

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50369460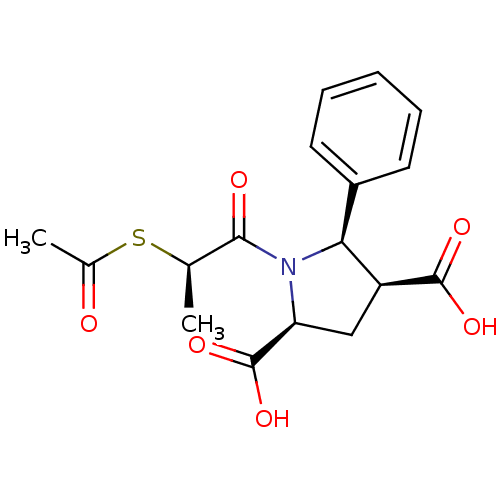 (CHEMBL1788109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Enzyme inhibitory activity towards Angiotensin I converting enzyme | J Med Chem 42: 3743-78 (1999) BindingDB Entry DOI: 10.7270/Q22Z167W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM21842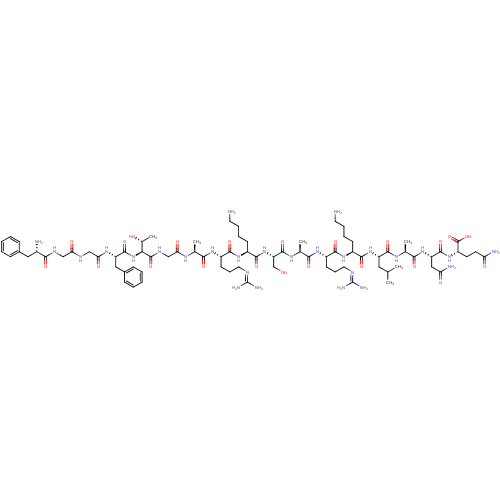 ((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071073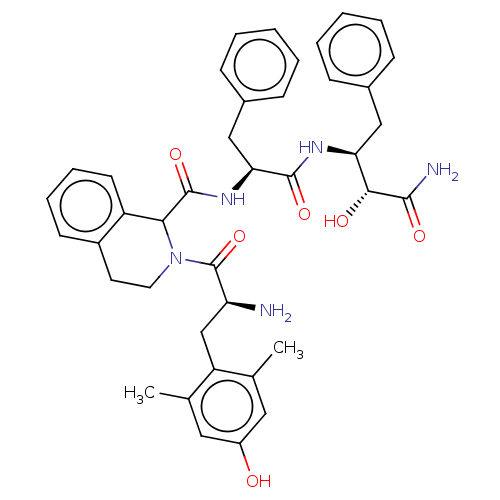 (CHEMBL3409763) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85194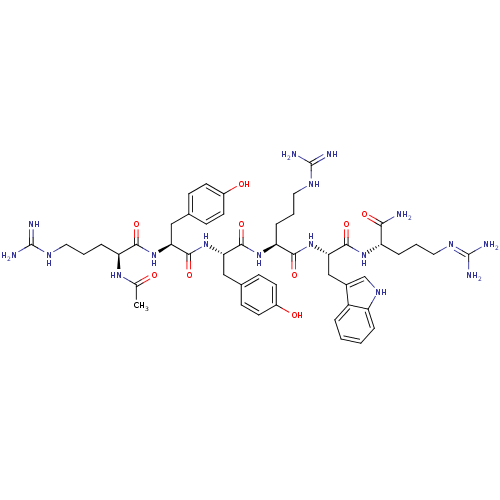 (Ac-RYYRWR-NH2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071072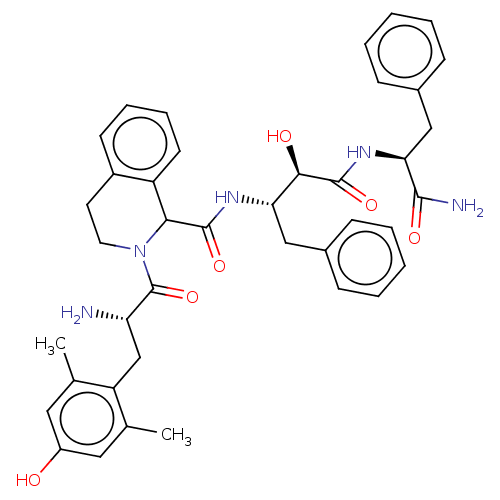 (CHEMBL3409762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85191 (Ac-RYYRWK-NH2 | CAS_200959-47-3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071071 (CHEMBL3409761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140041 (CHEMBL3765059) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071095 (CHEMBL3409766) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071094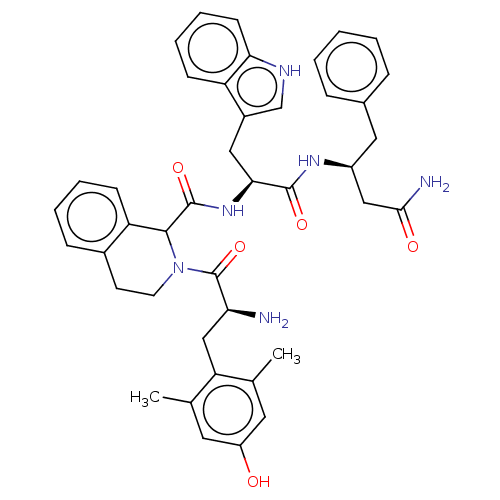 (CHEMBL3409765) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071070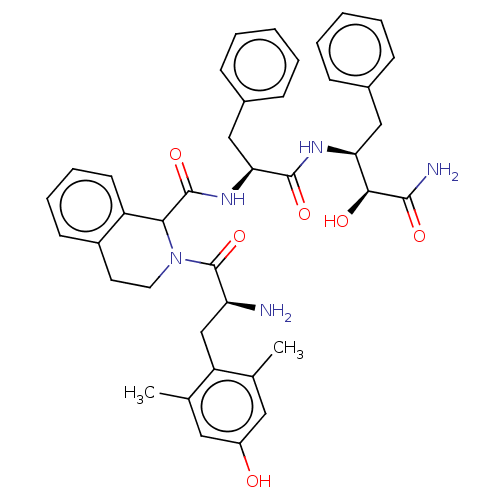 (CHEMBL3409760) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071093 (CHEMBL3409764) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85193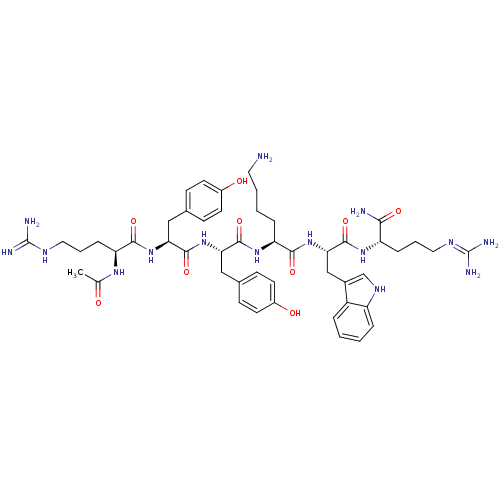 (Ac-RYYKWR-NH2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (MOUSE) | BDBM85192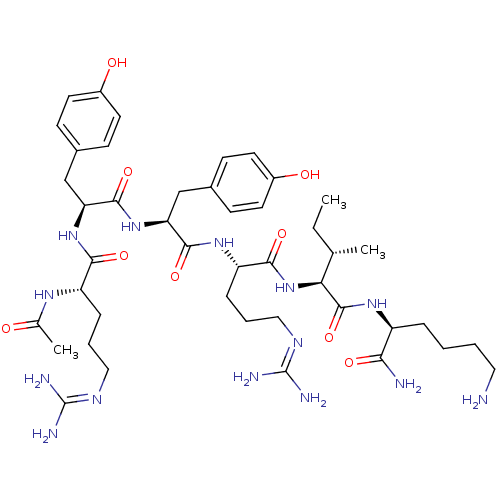 (Ac-RYYRIK-NH2 | CAS_200959-48-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by PDSP Ki Database | J Pharmacol Exp Ther 283: 735-41 (1997) BindingDB Entry DOI: 10.7270/Q2JW8CDH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071069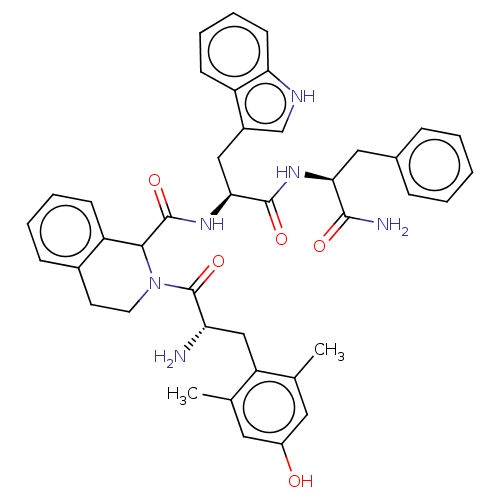 (CHEMBL3409759) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140039 (CHEMBL3764556) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071068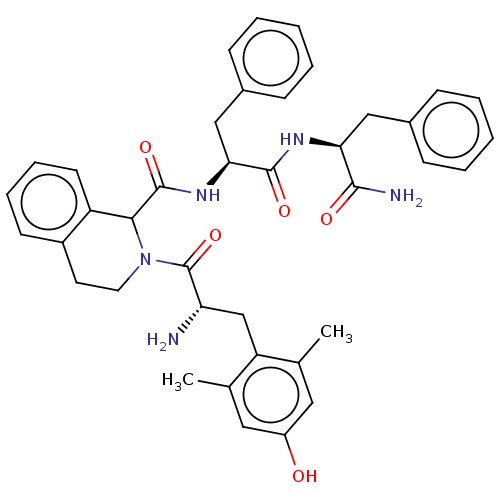 (CHEMBL3409758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortex delta opioid receptor after 2.5 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139929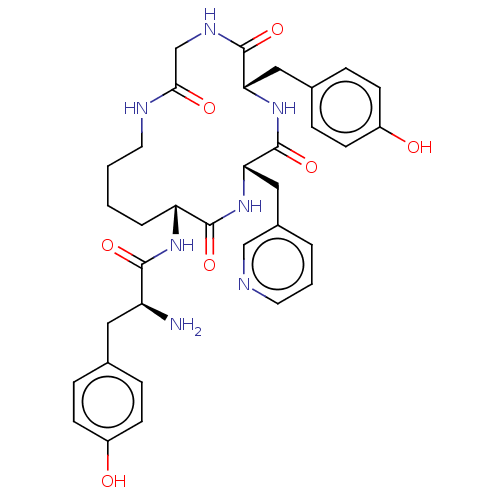 (CHEMBL3763920) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071071 (CHEMBL3409761) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071069 (CHEMBL3409759) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071068 (CHEMBL3409758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071095 (CHEMBL3409766) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071072 (CHEMBL3409762) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071076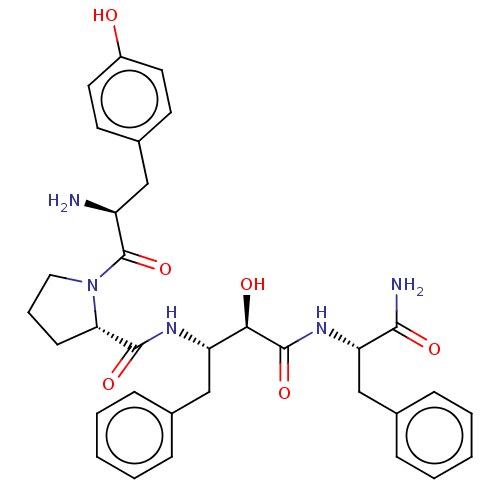 (CHEMBL3409749) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139926 (CHEMBL3764157) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139928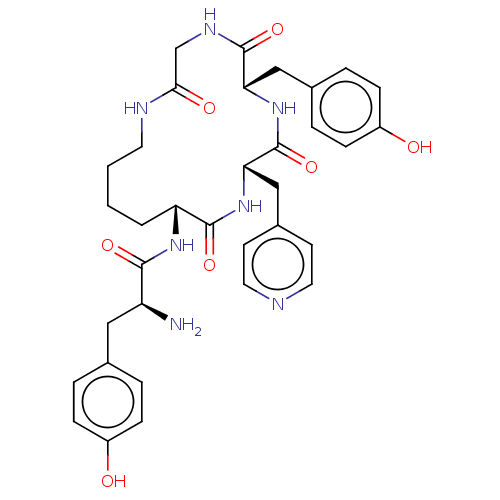 (CHEMBL3763985) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071086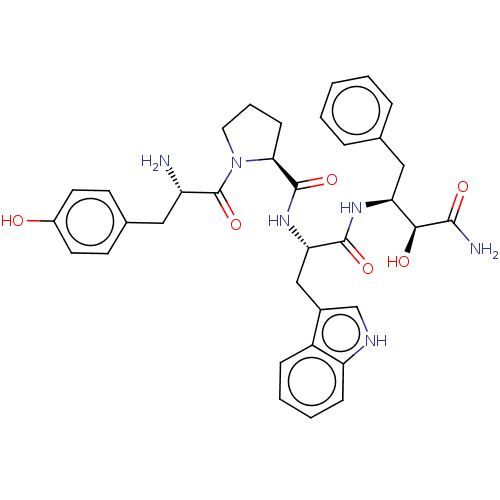 (CHEMBL3409741) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50114010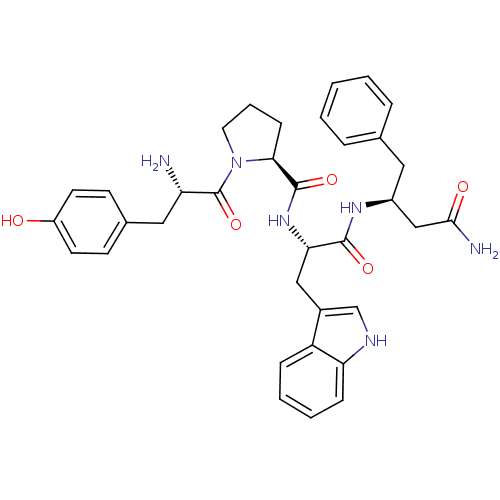 (1-[2-Amino-3-(4-hydroxy-phenyl)-propionyl]-pyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071070 (CHEMBL3409760) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071092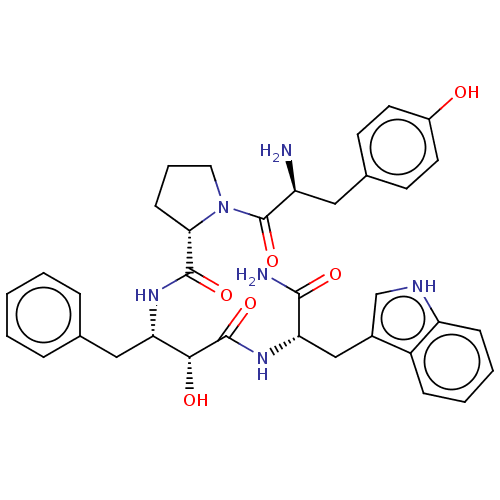 (CHEMBL3409755) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071094 (CHEMBL3409765) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140042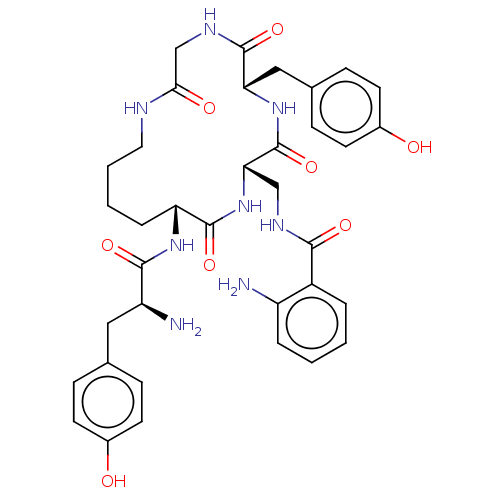 (CHEMBL3763495) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071073 (CHEMBL3409763) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071093 (CHEMBL3409764) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071090 (CHEMBL3409745) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139927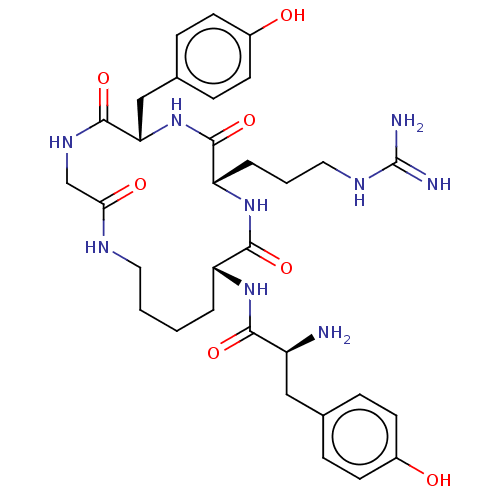 (CHEMBL3765741) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071075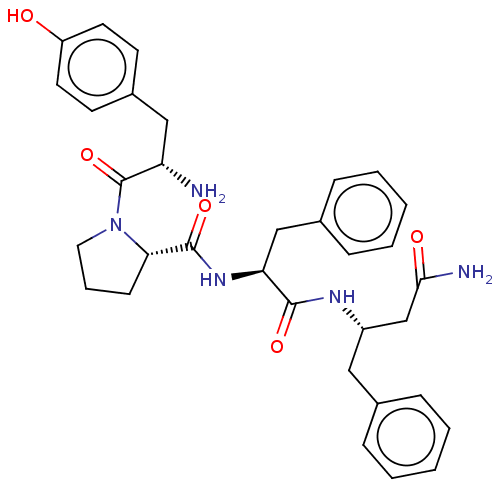 (CHEMBL3409748) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50094772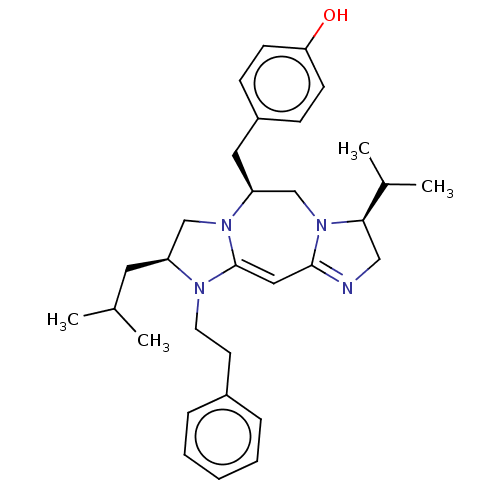 (CHEMBL3589796) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from kappa opioid receptor in guinea pig cortices and cerebella by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071067 (CHEMBL3409757) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071085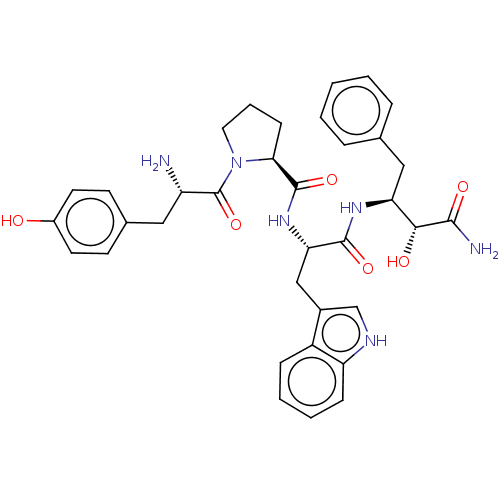 (CHEMBL3409740) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50071093 (CHEMBL3409764) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from guinea pig cortex/cerebella kappa opioid receptor after 2 hrs by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094772 (CHEMBL3589796) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071065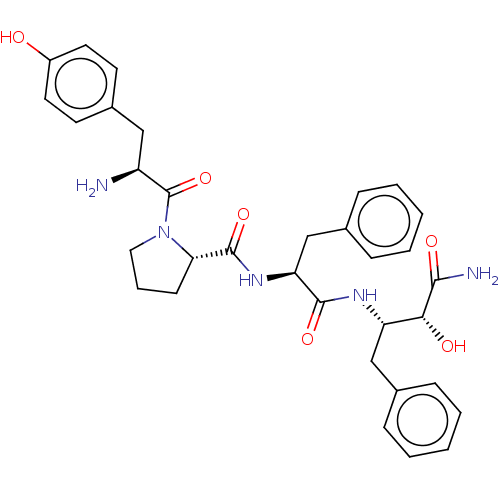 (CHEMBL3409744) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094764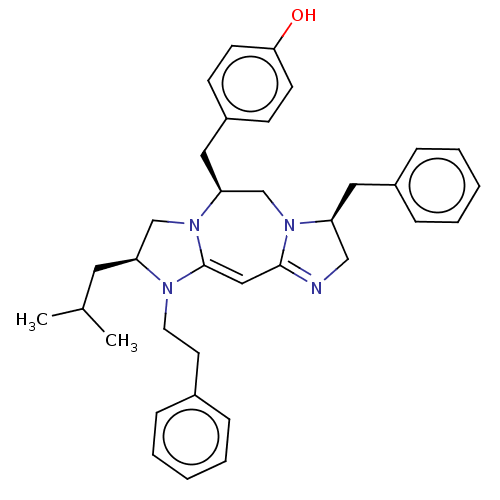 (CHEMBL3589705) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50140041 (CHEMBL3765059) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting method | J Med Chem 59: 1239-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01899 BindingDB Entry DOI: 10.7270/Q2CV4KKT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50071088 (CHEMBL3409753) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from rat cortex mu opioid receptor after 30 mins by liquid scintillation counting analysis | Eur J Med Chem 92: 270-81 (2015) Article DOI: 10.1016/j.ejmech.2014.12.049 BindingDB Entry DOI: 10.7270/Q22B90RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50094774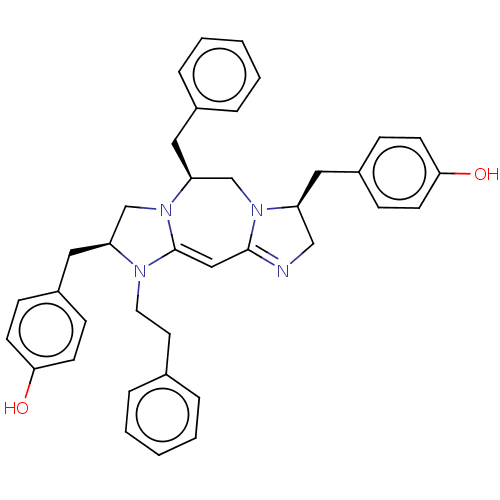 (CHEMBL3589798) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta opioid receptor in rat cortices by competition binding assay | J Med Chem 58: 4905-17 (2015) Article DOI: 10.1021/jm501637c BindingDB Entry DOI: 10.7270/Q2TB18NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 633 total ) | Next | Last >> |