 Found 149 hits with Last Name = 'dove' and Initial = 'j'
Found 149 hits with Last Name = 'dove' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26023
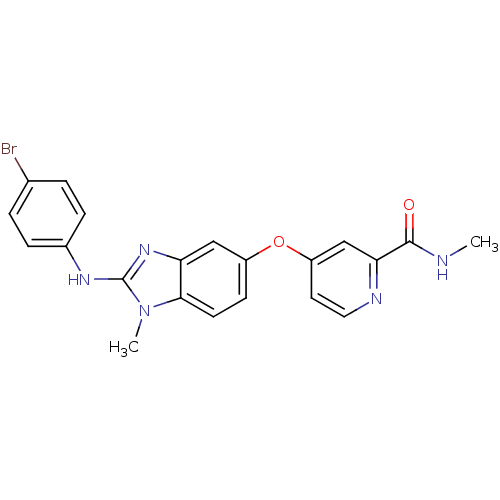
(4-({2-[(4-bromophenyl)(methyl)amino]-1H-1,3-benzod...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C21H18BrN5O2/c1-23-20(28)18-12-16(9-10-24-18)29-15-7-8-19-17(11-15)26-21(27(19)2)25-14-5-3-13(22)4-6-14/h3-12H,1-2H3,(H,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26034
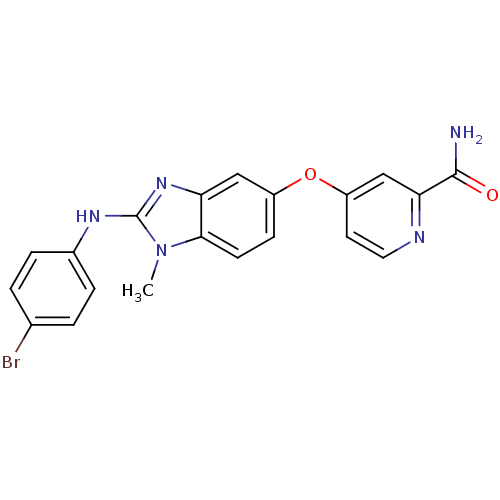
(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES Cn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(N)=O)ccc12 Show InChI InChI=1S/C20H16BrN5O2/c1-26-18-7-6-14(28-15-8-9-23-17(11-15)19(22)27)10-16(18)25-20(26)24-13-4-2-12(21)3-5-13/h2-11H,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50400752
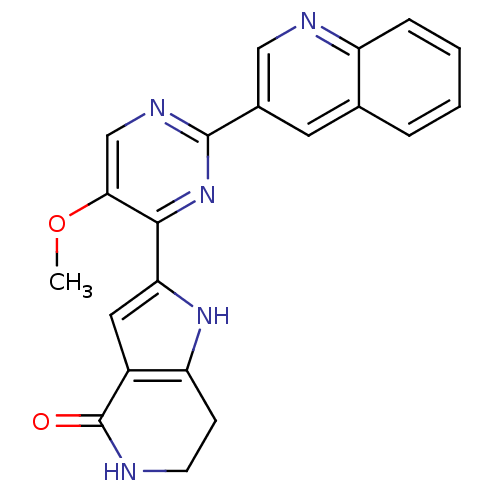
(CHEMBL2203552)Show SMILES COc1cnc(nc1-c1cc2c(CCNC2=O)[nH]1)-c1cnc2ccccc2c1 Show InChI InChI=1S/C21H17N5O2/c1-28-18-11-24-20(13-8-12-4-2-3-5-15(12)23-10-13)26-19(18)17-9-14-16(25-17)6-7-22-21(14)27/h2-5,8-11,25H,6-7H2,1H3,(H,22,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26023

(4-({2-[(4-bromophenyl)(methyl)amino]-1H-1,3-benzod...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C21H18BrN5O2/c1-23-20(28)18-12-16(9-10-24-18)29-15-7-8-19-17(11-15)26-21(27(19)2)25-14-5-3-13(22)4-6-14/h3-12H,1-2H3,(H,23,28)(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-2
(Homo sapiens (Human)) | BDBM50400754

(CHEMBL2203564)Show SMILES COc1cnc(COc2ccc(F)cc2C)cc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C21H20FN3O3/c1-12-7-13(22)3-4-19(12)28-11-14-8-15(20(27-2)10-24-14)18-9-16-17(25-18)5-6-23-21(16)26/h3-4,7-10,25H,5-6,11H2,1-2H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma 2 using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 32 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-2
(Homo sapiens (Human)) | BDBM50400756
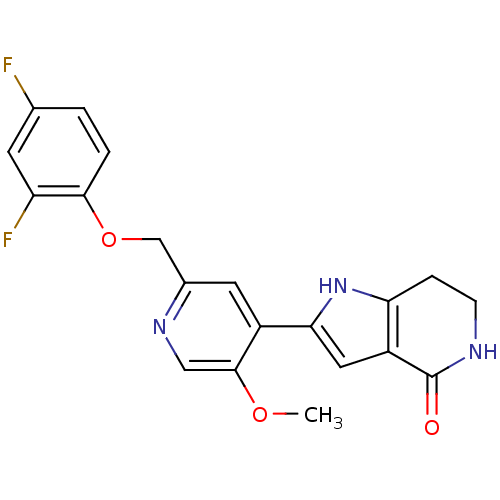
(CHEMBL2203562)Show SMILES COc1cnc(COc2ccc(F)cc2F)cc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C20H17F2N3O3/c1-27-19-9-24-12(10-28-18-3-2-11(21)6-15(18)22)7-13(19)17-8-14-16(25-17)4-5-23-20(14)26/h2-3,6-9,25H,4-5,10H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma 2 using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 32 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26037
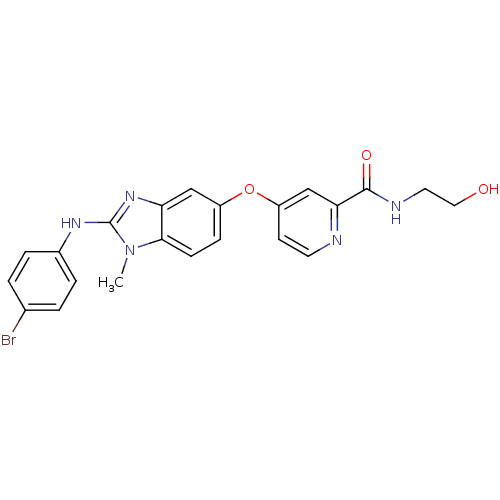
(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES Cn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(=O)NCCO)ccc12 Show InChI InChI=1S/C22H20BrN5O3/c1-28-20-7-6-16(31-17-8-9-24-19(13-17)21(30)25-10-11-29)12-18(20)27-22(28)26-15-4-2-14(23)3-5-15/h2-9,12-13,29H,10-11H2,1H3,(H,25,30)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26028
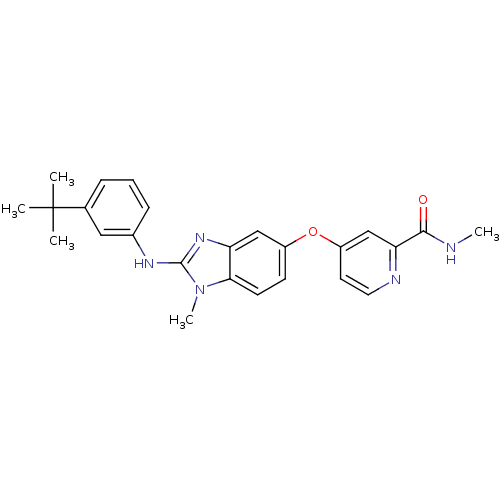
(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-2
(Homo sapiens (Human)) | BDBM50400753
(CHEMBL2203565)Show SMILES COc1cnc(COc2cc(F)c(F)cc2C)cc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C21H19F2N3O3/c1-11-5-15(22)16(23)8-19(11)29-10-12-6-13(20(28-2)9-25-12)18-7-14-17(26-18)3-4-24-21(14)27/h5-9,26H,3-4,10H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma 2 using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 32 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26022
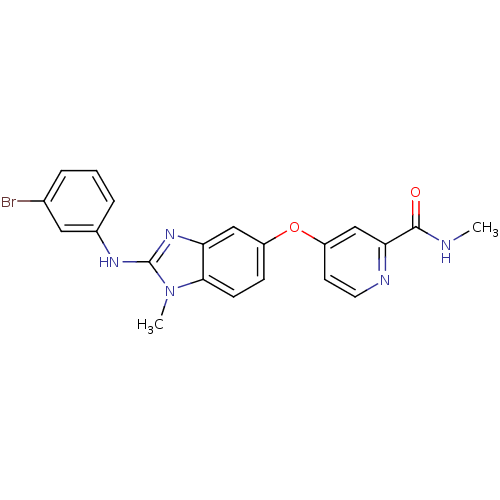
(4-({2-[(3-bromophenyl)(methyl)amino]-1H-1,3-benzod...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(Br)c4)nc3c2)ccn1 Show InChI InChI=1S/C21H18BrN5O2/c1-23-20(28)18-12-16(8-9-24-18)29-15-6-7-19-17(11-15)26-21(27(19)2)25-14-5-3-4-13(22)10-14/h3-12H,1-2H3,(H,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-2
(Homo sapiens (Human)) | BDBM50400755

(CHEMBL2203563)Show SMILES COc1cnc(COc2ccc(F)c(F)c2)cc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C20H17F2N3O3/c1-27-19-9-24-11(10-28-12-2-3-15(21)16(22)7-12)6-13(19)18-8-14-17(25-18)4-5-23-20(14)26/h2-3,6-9,25H,4-5,10H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma 2 using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 32 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase I isoform gamma-2
(Homo sapiens (Human)) | BDBM50400757
(CHEMBL2203561)Show SMILES COc1cnc(COc2ccc(F)cc2)cc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C20H18FN3O3/c1-26-19-10-23-13(11-27-14-4-2-12(21)3-5-14)8-15(19)18-9-16-17(24-18)6-7-22-20(16)25/h2-5,8-10,24H,6-7,11H2,1H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma 2 using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 32 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26025

(N-methyl-4-[(2-{methyl[3-(trifluoromethyl)phenyl]a...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O2/c1-26-20(31)18-12-16(8-9-27-18)32-15-6-7-19-17(11-15)29-21(30(19)2)28-14-5-3-4-13(10-14)22(23,24)25/h3-12H,1-2H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26038

(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES CCCC1CCCCN1NC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C28H31BrN6O2/c1-3-6-21-7-4-5-16-35(21)33-27(36)25-18-23(14-15-30-25)37-22-12-13-26-24(17-22)32-28(34(26)2)31-20-10-8-19(29)9-11-20/h8-15,17-18,21H,3-7,16H2,1-2H3,(H,31,32)(H,33,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-2
(Homo sapiens (Human)) | BDBM50400748
(CHEMBL2203556)Show InChI InChI=1S/C18H17N5O2/c1-25-15-10-20-18(21-11-5-3-2-4-6-11)23-16(15)14-9-12-13(22-14)7-8-19-17(12)24/h2-6,9-10,22H,7-8H2,1H3,(H,19,24)(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma 2 using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 32 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26025

(N-methyl-4-[(2-{methyl[3-(trifluoromethyl)phenyl]a...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O2/c1-26-20(31)18-12-16(8-9-27-18)32-15-6-7-19-17(11-15)29-21(30(19)2)28-14-5-3-4-13(10-14)22(23,24)25/h3-12H,1-2H3,(H,26,31)(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26017

(4-({2-[(3-tert-butylphenyl)amino]-1H-1,3-benzodiaz...)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(Nc4cccc(c4)C(C)(C)C)[nH]c3c2)ccn1 Show InChI InChI=1S/C24H25N5O2/c1-24(2,3)15-6-5-7-16(12-15)27-23-28-19-9-8-17(13-20(19)29-23)31-18-10-11-26-21(14-18)22(30)25-4/h5-14H,1-4H3,(H,25,30)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-2
(Homo sapiens (Human)) | BDBM50400752

(CHEMBL2203552)Show SMILES COc1cnc(nc1-c1cc2c(CCNC2=O)[nH]1)-c1cnc2ccccc2c1 Show InChI InChI=1S/C21H17N5O2/c1-28-18-11-24-20(13-8-12-4-2-3-5-15(12)23-10-13)26-19(18)17-9-14-16(25-17)6-7-22-21(14)27/h2-5,8-11,25H,6-7H2,1H3,(H,22,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma 2 using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 32 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase I isoform alpha
(Homo sapiens (Human)) | BDBM50400752

(CHEMBL2203552)Show SMILES COc1cnc(nc1-c1cc2c(CCNC2=O)[nH]1)-c1cnc2ccccc2c1 Show InChI InChI=1S/C21H17N5O2/c1-28-18-11-24-20(13-8-12-4-2-3-5-15(12)23-10-13)26-19(18)17-9-14-16(25-17)6-7-22-21(14)27/h2-5,8-11,25H,6-7H2,1H3,(H,22,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-alpha using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 8 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26029
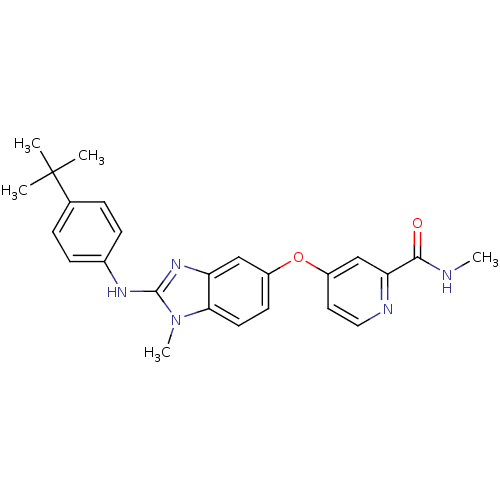
(4-({2-[(4-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(cc4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-6-8-17(9-7-16)28-24-29-20-14-18(10-11-22(20)30(24)5)32-19-12-13-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26026

(N-methyl-4-[(2-{methyl[4-(trifluoromethyl)phenyl]a...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(cc4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O2/c1-26-20(31)18-12-16(9-10-27-18)32-15-7-8-19-17(11-15)29-21(30(19)2)28-14-5-3-13(4-6-14)22(23,24)25/h3-12H,1-2H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26032
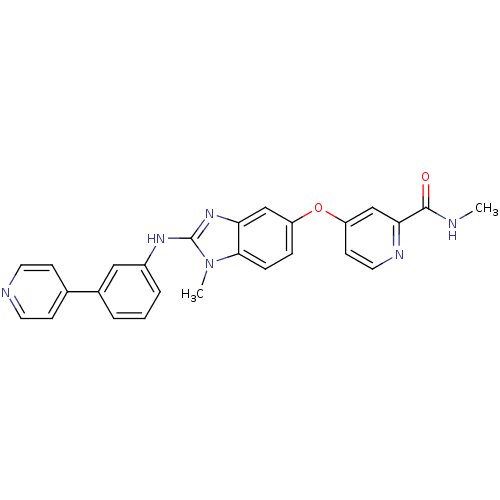
(N-methyl-4-[(2-{methyl[3-(pyridin-4-yl)phenyl]amin...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)-c4ccncc4)nc3c2)ccn1 Show InChI InChI=1S/C26H22N6O2/c1-27-25(33)23-16-21(10-13-29-23)34-20-6-7-24-22(15-20)31-26(32(24)2)30-19-5-3-4-18(14-19)17-8-11-28-12-9-17/h3-16H,1-2H3,(H,27,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26021
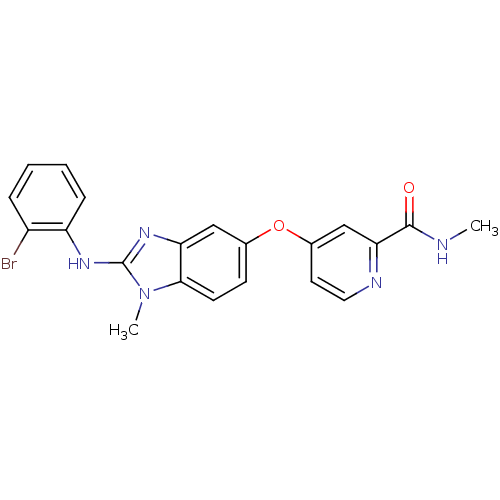
(4-({2-[(2-bromophenyl)(methyl)amino]-1H-1,3-benzod...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccccc4Br)nc3c2)ccn1 Show InChI InChI=1S/C21H18BrN5O2/c1-23-20(28)18-12-14(9-10-24-18)29-13-7-8-19-17(11-13)26-21(27(19)2)25-16-6-4-3-5-15(16)22/h3-12H,1-2H3,(H,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26016

(N-methyl-4-[(2-{[4-(trifluoromethyl)phenyl]amino}-...)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(Nc4ccc(cc4)C(F)(F)F)[nH]c3c2)ccn1 Show InChI InChI=1S/C21H16F3N5O2/c1-25-19(30)18-11-15(8-9-26-18)31-14-6-7-16-17(10-14)29-20(28-16)27-13-4-2-12(3-5-13)21(22,23)24/h2-11H,1H3,(H,25,30)(H2,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
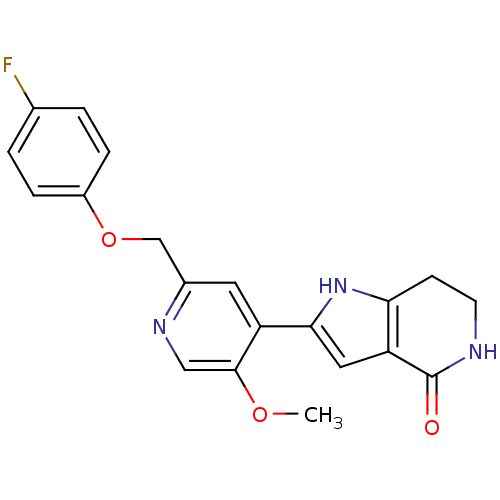
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26037

(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES Cn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(=O)NCCO)ccc12 Show InChI InChI=1S/C22H20BrN5O3/c1-28-20-7-6-16(31-17-8-9-24-19(13-17)21(30)25-10-11-29)12-18(20)27-22(28)26-15-4-2-14(23)3-5-15/h2-9,12-13,29H,10-11H2,1H3,(H,25,30)(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26035
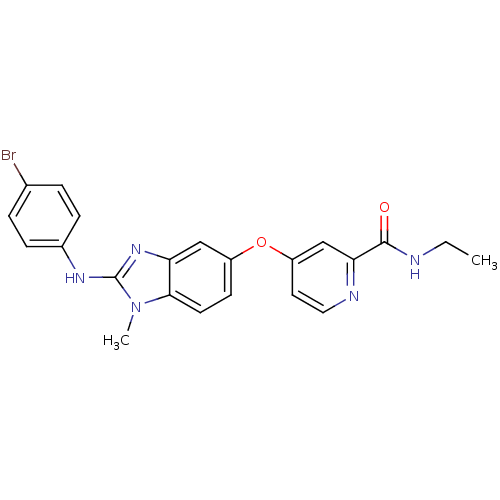
(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES CCNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C22H20BrN5O2/c1-3-24-21(29)19-13-17(10-11-25-19)30-16-8-9-20-18(12-16)27-22(28(20)2)26-15-6-4-14(23)5-7-15/h4-13H,3H2,1-2H3,(H,24,29)(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26035

(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES CCNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C22H20BrN5O2/c1-3-24-21(29)19-13-17(10-11-25-19)30-16-8-9-20-18(12-16)27-22(28(20)2)26-15-6-4-14(23)5-7-15/h4-13H,3H2,1-2H3,(H,24,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
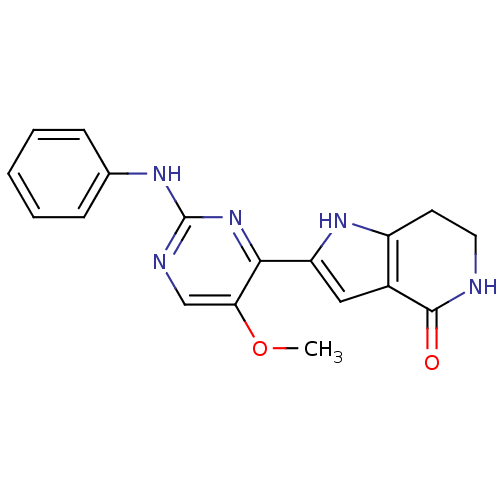
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26032

(N-methyl-4-[(2-{methyl[3-(pyridin-4-yl)phenyl]amin...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)-c4ccncc4)nc3c2)ccn1 Show InChI InChI=1S/C26H22N6O2/c1-27-25(33)23-16-21(10-13-29-23)34-20-6-7-24-22(15-20)31-26(32(24)2)30-19-5-3-4-18(14-19)17-8-11-28-12-9-17/h3-16H,1-2H3,(H,27,33)(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26020
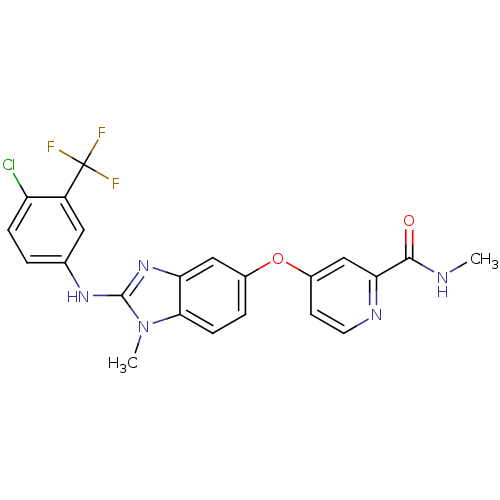
(4-[(2-{[4-chloro-3-(trifluoromethyl)phenyl](methyl...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Cl)c(c4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H17ClF3N5O2/c1-27-20(32)18-11-14(7-8-28-18)33-13-4-6-19-17(10-13)30-21(31(19)2)29-12-3-5-16(23)15(9-12)22(24,25)26/h3-11H,1-2H3,(H,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26018

(4-({2-[(4-tert-butylphenyl)amino]-1H-1,3-benzodiaz...)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(Nc4ccc(cc4)C(C)(C)C)[nH]c3c2)ccn1 Show InChI InChI=1S/C24H25N5O2/c1-24(2,3)15-5-7-16(8-6-15)27-23-28-19-10-9-17(13-20(19)29-23)31-18-11-12-26-21(14-18)22(30)25-4/h5-14H,1-4H3,(H,25,30)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50400751
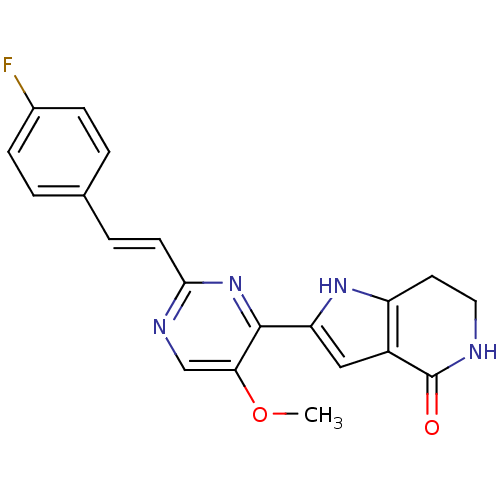
(CHEMBL2203553)Show SMILES COc1cnc(\C=C\c2ccc(F)cc2)nc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C20H17FN4O2/c1-27-17-11-23-18(7-4-12-2-5-13(21)6-3-12)25-19(17)16-10-14-15(24-16)8-9-22-20(14)26/h2-7,10-11,24H,8-9H2,1H3,(H,22,26)/b7-4+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-delta using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 11 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50400748

(CHEMBL2203556)Show InChI InChI=1S/C18H17N5O2/c1-25-15-10-20-18(21-11-5-3-2-4-6-11)23-16(15)14-9-12-13(22-14)7-8-19-17(12)24/h2-6,9-10,22H,7-8H2,1H3,(H,19,24)(H,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26036
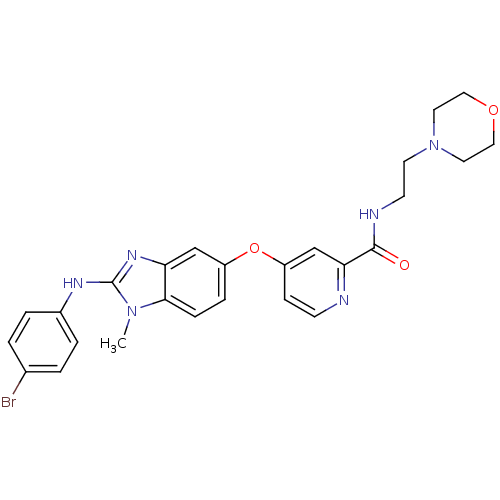
(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES Cn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(=O)NCCN3CCOCC3)ccc12 Show InChI InChI=1S/C26H27BrN6O3/c1-32-24-7-6-20(16-22(24)31-26(32)30-19-4-2-18(27)3-5-19)36-21-8-9-28-23(17-21)25(34)29-10-11-33-12-14-35-15-13-33/h2-9,16-17H,10-15H2,1H3,(H,29,34)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-2
(Homo sapiens (Human)) | BDBM50400750
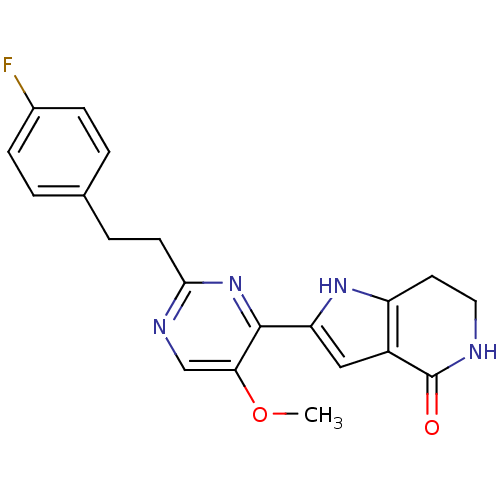
(CHEMBL2203554)Show SMILES COc1cnc(CCc2ccc(F)cc2)nc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C20H19FN4O2/c1-27-17-11-23-18(7-4-12-2-5-13(21)6-3-12)25-19(17)16-10-14-15(24-16)8-9-22-20(14)26/h2-3,5-6,10-11,24H,4,7-9H2,1H3,(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma 2 using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 32 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26033
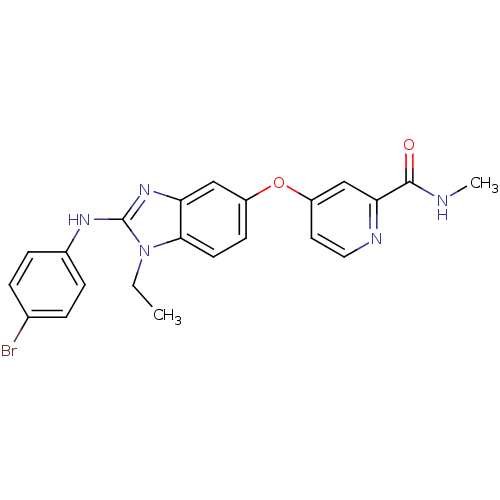
(4-({2-[(4-bromophenyl)(ethyl)amino]-1H-1,3-benzodi...)Show SMILES CCn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(=O)NC)ccc12 Show InChI InChI=1S/C22H20BrN5O2/c1-3-28-20-9-8-16(30-17-10-11-25-19(13-17)21(29)24-2)12-18(20)27-22(28)26-15-6-4-14(23)5-7-15/h4-13H,3H2,1-2H3,(H,24,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform alpha
(Homo sapiens (Human)) | BDBM50400749

(CHEMBL2203555)Show SMILES COc1cnc(NCc2ccccc2)nc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C19H19N5O2/c1-26-16-11-22-19(21-10-12-5-3-2-4-6-12)24-17(16)15-9-13-14(23-15)7-8-20-18(13)25/h2-6,9,11,23H,7-8,10H2,1H3,(H,20,25)(H,21,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-alpha using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 8 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26013
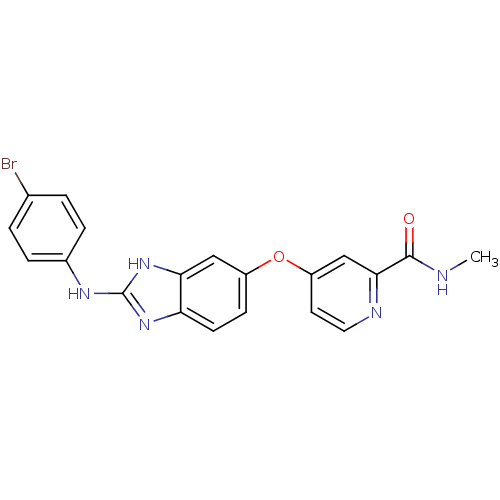
(4-({2-[(4-bromophenyl)amino]-1H-1,3-benzodiazol-5-...)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(Nc4ccc(Br)cc4)[nH]c3c2)ccn1 Show InChI InChI=1S/C20H16BrN5O2/c1-22-19(27)18-11-15(8-9-23-18)28-14-6-7-16-17(10-14)26-20(25-16)24-13-4-2-12(21)3-5-13/h2-11H,1H3,(H,22,27)(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26016

(N-methyl-4-[(2-{[4-(trifluoromethyl)phenyl]amino}-...)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(Nc4ccc(cc4)C(F)(F)F)[nH]c3c2)ccn1 Show InChI InChI=1S/C21H16F3N5O2/c1-25-19(30)18-11-15(8-9-26-18)31-14-6-7-16-17(10-14)29-20(28-16)27-13-4-2-12(3-5-13)21(22,23)24/h2-11H,1H3,(H,25,30)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50400757

(CHEMBL2203561)Show SMILES COc1cnc(COc2ccc(F)cc2)cc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C20H18FN3O3/c1-26-19-10-23-13(11-27-14-4-2-12(21)3-5-14)8-15(19)18-9-16-17(24-18)6-7-22-20(16)25/h2-5,8-10,24H,6-7,11H2,1H3,(H,22,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-delta using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 11 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50400748

(CHEMBL2203556)Show InChI InChI=1S/C18H17N5O2/c1-25-15-10-20-18(21-11-5-3-2-4-6-11)23-16(15)14-9-12-13(22-14)7-8-19-17(12)24/h2-6,9-10,22H,7-8H2,1H3,(H,19,24)(H,20,21,23) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-delta using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 11 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50400749

(CHEMBL2203555)Show SMILES COc1cnc(NCc2ccccc2)nc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C19H19N5O2/c1-26-16-11-22-19(21-10-12-5-3-2-4-6-12)24-17(16)15-9-13-14(23-15)7-8-20-18(13)25/h2-6,9,11,23H,7-8,10H2,1H3,(H,20,25)(H,21,22,24) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-delta using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 11 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-2
(Homo sapiens (Human)) | BDBM50400745
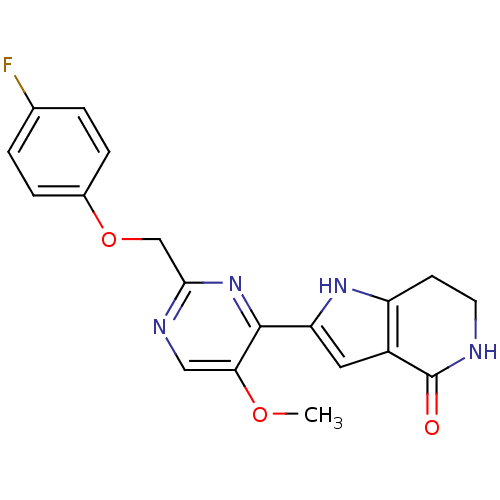
(CHEMBL2203559)Show SMILES COc1cnc(COc2ccc(F)cc2)nc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C19H17FN4O3/c1-26-16-9-22-17(10-27-12-4-2-11(20)3-5-12)24-18(16)15-8-13-14(23-15)6-7-21-19(13)25/h2-5,8-9,23H,6-7,10H2,1H3,(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma 2 using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 32 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26022

(4-({2-[(3-bromophenyl)(methyl)amino]-1H-1,3-benzod...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(Br)c4)nc3c2)ccn1 Show InChI InChI=1S/C21H18BrN5O2/c1-23-20(28)18-12-16(8-9-24-18)29-15-6-7-19-17(11-15)26-21(27(19)2)25-14-5-3-4-13(22)10-14/h3-12H,1-2H3,(H,23,28)(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26017

(4-({2-[(3-tert-butylphenyl)amino]-1H-1,3-benzodiaz...)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(Nc4cccc(c4)C(C)(C)C)[nH]c3c2)ccn1 Show InChI InChI=1S/C24H25N5O2/c1-24(2,3)15-6-5-7-16(12-15)27-23-28-19-9-8-17(13-20(19)29-23)31-18-10-11-26-21(14-18)22(30)25-4/h5-14H,1-4H3,(H,25,30)(H2,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform gamma-2
(Homo sapiens (Human)) | BDBM50400744
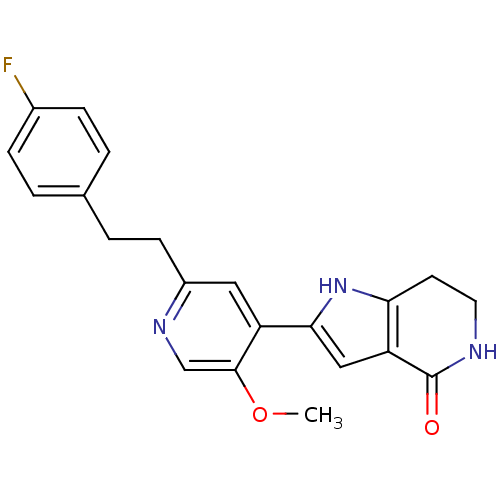
(CHEMBL2203560)Show SMILES COc1cnc(CCc2ccc(F)cc2)cc1-c1cc2c(CCNC2=O)[nH]1 Show InChI InChI=1S/C21H20FN3O2/c1-27-20-12-24-15(7-4-13-2-5-14(22)6-3-13)10-16(20)19-11-17-18(25-19)8-9-23-21(17)26/h2-3,5-6,10-12,25H,4,7-9H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CK1-gamma 2 using biotin[long chain]-KKRRRAL{pS}VATLPGL substrate in presence of 32 uM ATP |
ACS Med Chem Lett 3: 1059-1064 (2012)
Article DOI: 10.1021/ml300278f
BindingDB Entry DOI: 10.7270/Q21V5G41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26020

(4-[(2-{[4-chloro-3-(trifluoromethyl)phenyl](methyl...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4ccc(Cl)c(c4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H17ClF3N5O2/c1-27-20(32)18-11-14(7-8-28-18)33-13-4-6-19-17(10-13)30-21(31(19)2)29-12-3-5-16(23)15(9-12)22(24,25)26/h3-11H,1-2H3,(H,27,32)(H,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM26031
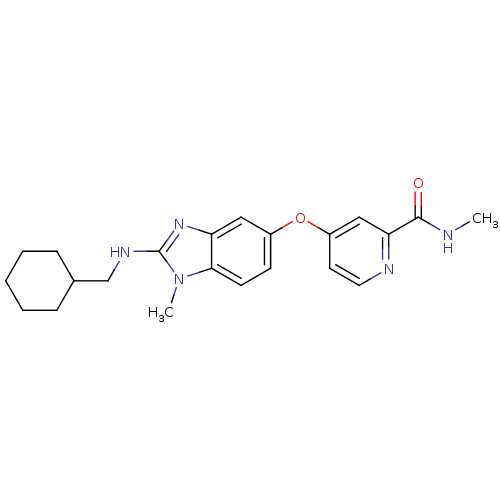
(4-({2-[(cyclohexylmethyl)(methyl)amino]-1H-1,3-ben...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(NCC4CCCCC4)nc3c2)ccn1 Show InChI InChI=1S/C22H27N5O2/c1-23-21(28)19-13-17(10-11-24-19)29-16-8-9-20-18(12-16)26-22(27(20)2)25-14-15-6-4-3-5-7-15/h8-13,15H,3-7,14H2,1-2H3,(H,23,28)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf [V600E]
(Homo sapiens (Human)) | BDBM26036

(4-({2-[(4-bromophenyl)amino]-1-methyl-1H-1,3-benzo...)Show SMILES Cn1c(Nc2ccc(Br)cc2)nc2cc(Oc3ccnc(c3)C(=O)NCCN3CCOCC3)ccc12 Show InChI InChI=1S/C26H27BrN6O3/c1-32-24-7-6-20(16-22(24)31-26(32)30-19-4-2-18(27)3-5-19)36-21-8-9-28-23(17-21)25(34)29-10-11-33-12-14-35-15-13-33/h2-9,16-17H,10-15H2,1H3,(H,29,34)(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis
| Assay Description
To measure compound inhibitor effects on the ability of Raf enzymes to phosphorylate the Mek substrate, a capture ELISA utilizing streptavidin-coated... |
J Med Chem 51: 7049-52 (2008)
Article DOI: 10.1021/jm801050k
BindingDB Entry DOI: 10.7270/Q2B56H1C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data