Entry DOI: 10.7270/Q2W66KM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50263187 (9-(3-chloro-2,6-difluorobenzyl)-2-butoxy-N-(pyridi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis MurI | Bioorg Med Chem Lett 18: 4368-72 (2008) Article DOI: 10.1016/j.bmcl.2008.06.068 BindingDB Entry DOI: 10.7270/Q28052D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50245950 (2-(butylthio)-8-(4-fluorobenzyl)-4-(3-hydroxyazeti...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis murI assessed as effect on conversion of D-glutamate to L-glutamate at pH 8.0 by HPLC | Bioorg Med Chem Lett 18: 6100-3 (2008) Article DOI: 10.1016/j.bmcl.2008.10.022 BindingDB Entry DOI: 10.7270/Q2W66KM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102662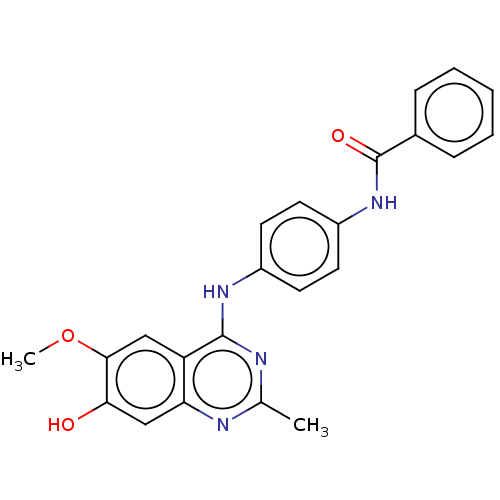 (CHEMBL3343034) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50263107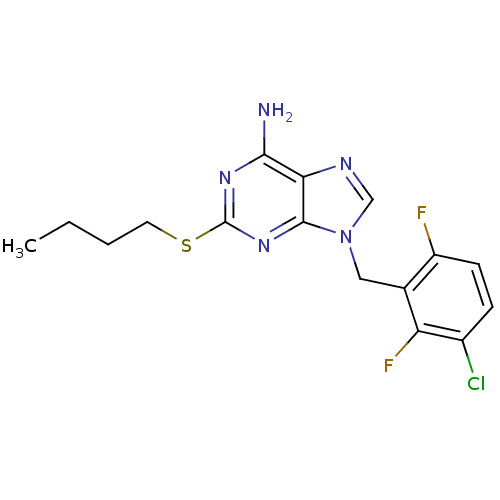 (9-(3-chloro-2,6-difluorobenzyl)-2-(butylthio)-9H-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis MurI | Bioorg Med Chem Lett 18: 4368-72 (2008) Article DOI: 10.1016/j.bmcl.2008.06.068 BindingDB Entry DOI: 10.7270/Q28052D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50246529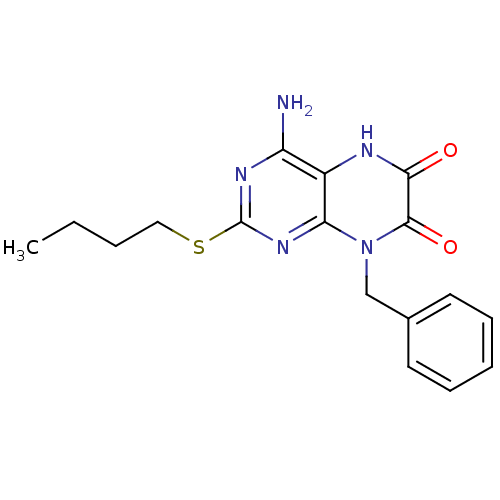 (4-amino-8-benzyl-2-(butylthio)pteridine-6,7(5H,8H)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis murI assessed as effect on conversion of D-glutamate to L-glutamate at pH 8.0 by HPLC | Bioorg Med Chem Lett 18: 6100-3 (2008) Article DOI: 10.1016/j.bmcl.2008.10.022 BindingDB Entry DOI: 10.7270/Q2W66KM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50245993 (2-(butylthio)-8-(4-fluorobenzyl)-4-(2-morpholinoet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis murI assessed as effect on conversion of D-glutamate to L-glutamate at pH 8.0 by HPLC | Bioorg Med Chem Lett 18: 6100-3 (2008) Article DOI: 10.1016/j.bmcl.2008.10.022 BindingDB Entry DOI: 10.7270/Q2W66KM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102679 (CHEMBL3343035) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102669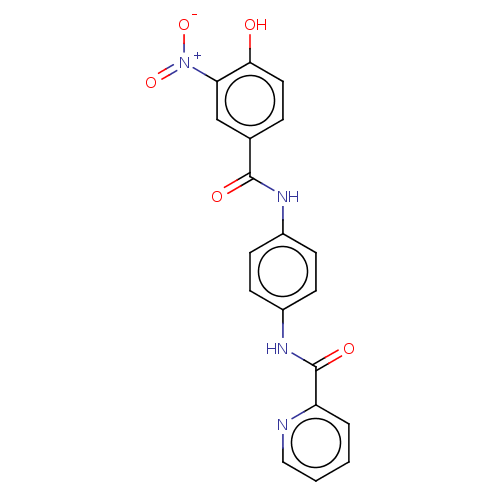 (CHEMBL3343045) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50245847 (4-amino-2-(butylthio)-8-(4-fluorobenzyl)pteridine-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis murI assessed as effect on conversion of D-glutamate to L-glutamate at pH 8.0 by HPLC | Bioorg Med Chem Lett 18: 6100-3 (2008) Article DOI: 10.1016/j.bmcl.2008.10.022 BindingDB Entry DOI: 10.7270/Q2W66KM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50103542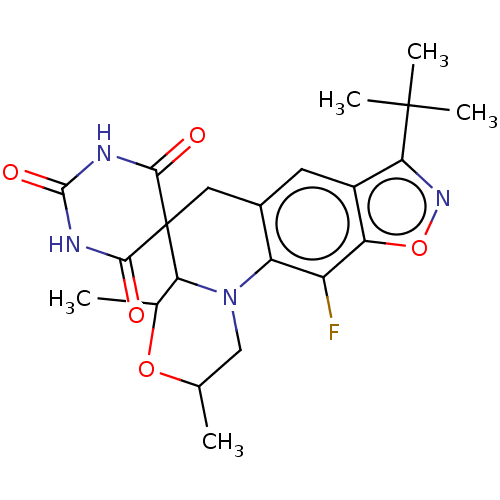 (CHEMBL3357989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase using fluorescence-tagged DNA by fluorescenct polarization assay | J Med Chem 57: 9078-95 (2014) Article DOI: 10.1021/jm501174m BindingDB Entry DOI: 10.7270/Q2F76FB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50263106 (9-(2,6-difluorobenzyl)-2-(butylthio)-9H-purin-6-am...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis MurI | Bioorg Med Chem Lett 18: 4368-72 (2008) Article DOI: 10.1016/j.bmcl.2008.06.068 BindingDB Entry DOI: 10.7270/Q28052D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional protein GlmU (Escherichia coli) | BDBM50102725 (CHEMBL3343056) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Escherichia coli ATCC 27325 GlmU expressed in Escherichia coli HMS174(DE3) incubated for 15 mins prior to MgCl2 addition measured after... | Bioorg Med Chem 22: 6256-69 (2014) Article DOI: 10.1016/j.bmc.2014.08.017 BindingDB Entry DOI: 10.7270/Q2SN0BR9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Enterococcus faecalis) | BDBM50263141 (9-(2,6-difluoro-3-methylbenzyl)-2-(1,1,1,2,2-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston Curated by ChEMBL | Assay Description Inhibition of Enterococcus faecalis MurI | Bioorg Med Chem Lett 18: 4368-72 (2008) Article DOI: 10.1016/j.bmcl.2008.06.068 BindingDB Entry DOI: 10.7270/Q28052D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 239 total ) | Next | Last >> |