 Found 52 hits with Last Name = 'doring' and Initial = 'k'
Found 52 hits with Last Name = 'doring' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227815
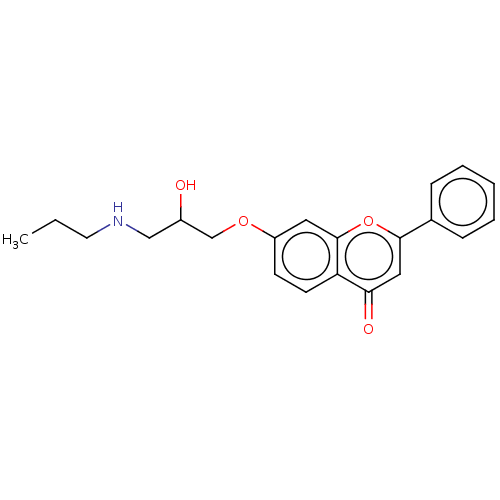
(Flavodilol)Show InChI InChI=1S/C21H23NO4/c1-2-10-22-13-16(23)14-25-17-8-9-18-19(24)12-20(26-21(18)11-17)15-6-4-3-5-7-15/h3-9,11-12,16,22-23H,2,10,13-14H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
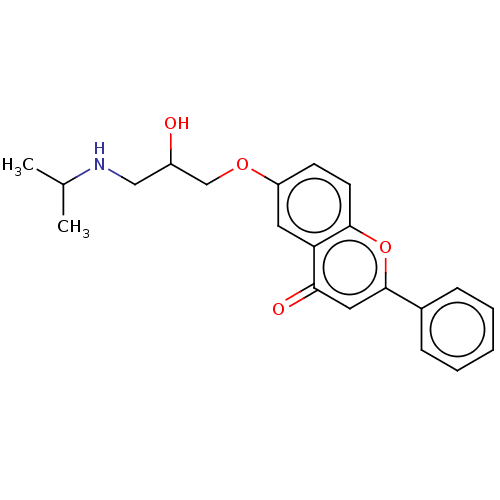
(Rattus norvegicus (Rat)) | BDBM50227820
(CHEMBL57544)Show InChI InChI=1S/C21H21NO4/c23-16(12-22-15-6-7-15)13-25-17-8-9-18-19(24)11-20(26-21(18)10-17)14-4-2-1-3-5-14/h1-5,8-11,15-16,22-23H,6-7,12-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM25761

(Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...)Show InChI InChI=1S/C16H21NO2/c1-12(2)17-10-14(18)11-19-16-9-5-7-13-6-3-4-8-15(13)16/h3-9,12,14,17-18H,10-11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
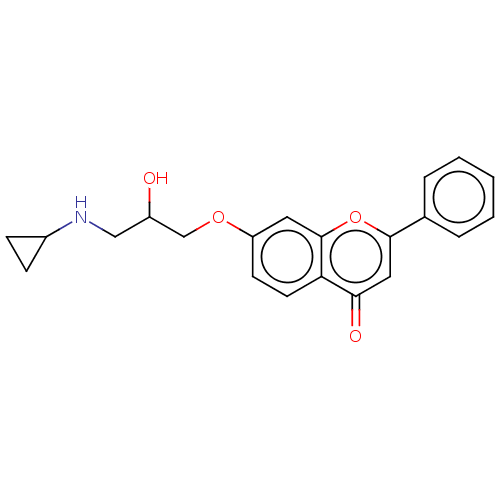
(Rattus norvegicus (Rat)) | BDBM50227814
(CHEMBL291999)Show InChI InChI=1S/C21H23NO4/c1-14(2)22-12-16(23)13-25-17-8-9-20-18(10-17)19(24)11-21(26-20)15-6-4-3-5-7-15/h3-11,14,16,22-23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227817
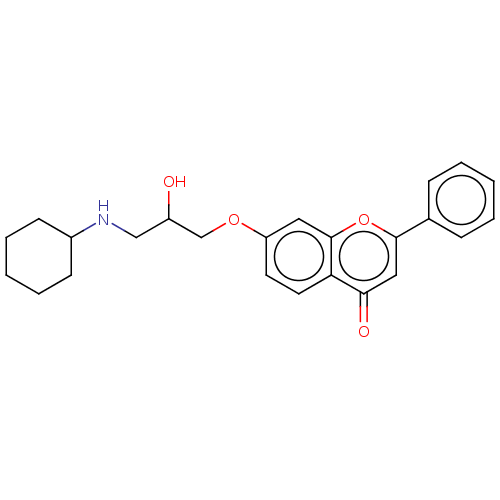
(CHEMBL57611)Show SMILES OC(CNC1CCCCC1)COc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C24H27NO4/c26-19(15-25-18-9-5-2-6-10-18)16-28-20-11-12-21-22(27)14-23(29-24(21)13-20)17-7-3-1-4-8-17/h1,3-4,7-8,11-14,18-19,25-26H,2,5-6,9-10,15-16H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant obtained from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227816
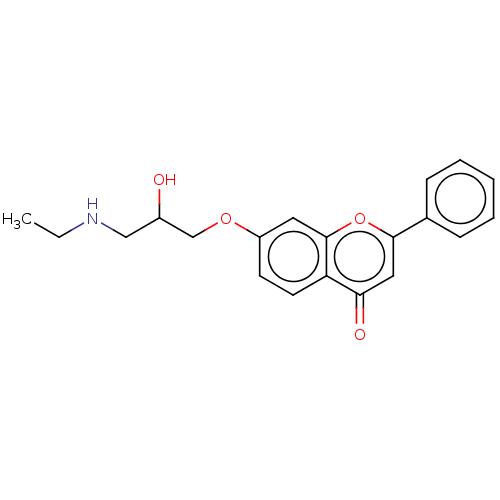
(CHEMBL57659)Show InChI InChI=1S/C20H21NO4/c1-2-21-12-15(22)13-24-16-8-9-17-18(23)11-19(25-20(17)10-16)14-6-4-3-5-7-14/h3-11,15,21-22H,2,12-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227819
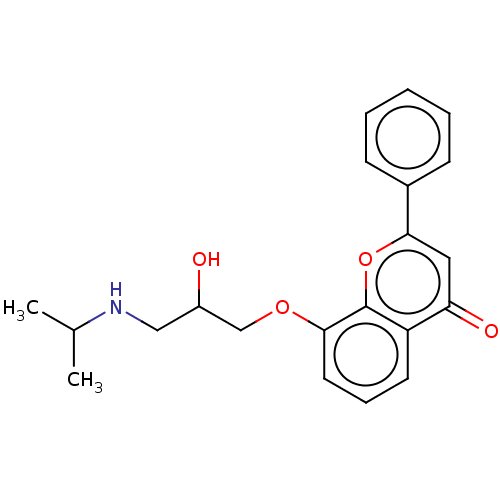
(CHEMBL273690)Show InChI InChI=1S/C21H23NO4/c1-14(2)22-12-16(23)13-25-19-10-6-9-17-18(24)11-20(26-21(17)19)15-7-4-3-5-8-15/h3-11,14,16,22-23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227818
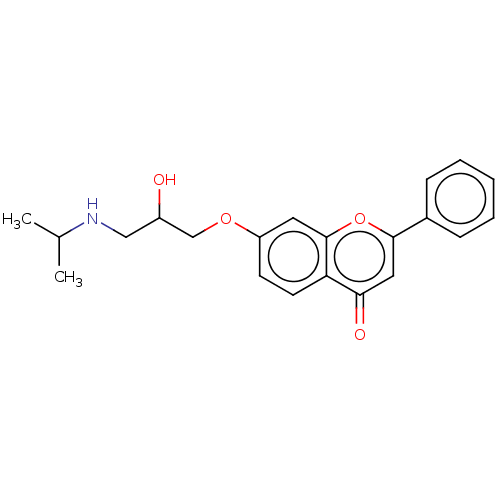
(CHEMBL56023)Show InChI InChI=1S/C21H23NO4/c1-14(2)22-12-16(23)13-25-17-8-9-18-19(24)11-20(26-21(18)10-17)15-6-4-3-5-7-15/h3-11,14,16,22-23H,12-13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227813

(CHEMBL293974)Show SMILES CC(C)(C)NCC(O)COc1ccc2c(c1)oc(cc2=O)-c1ccccc1 Show InChI InChI=1S/C22H25NO4/c1-22(2,3)23-13-16(24)14-26-17-9-10-18-19(25)12-20(27-21(18)11-17)15-7-5-4-6-8-15/h4-12,16,23-24H,13-14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Beta-1/Beta-2/Beta-3 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50227821
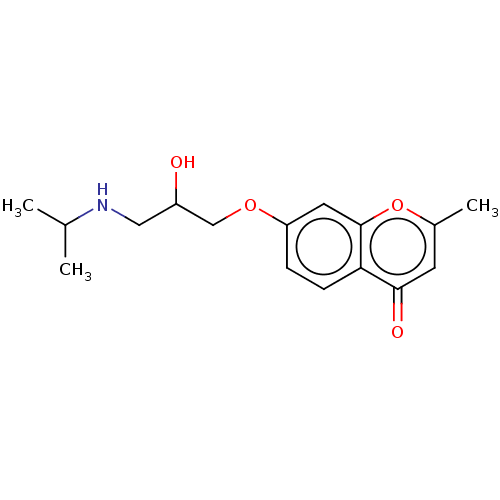
(CHEMBL56492)Show InChI InChI=1S/C16H21NO4/c1-10(2)17-8-12(18)9-20-13-4-5-14-15(19)6-11(3)21-16(14)7-13/h4-7,10,12,17-18H,8-9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennwalt Corporation
Curated by ChEMBL
| Assay Description
Inhibition constant from beta adrenergic receptor binding assay |
J Med Chem 32: 183-92 (1989)
BindingDB Entry DOI: 10.7270/Q2474D33 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362312
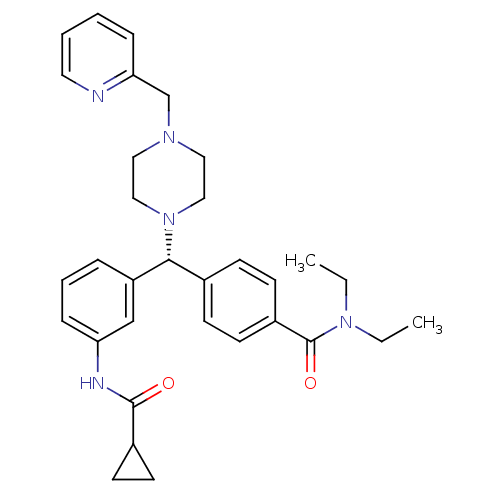
(CHEMBL1939740)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2ccccn2)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C32H39N5O2/c1-3-36(4-2)32(39)26-15-11-24(12-16-26)30(27-8-7-10-28(22-27)34-31(38)25-13-14-25)37-20-18-35(19-21-37)23-29-9-5-6-17-33-29/h5-12,15-17,22,25,30H,3-4,13-14,18-21,23H2,1-2H3,(H,34,38)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362304
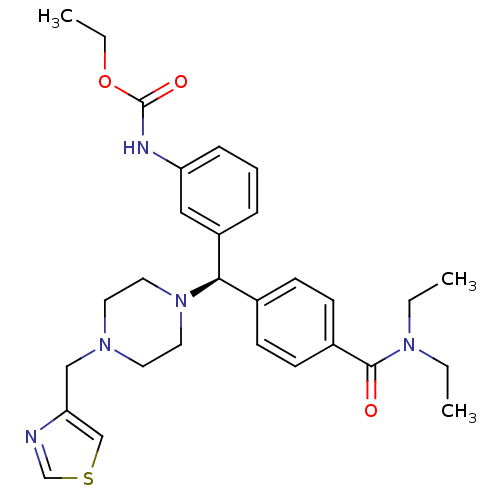
(CHEMBL1939746)Show SMILES CCOC(=O)Nc1cccc(c1)[C@H](N1CCN(Cc2cscn2)CC1)c1ccc(cc1)C(=O)N(CC)CC |r| Show InChI InChI=1S/C29H37N5O3S/c1-4-33(5-2)28(35)23-12-10-22(11-13-23)27(24-8-7-9-25(18-24)31-29(36)37-6-3)34-16-14-32(15-17-34)19-26-20-38-21-30-26/h7-13,18,20-21,27H,4-6,14-17,19H2,1-3H3,(H,31,36)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362299
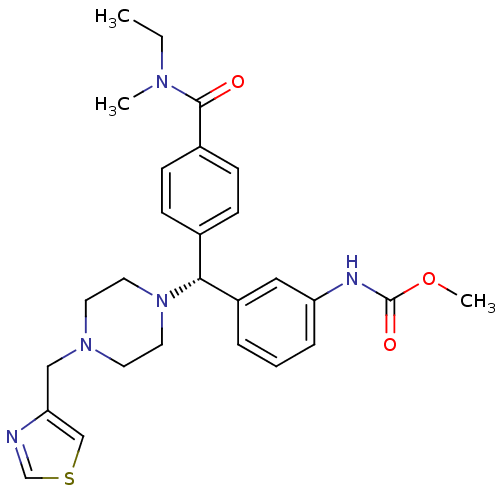
(CHEMBL1939751)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2cscn2)CC1)c1cccc(NC(=O)OC)c1 |r| Show InChI InChI=1S/C27H33N5O3S/c1-4-30(2)26(33)21-10-8-20(9-11-21)25(22-6-5-7-23(16-22)29-27(34)35-3)32-14-12-31(13-15-32)17-24-18-36-19-28-24/h5-11,16,18-19,25H,4,12-15,17H2,1-3H3,(H,29,34)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362301
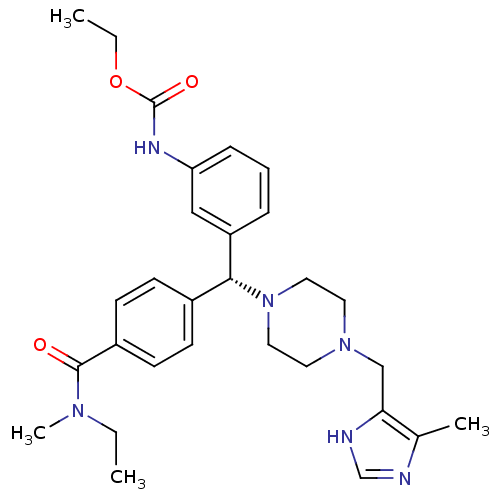
(CHEMBL1939749)Show SMILES CCOC(=O)Nc1cccc(c1)[C@H](N1CCN(Cc2[nH]cnc2C)CC1)c1ccc(cc1)C(=O)N(C)CC |r| Show InChI InChI=1S/C29H38N6O3/c1-5-33(4)28(36)23-12-10-22(11-13-23)27(24-8-7-9-25(18-24)32-29(37)38-6-2)35-16-14-34(15-17-35)19-26-21(3)30-20-31-26/h7-13,18,20,27H,5-6,14-17,19H2,1-4H3,(H,30,31)(H,32,37)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362293
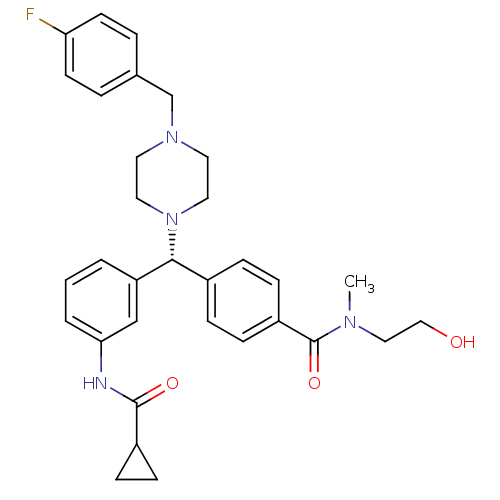
(CHEMBL1939738)Show SMILES CN(CCO)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2ccc(F)cc2)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C32H37FN4O3/c1-35(19-20-38)32(40)26-11-7-24(8-12-26)30(27-3-2-4-29(21-27)34-31(39)25-9-10-25)37-17-15-36(16-18-37)22-23-5-13-28(33)14-6-23/h2-8,11-14,21,25,30,38H,9-10,15-20,22H2,1H3,(H,34,39)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362294

(CHEMBL1939737)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2ccc(F)cc2)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C33H39FN4O2/c1-3-37(4-2)33(40)27-14-10-25(11-15-27)31(28-6-5-7-30(22-28)35-32(39)26-12-13-26)38-20-18-36(19-21-38)23-24-8-16-29(34)17-9-24/h5-11,14-17,22,26,31H,3-4,12-13,18-21,23H2,1-2H3,(H,35,39)/t31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362307
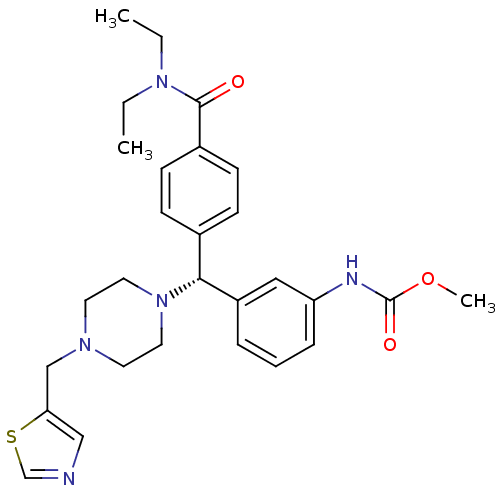
(CHEMBL1939736)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2cncs2)CC1)c1cccc(NC(=O)OC)c1 |r| Show InChI InChI=1S/C28H35N5O3S/c1-4-32(5-2)27(34)22-11-9-21(10-12-22)26(23-7-6-8-24(17-23)30-28(35)36-3)33-15-13-31(14-16-33)19-25-18-29-20-37-25/h6-12,17-18,20,26H,4-5,13-16,19H2,1-3H3,(H,30,35)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362306

(CHEMBL1939735)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2cccnc2)CC1)c1cccc(NC(=O)OC)c1 |r| Show InChI InChI=1S/C30H37N5O3/c1-4-34(5-2)29(36)25-13-11-24(12-14-25)28(26-9-6-10-27(20-26)32-30(37)38-3)35-18-16-33(17-19-35)22-23-8-7-15-31-21-23/h6-15,20-21,28H,4-5,16-19,22H2,1-3H3,(H,32,37)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362300

(CHEMBL1939750)Show SMILES CCOC(=O)Nc1cccc(c1)[C@H](N1CCN(Cc2cnc(C)[nH]2)CC1)c1ccc(cc1)C(=O)N(C)CC |r| Show InChI InChI=1S/C29H38N6O3/c1-5-33(4)28(36)23-12-10-22(11-13-23)27(24-8-7-9-25(18-24)32-29(37)38-6-2)35-16-14-34(15-17-35)20-26-19-30-21(3)31-26/h7-13,18-19,27H,5-6,14-17,20H2,1-4H3,(H,30,31)(H,32,37)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362305
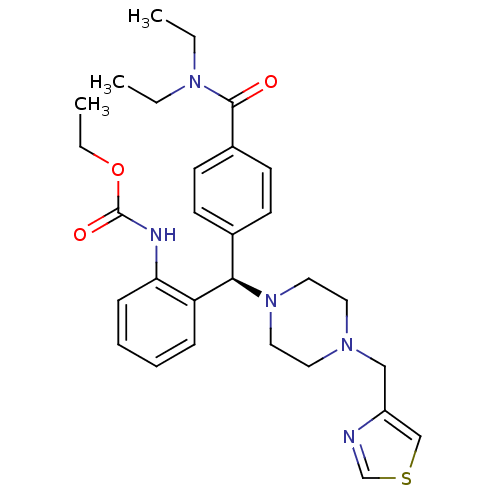
(CHEMBL1939745)Show SMILES CCOC(=O)Nc1ccccc1[C@H](N1CCN(Cc2cscn2)CC1)c1ccc(cc1)C(=O)N(CC)CC |r| Show InChI InChI=1S/C29H37N5O3S/c1-4-33(5-2)28(35)23-13-11-22(12-14-23)27(25-9-7-8-10-26(25)31-29(36)37-6-3)34-17-15-32(16-18-34)19-24-20-38-21-30-24/h7-14,20-21,27H,4-6,15-19H2,1-3H3,(H,31,36)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362313
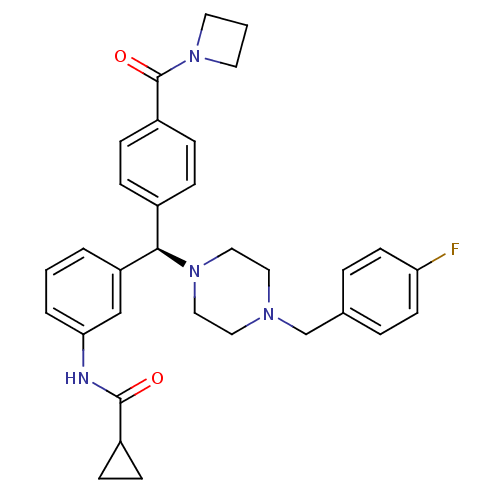
(CHEMBL1939739)Show SMILES Fc1ccc(CN2CCN(CC2)[C@H](c2ccc(cc2)C(=O)N2CCC2)c2cccc(NC(=O)C3CC3)c2)cc1 |r| Show InChI InChI=1S/C32H35FN4O2/c33-28-13-5-23(6-14-28)22-35-17-19-36(20-18-35)30(24-7-11-26(12-8-24)32(39)37-15-2-16-37)27-3-1-4-29(21-27)34-31(38)25-9-10-25/h1,3-8,11-14,21,25,30H,2,9-10,15-20,22H2,(H,34,38)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362310
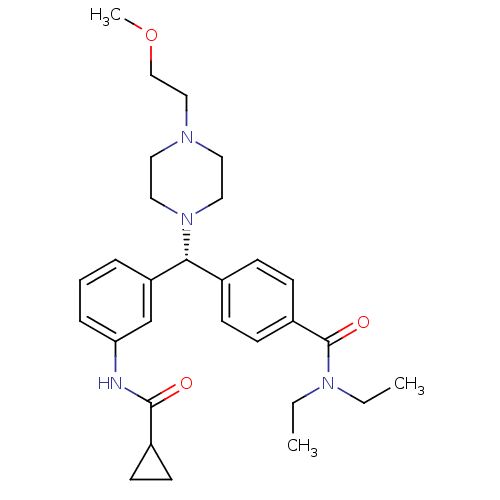
(CHEMBL1939743)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1CCN(CCOC)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C29H40N4O3/c1-4-32(5-2)29(35)24-13-9-22(10-14-24)27(33-17-15-31(16-18-33)19-20-36-3)25-7-6-8-26(21-25)30-28(34)23-11-12-23/h6-10,13-14,21,23,27H,4-5,11-12,15-20H2,1-3H3,(H,30,34)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362303

(CHEMBL1939747)Show SMILES CCOC(=O)Nc1ccc(cc1)[C@H](N1CCN(Cc2cscn2)CC1)c1ccc(cc1)C(=O)N(CC)CC |r| Show InChI InChI=1S/C29H37N5O3S/c1-4-33(5-2)28(35)24-9-7-22(8-10-24)27(23-11-13-25(14-12-23)31-29(36)37-6-3)34-17-15-32(16-18-34)19-26-20-38-21-30-26/h7-14,20-21,27H,4-6,15-19H2,1-3H3,(H,31,36)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362302

(CHEMBL1939748)Show SMILES CCOC(=O)Nc1ccccc1[C@H](N1CCN(Cc2ccccn2)CC1)c1ccc(cc1)C(=O)N(CC)CC |r| Show InChI InChI=1S/C31H39N5O3/c1-4-35(5-2)30(37)25-16-14-24(15-17-25)29(27-12-7-8-13-28(27)33-31(38)39-6-3)36-21-19-34(20-22-36)23-26-11-9-10-18-32-26/h7-18,29H,4-6,19-23H2,1-3H3,(H,33,38)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362296
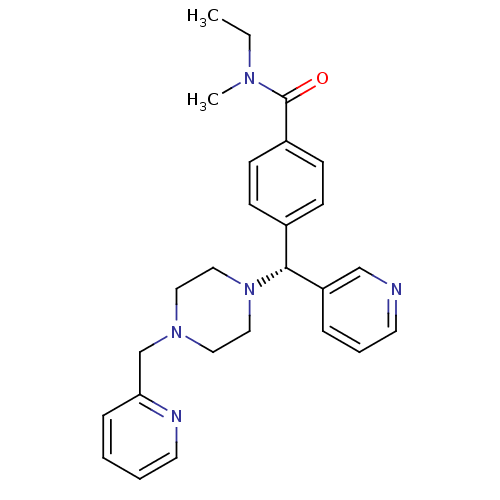
(CHEMBL1939753)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2ccccn2)CC1)c1cccnc1 |r| Show InChI InChI=1S/C26H31N5O/c1-3-29(2)26(32)22-11-9-21(10-12-22)25(23-7-6-13-27-19-23)31-17-15-30(16-18-31)20-24-8-4-5-14-28-24/h4-14,19,25H,3,15-18,20H2,1-2H3/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362298
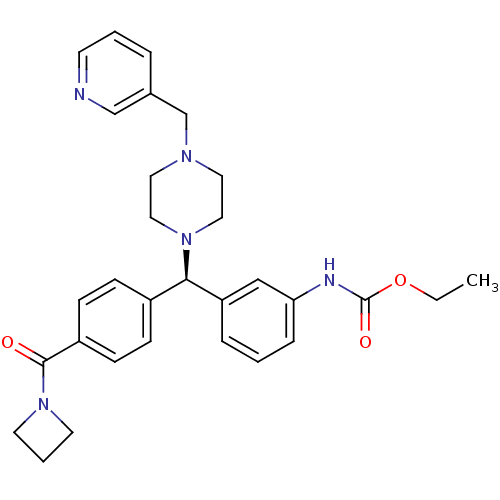
(CHEMBL1939752)Show SMILES CCOC(=O)Nc1cccc(c1)[C@H](N1CCN(Cc2cccnc2)CC1)c1ccc(cc1)C(=O)N1CCC1 |r| Show InChI InChI=1S/C30H35N5O3/c1-2-38-30(37)32-27-8-3-7-26(20-27)28(24-9-11-25(12-10-24)29(36)35-14-5-15-35)34-18-16-33(17-19-34)22-23-6-4-13-31-21-23/h3-4,6-13,20-21,28H,2,5,14-19,22H2,1H3,(H,32,37)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362297

(CHEMBL1938410)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2cscn2)CC1)c1cccnc1 |r| Show InChI InChI=1S/C24H29N5OS/c1-3-27(2)24(30)20-8-6-19(7-9-20)23(21-5-4-10-25-15-21)29-13-11-28(12-14-29)16-22-17-31-18-26-22/h4-10,15,17-18,23H,3,11-14,16H2,1-2H3/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362295

(CHEMBL1939754)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2ccccn2)CC1)c1ccccn1 |r| Show InChI InChI=1S/C26H31N5O/c1-3-29(2)26(32)22-12-10-21(11-13-22)25(24-9-5-7-15-28-24)31-18-16-30(17-19-31)20-23-8-4-6-14-27-23/h4-15,25H,3,16-20H2,1-2H3/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362308
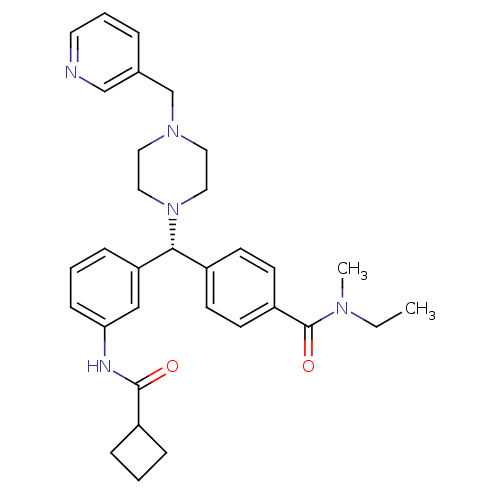
(CHEMBL1939741)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2cccnc2)CC1)c1cccc(NC(=O)C2CCC2)c1 |r| Show InChI InChI=1S/C32H39N5O2/c1-3-35(2)32(39)27-14-12-25(13-15-27)30(28-10-5-11-29(21-28)34-31(38)26-8-4-9-26)37-19-17-36(18-20-37)23-24-7-6-16-33-22-24/h5-7,10-16,21-22,26,30H,3-4,8-9,17-20,23H2,1-2H3,(H,34,38)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362309
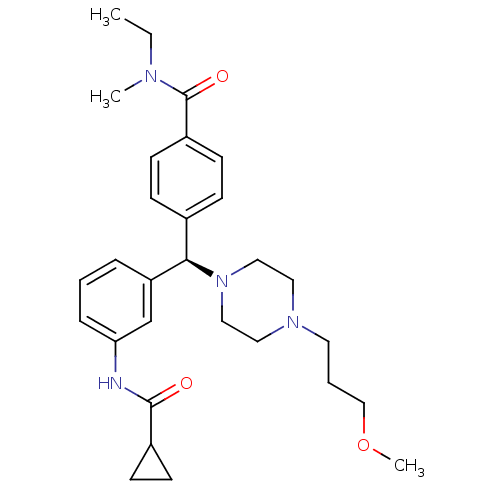
(CHEMBL1939744)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(CCCOC)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C29H40N4O3/c1-4-31(2)29(35)24-13-9-22(10-14-24)27(33-18-16-32(17-19-33)15-6-20-36-3)25-7-5-8-26(21-25)30-28(34)23-11-12-23/h5,7-10,13-14,21,23,27H,4,6,11-12,15-20H2,1-3H3,(H,30,34)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50362311
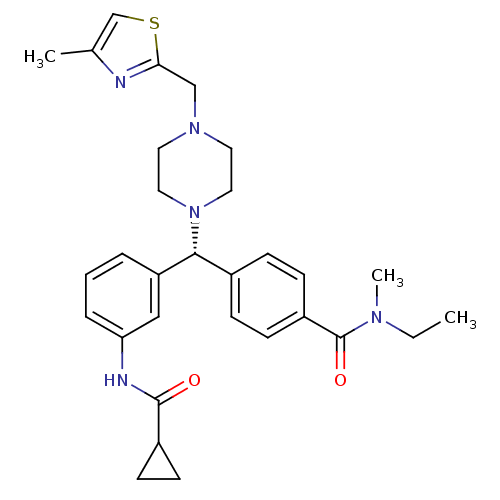
(CHEMBL1939742)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2nc(C)cs2)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C30H37N5O2S/c1-4-33(3)30(37)24-12-8-22(9-13-24)28(25-6-5-7-26(18-25)32-29(36)23-10-11-23)35-16-14-34(15-17-35)19-27-31-21(2)20-38-27/h5-9,12-13,18,20,23,28H,4,10-11,14-17,19H2,1-3H3,(H,32,36)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [I125]deltorphin from delta-opioid receptor overexpressed in human HEK293 cells |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362313

(CHEMBL1939739)Show SMILES Fc1ccc(CN2CCN(CC2)[C@H](c2ccc(cc2)C(=O)N2CCC2)c2cccc(NC(=O)C3CC3)c2)cc1 |r| Show InChI InChI=1S/C32H35FN4O2/c33-28-13-5-23(6-14-28)22-35-17-19-36(20-18-35)30(24-7-11-26(12-8-24)32(39)37-15-2-16-37)27-3-1-4-29(21-27)34-31(38)25-9-10-25/h1,3-8,11-14,21,25,30H,2,9-10,15-20,22H2,(H,34,38)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362304

(CHEMBL1939746)Show SMILES CCOC(=O)Nc1cccc(c1)[C@H](N1CCN(Cc2cscn2)CC1)c1ccc(cc1)C(=O)N(CC)CC |r| Show InChI InChI=1S/C29H37N5O3S/c1-4-33(5-2)28(35)23-12-10-22(11-13-23)27(24-8-7-9-25(18-24)31-29(36)37-6-3)34-16-14-32(15-17-34)19-26-20-38-21-30-26/h7-13,18,20-21,27H,4-6,14-17,19H2,1-3H3,(H,31,36)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362303

(CHEMBL1939747)Show SMILES CCOC(=O)Nc1ccc(cc1)[C@H](N1CCN(Cc2cscn2)CC1)c1ccc(cc1)C(=O)N(CC)CC |r| Show InChI InChI=1S/C29H37N5O3S/c1-4-33(5-2)28(35)24-9-7-22(8-10-24)27(23-11-13-25(14-12-23)31-29(36)37-6-3)34-17-15-32(16-18-34)19-26-20-38-21-30-26/h7-14,20-21,27H,4-6,15-19H2,1-3H3,(H,31,36)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362305

(CHEMBL1939745)Show SMILES CCOC(=O)Nc1ccccc1[C@H](N1CCN(Cc2cscn2)CC1)c1ccc(cc1)C(=O)N(CC)CC |r| Show InChI InChI=1S/C29H37N5O3S/c1-4-33(5-2)28(35)23-13-11-22(12-14-23)27(25-9-7-8-10-26(25)31-29(36)37-6-3)34-17-15-32(16-18-34)19-24-20-38-21-30-24/h7-14,20-21,27H,4-6,15-19H2,1-3H3,(H,31,36)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362302

(CHEMBL1939748)Show SMILES CCOC(=O)Nc1ccccc1[C@H](N1CCN(Cc2ccccn2)CC1)c1ccc(cc1)C(=O)N(CC)CC |r| Show InChI InChI=1S/C31H39N5O3/c1-4-35(5-2)30(37)25-16-14-24(15-17-25)29(27-12-7-8-13-28(27)33-31(38)39-6-3)36-21-19-34(20-22-36)23-26-11-9-10-18-32-26/h7-18,29H,4-6,19-23H2,1-3H3,(H,33,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362294

(CHEMBL1939737)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2ccc(F)cc2)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C33H39FN4O2/c1-3-37(4-2)33(40)27-14-10-25(11-15-27)31(28-6-5-7-30(22-28)35-32(39)26-12-13-26)38-20-18-36(19-21-38)23-24-8-16-29(34)17-9-24/h5-11,14-17,22,26,31H,3-4,12-13,18-21,23H2,1-2H3,(H,35,39)/t31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362306

(CHEMBL1939735)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2cccnc2)CC1)c1cccc(NC(=O)OC)c1 |r| Show InChI InChI=1S/C30H37N5O3/c1-4-34(5-2)29(36)25-13-11-24(12-14-25)28(26-9-6-10-27(20-26)32-30(37)38-3)35-18-16-33(17-19-35)22-23-8-7-15-31-21-23/h6-15,20-21,28H,4-5,16-19,22H2,1-3H3,(H,32,37)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362298

(CHEMBL1939752)Show SMILES CCOC(=O)Nc1cccc(c1)[C@H](N1CCN(Cc2cccnc2)CC1)c1ccc(cc1)C(=O)N1CCC1 |r| Show InChI InChI=1S/C30H35N5O3/c1-2-38-30(37)32-27-8-3-7-26(20-27)28(24-9-11-25(12-10-24)29(36)35-14-5-15-35)34-18-16-33(17-19-34)22-23-6-4-13-31-21-23/h3-4,6-13,20-21,28H,2,5,14-19,22H2,1H3,(H,32,37)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362309

(CHEMBL1939744)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(CCCOC)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C29H40N4O3/c1-4-31(2)29(35)24-13-9-22(10-14-24)27(33-18-16-32(17-19-33)15-6-20-36-3)25-7-5-8-26(21-25)30-28(34)23-11-12-23/h5,7-10,13-14,21,23,27H,4,6,11-12,15-20H2,1-3H3,(H,30,34)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362296

(CHEMBL1939753)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2ccccn2)CC1)c1cccnc1 |r| Show InChI InChI=1S/C26H31N5O/c1-3-29(2)26(32)22-11-9-21(10-12-22)25(23-7-6-13-27-19-23)31-17-15-30(16-18-31)20-24-8-4-5-14-28-24/h4-14,19,25H,3,15-18,20H2,1-2H3/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362307

(CHEMBL1939736)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2cncs2)CC1)c1cccc(NC(=O)OC)c1 |r| Show InChI InChI=1S/C28H35N5O3S/c1-4-32(5-2)27(34)22-11-9-21(10-12-22)26(23-7-6-8-24(17-23)30-28(35)36-3)33-15-13-31(14-16-33)19-25-18-29-20-37-25/h6-12,17-18,20,26H,4-5,13-16,19H2,1-3H3,(H,30,35)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362293

(CHEMBL1939738)Show SMILES CN(CCO)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2ccc(F)cc2)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C32H37FN4O3/c1-35(19-20-38)32(40)26-11-7-24(8-12-26)30(27-3-2-4-29(21-27)34-31(39)25-9-10-25)37-17-15-36(16-18-37)22-23-5-13-28(33)14-6-23/h2-8,11-14,21,25,30,38H,9-10,15-20,22H2,1H3,(H,34,39)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362297

(CHEMBL1938410)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2cscn2)CC1)c1cccnc1 |r| Show InChI InChI=1S/C24H29N5OS/c1-3-27(2)24(30)20-8-6-19(7-9-20)23(21-5-4-10-25-15-21)29-13-11-28(12-14-29)16-22-17-31-18-26-22/h4-10,15,17-18,23H,3,11-14,16H2,1-2H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362299

(CHEMBL1939751)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2cscn2)CC1)c1cccc(NC(=O)OC)c1 |r| Show InChI InChI=1S/C27H33N5O3S/c1-4-30(2)26(33)21-10-8-20(9-11-21)25(22-6-5-7-23(16-22)29-27(34)35-3)32-14-12-31(13-15-32)17-24-18-36-19-28-24/h5-11,16,18-19,25H,4,12-15,17H2,1-3H3,(H,29,34)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362311

(CHEMBL1939742)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2nc(C)cs2)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C30H37N5O2S/c1-4-33(3)30(37)24-12-8-22(9-13-24)28(25-6-5-7-26(18-25)32-29(36)23-10-11-23)35-16-14-34(15-17-35)19-27-31-21(2)20-38-27/h5-9,12-13,18,20,23,28H,4,10-11,14-17,19H2,1-3H3,(H,32,36)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362312

(CHEMBL1939740)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2ccccn2)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C32H39N5O2/c1-3-36(4-2)32(39)26-15-11-24(12-16-26)30(27-8-7-10-28(22-27)34-31(38)25-13-14-25)37-20-18-35(19-21-37)23-29-9-5-6-17-33-29/h5-12,15-17,22,25,30H,3-4,13-14,18-21,23H2,1-2H3,(H,34,38)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362308

(CHEMBL1939741)Show SMILES CCN(C)C(=O)c1ccc(cc1)[C@@H](N1CCN(Cc2cccnc2)CC1)c1cccc(NC(=O)C2CCC2)c1 |r| Show InChI InChI=1S/C32H39N5O2/c1-3-35(2)32(39)27-14-12-25(13-15-27)30(28-10-5-11-29(21-28)34-31(38)26-8-4-9-26)37-19-17-36(18-20-37)23-24-7-6-16-33-22-24/h5-7,10-16,21-22,26,30H,3-4,8-9,17-20,23H2,1-2H3,(H,34,38)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362310

(CHEMBL1939743)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1CCN(CCOC)CC1)c1cccc(NC(=O)C2CC2)c1 |r| Show InChI InChI=1S/C29H40N4O3/c1-4-32(5-2)29(35)24-13-9-22(10-14-24)27(33-17-15-31(16-18-33)19-20-36-3)25-7-6-8-26(21-25)30-28(34)23-11-12-23/h6-10,13-14,21,23,27H,4-5,11-12,15-20H2,1-3H3,(H,30,34)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50362301

(CHEMBL1939749)Show SMILES CCOC(=O)Nc1cccc(c1)[C@H](N1CCN(Cc2[nH]cnc2C)CC1)c1ccc(cc1)C(=O)N(C)CC |r| Show InChI InChI=1S/C29H38N6O3/c1-5-33(4)28(36)23-12-10-22(11-13-23)27(24-8-7-9-25(18-24)32-29(37)38-6-2)35-16-14-34(15-17-35)19-26-21(3)30-20-31-26/h7-13,18,20,27H,5-6,14-17,19H2,1-4H3,(H,30,31)(H,32,37)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human Erg |
Bioorg Med Chem Lett 22: 1169-73 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.088
BindingDB Entry DOI: 10.7270/Q269741B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data