 Found 857 hits with Last Name = 'dudley' and Initial = 'd'
Found 857 hits with Last Name = 'dudley' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433530
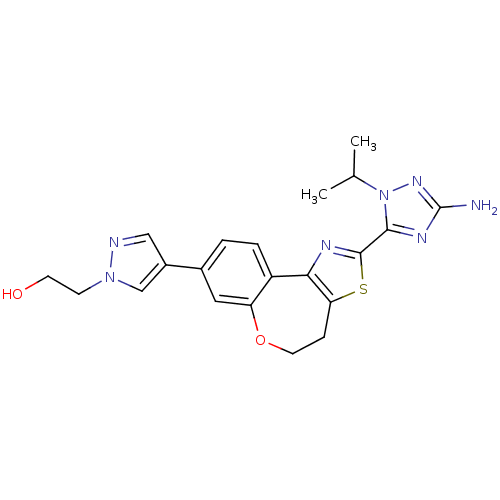
(CHEMBL2381382)Show SMILES CC(C)n1nc(N)nc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H23N7O2S/c1-12(2)28-19(25-21(22)26-28)20-24-18-15-4-3-13(14-10-23-27(11-14)6-7-29)9-16(15)30-8-5-17(18)31-20/h3-4,9-12,29H,5-8H2,1-2H3,(H2,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433534
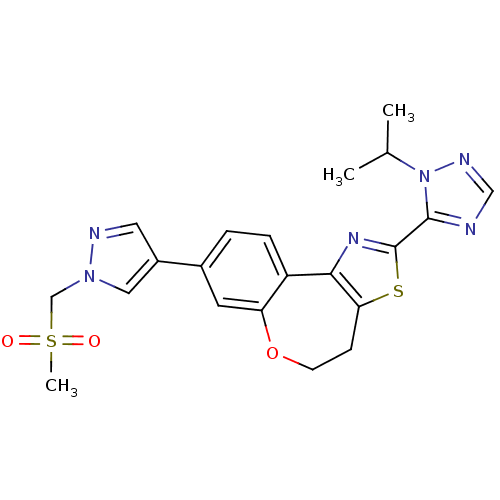
(CHEMBL2381375)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CS(C)(=O)=O)c2)s1 Show InChI InChI=1S/C21H22N6O3S2/c1-13(2)27-20(22-11-24-27)21-25-19-16-5-4-14(8-17(16)30-7-6-18(19)31-21)15-9-23-26(10-15)12-32(3,28)29/h4-5,8-11,13H,6-7,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433533
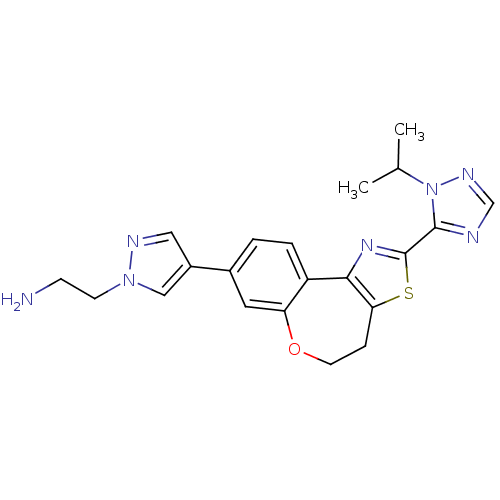
(CHEMBL2381376)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCN)c2)s1 Show InChI InChI=1S/C21H23N7OS/c1-13(2)28-20(23-12-25-28)21-26-19-16-4-3-14(15-10-24-27(11-15)7-6-22)9-17(16)29-8-5-18(19)30-21/h3-4,9-13H,5-8,22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434810
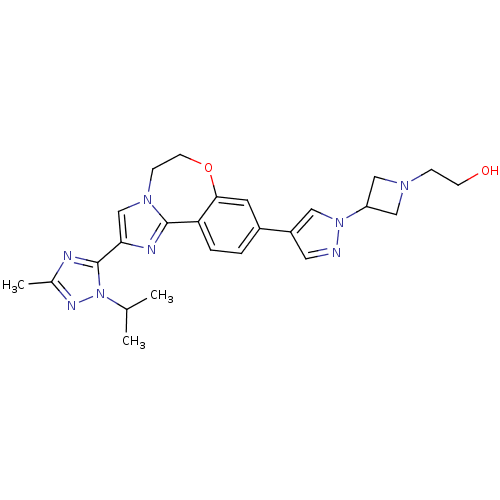
(CHEMBL2386970)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C1CN(CCO)C1 Show InChI InChI=1S/C25H30N8O2/c1-16(2)33-25(27-17(3)29-33)22-15-31-7-9-35-23-10-18(4-5-21(23)24(31)28-22)19-11-26-32(12-19)20-13-30(14-20)6-8-34/h4-5,10-12,15-16,20,34H,6-9,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433532
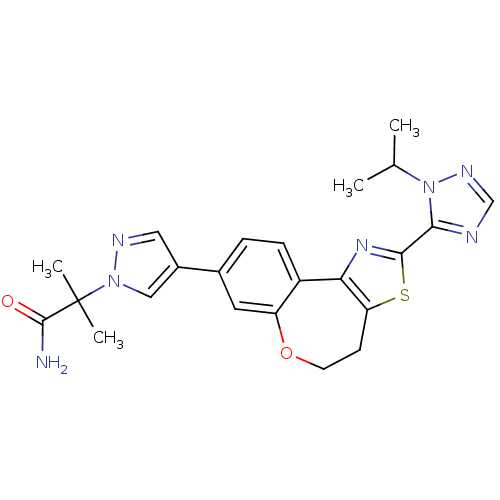
(CHEMBL2381377)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(c2)C(C)(C)C(N)=O)s1 Show InChI InChI=1S/C23H25N7O2S/c1-13(2)30-20(25-12-27-30)21-28-19-16-6-5-14(9-17(16)32-8-7-18(19)33-21)15-10-26-29(11-15)23(3,4)22(24)31/h5-6,9-13H,7-8H2,1-4H3,(H2,24,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50434806
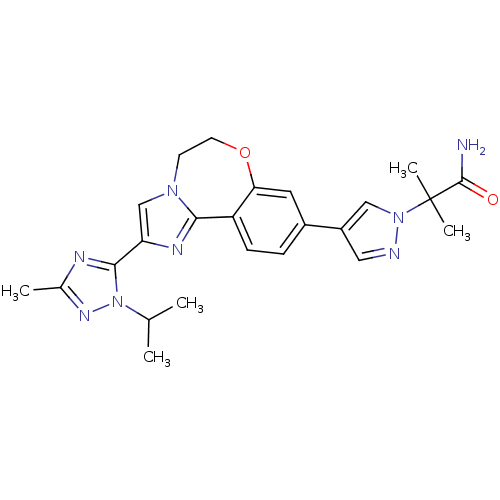
(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433529
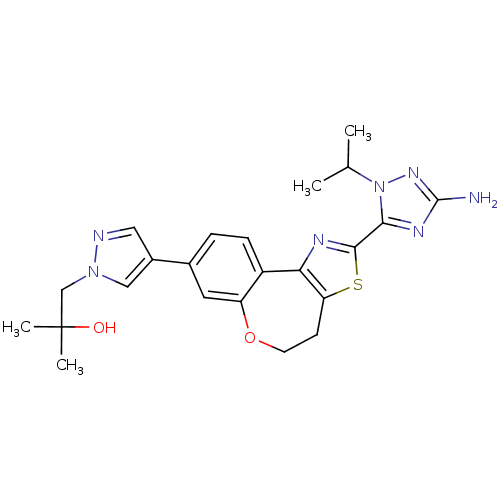
(CHEMBL2381380)Show SMILES CC(C)n1nc(N)nc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CC(C)(C)O)c2)s1 Show InChI InChI=1S/C23H27N7O2S/c1-13(2)30-20(27-22(24)28-30)21-26-19-16-6-5-14(9-17(16)32-8-7-18(19)33-21)15-10-25-29(11-15)12-23(3,4)31/h5-6,9-11,13,31H,7-8,12H2,1-4H3,(H2,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434807

(CHEMBL2387079)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CC(C)(C)O)c1 Show InChI InChI=1S/C23H27N7O2/c1-15(2)30-22(24-14-26-30)19-12-28-7-8-32-20-9-16(5-6-18(20)21(28)27-19)17-10-25-29(11-17)13-23(3,4)31/h5-6,9-12,14-15,31H,7-8,13H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433527

(CHEMBL2381379)Show SMILES CC(C)(O)Cn1cc(cn1)-c1ccc2-c3nc(sc3CCOc2c1)-c1ncnn1CC(F)(F)F Show InChI InChI=1S/C22H21F3N6O2S/c1-21(2,32)10-30-9-14(8-27-30)13-3-4-15-16(7-13)33-6-5-17-18(15)29-20(34-17)19-26-12-28-31(19)11-22(23,24)25/h3-4,7-9,12,32H,5-6,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433535
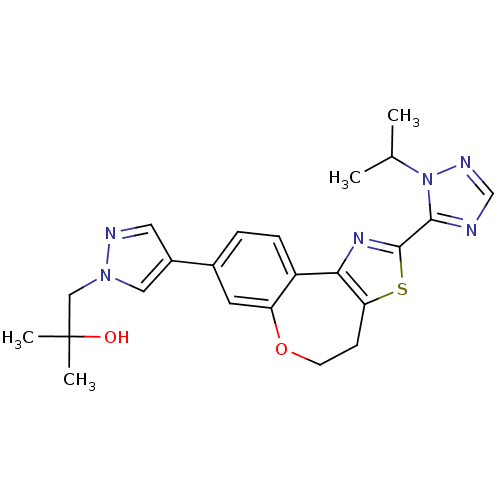
(CHEMBL2381374)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CC(C)(C)O)c2)s1 Show InChI InChI=1S/C23H26N6O2S/c1-14(2)29-21(24-13-26-29)22-27-20-17-6-5-15(9-18(17)31-8-7-19(20)32-22)16-10-25-28(11-16)12-23(3,4)30/h5-6,9-11,13-14,30H,7-8,12H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434812

(CHEMBL2387086)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C21H23N7O2/c1-14(2)28-21(22-13-24-28)18-12-26-6-8-30-19-9-15(3-4-17(19)20(26)25-18)16-10-23-27(11-16)5-7-29/h3-4,9-14,29H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433528
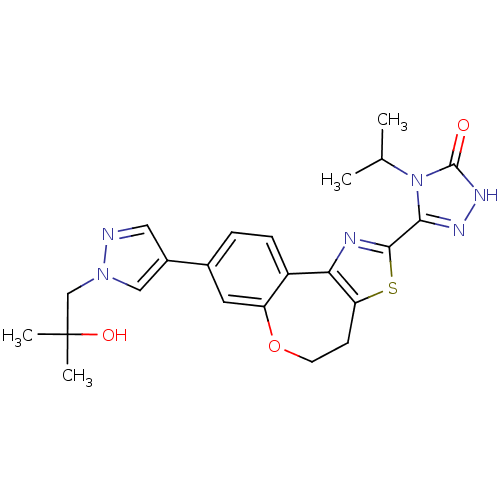
(CHEMBL2381381)Show SMILES CC(C)n1c(n[nH]c1=O)-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CC(C)(C)O)c2)s1 Show InChI InChI=1S/C23H26N6O3S/c1-13(2)29-20(26-27-22(29)30)21-25-19-16-6-5-14(9-17(16)32-8-7-18(19)33-21)15-10-24-28(11-15)12-23(3,4)31/h5-6,9-11,13,31H,7-8,12H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433531
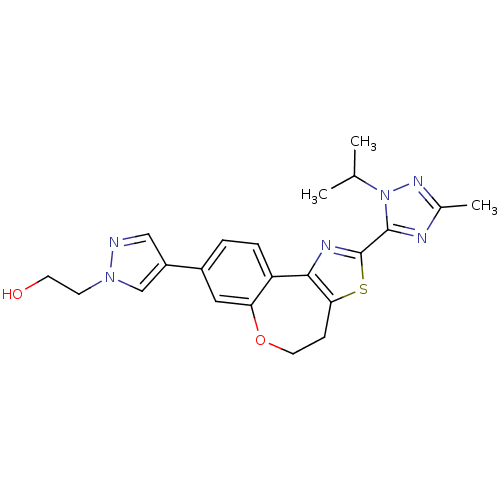
(CHEMBL2381378)Show SMILES CC(C)n1nc(C)nc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C22H24N6O2S/c1-13(2)28-21(24-14(3)26-28)22-25-20-17-5-4-15(16-11-23-27(12-16)7-8-29)10-18(17)30-9-6-19(20)31-22/h4-5,10-13,29H,6-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433536
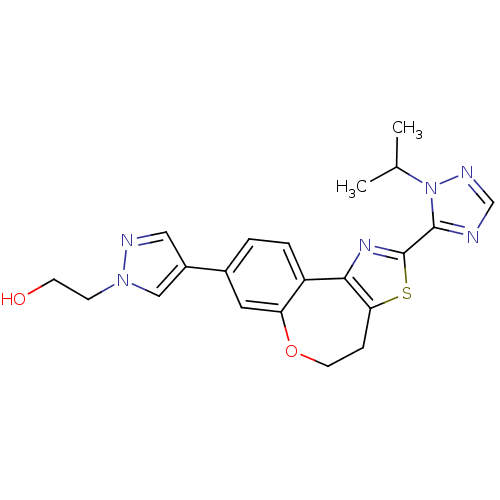
(CHEMBL2381373)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H22N6O2S/c1-13(2)27-20(22-12-24-27)21-25-19-16-4-3-14(15-10-23-26(11-15)6-7-28)9-17(16)29-8-5-18(19)30-21/h3-4,9-13,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434814

(CHEMBL2387082)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(C)c1 Show InChI InChI=1S/C21H23N7O/c1-13(2)28-21(23-14(3)25-28)18-12-27-7-8-29-19-9-15(16-10-22-26(4)11-16)5-6-17(19)20(27)24-18/h5-6,9-13H,7-8H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434808
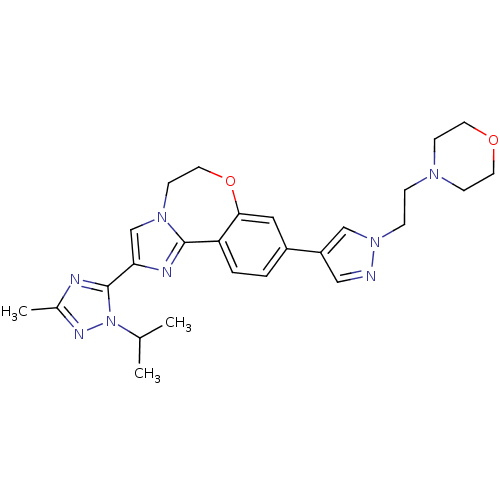
(CHEMBL2386972)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCN2CCOCC2)c1 Show InChI InChI=1S/C26H32N8O2/c1-18(2)34-26(28-19(3)30-34)23-17-32-10-13-36-24-14-20(4-5-22(24)25(32)29-23)21-15-27-33(16-21)7-6-31-8-11-35-12-9-31/h4-5,14-18H,6-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434817

(CHEMBL2387081)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cn[nH]c1 Show InChI InChI=1S/C19H19N7O/c1-12(2)26-19(20-11-23-26)16-10-25-5-6-27-17-7-13(14-8-21-22-9-14)3-4-15(17)18(25)24-16/h3-4,7-12H,5-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434809

(CHEMBL2386971)Show SMILES C[C@H](O)C(=O)N1CC(C1)n1cc(cn1)-c1ccc2-c3nc(cn3CCOc2c1)-c1nc(C)nn1C(C)C |r| Show InChI InChI=1S/C26H30N8O3/c1-15(2)34-25(28-17(4)30-34)22-14-31-7-8-37-23-9-18(5-6-21(23)24(31)29-22)19-10-27-33(11-19)20-12-32(13-20)26(36)16(3)35/h5-6,9-11,14-16,20,35H,7-8,12-13H2,1-4H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434813

(CHEMBL2387083)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C22H25N7O2/c1-14(2)29-22(24-15(3)26-29)19-13-27-7-9-31-20-10-16(4-5-18(20)21(27)25-19)17-11-23-28(12-17)6-8-30/h4-5,10-14,30H,6-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433537
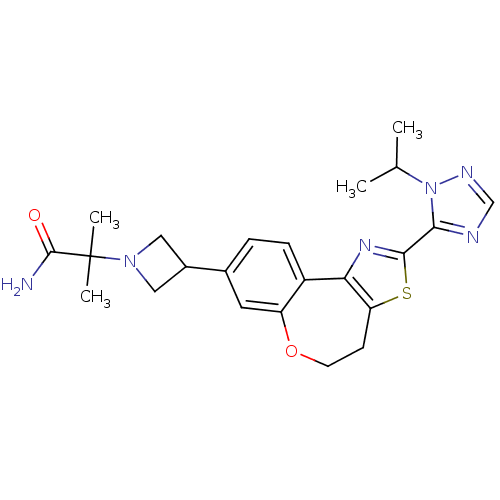
(CHEMBL2381279)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)C2CN(C2)C(C)(C)C(N)=O)s1 Show InChI InChI=1S/C23H28N6O2S/c1-13(2)29-20(25-12-26-29)21-27-19-16-6-5-14(9-17(16)31-8-7-18(19)32-21)15-10-28(11-15)23(3,4)22(24)30/h5-6,9,12-13,15H,7-8,10-11H2,1-4H3,(H2,24,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50433536

(CHEMBL2381373)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H22N6O2S/c1-13(2)27-20(22-12-24-27)21-25-19-16-4-3-14(15-10-23-26(11-15)6-7-28)9-17(16)29-8-5-18(19)30-21/h3-4,9-13,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kgamma expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433539
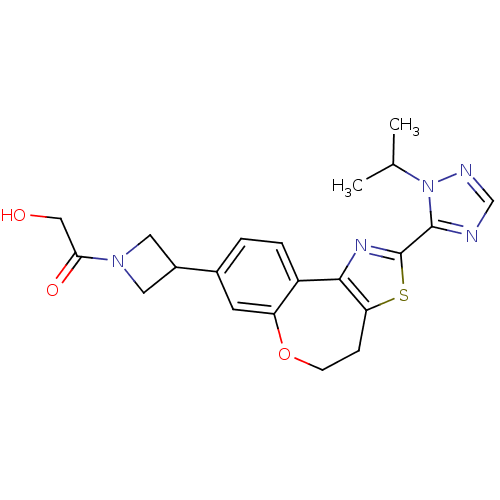
(CHEMBL2381277)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)C2CN(C2)C(=O)CO)s1 Show InChI InChI=1S/C21H23N5O3S/c1-12(2)26-20(22-11-23-26)21-24-19-15-4-3-13(14-8-25(9-14)18(28)10-27)7-16(15)29-6-5-17(19)30-21/h3-4,7,11-12,14,27H,5-6,8-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50433536

(CHEMBL2381373)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H22N6O2S/c1-13(2)27-20(22-12-24-27)21-25-19-16-4-3-14(15-10-23-26(11-15)6-7-28)9-17(16)29-8-5-18(19)30-21/h3-4,9-13,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kdelta expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433541
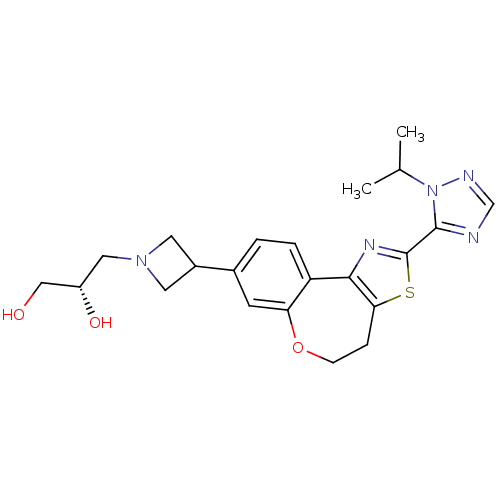
(CHEMBL2381275)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)C2CN(C[C@H](O)CO)C2)s1 |r| Show InChI InChI=1S/C22H27N5O3S/c1-13(2)27-21(23-12-24-27)22-25-20-17-4-3-14(7-18(17)30-6-5-19(20)31-22)15-8-26(9-15)10-16(29)11-28/h3-4,7,12-13,15-16,28-29H,5-6,8-11H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human Angiotensin receptor 1 |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433540
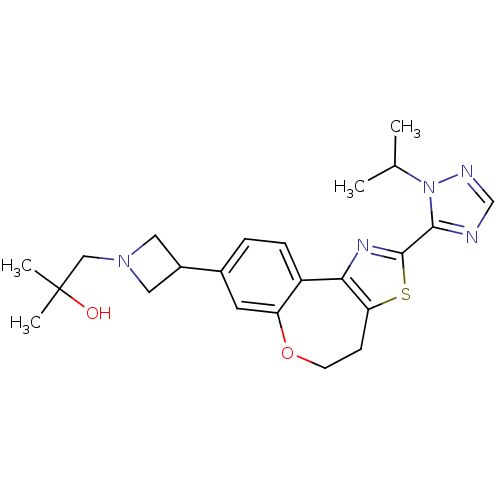
(CHEMBL2381276)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)C2CN(CC(C)(C)O)C2)s1 Show InChI InChI=1S/C23H29N5O2S/c1-14(2)28-21(24-13-25-28)22-26-20-17-6-5-15(9-18(17)30-8-7-19(20)31-22)16-10-27(11-16)12-23(3,4)29/h5-6,9,13-14,16,29H,7-8,10-12H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433538
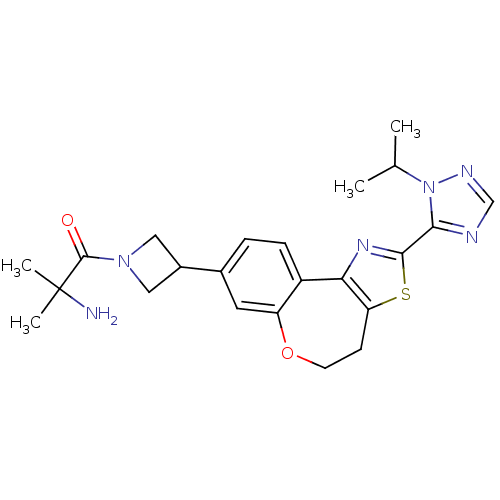
(CHEMBL2381278)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)C2CN(C2)C(=O)C(C)(C)N)s1 Show InChI InChI=1S/C23H28N6O2S/c1-13(2)29-20(25-12-26-29)21-27-19-16-6-5-14(9-17(16)31-8-7-18(19)32-21)15-10-28(11-15)22(30)23(3,4)24/h5-6,9,12-13,15H,7-8,10-11,24H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434811
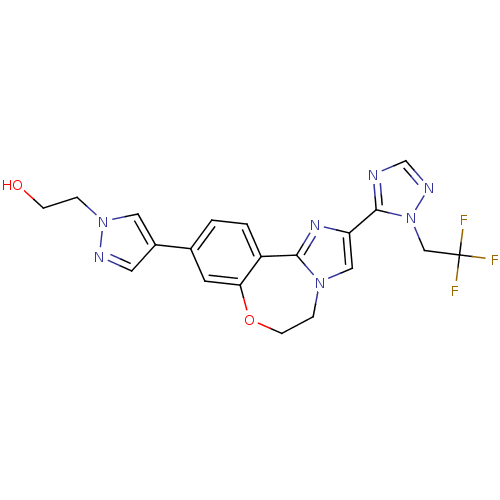
(CHEMBL2387087)Show SMILES OCCn1cc(cn1)-c1ccc2-c3nc(cn3CCOc2c1)-c1ncnn1CC(F)(F)F Show InChI InChI=1S/C20H18F3N7O2/c21-20(22,23)11-30-19(24-12-26-30)16-10-28-4-6-32-17-7-13(1-2-15(17)18(28)27-16)14-8-25-29(9-14)3-5-31/h1-2,7-10,12,31H,3-6,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25028
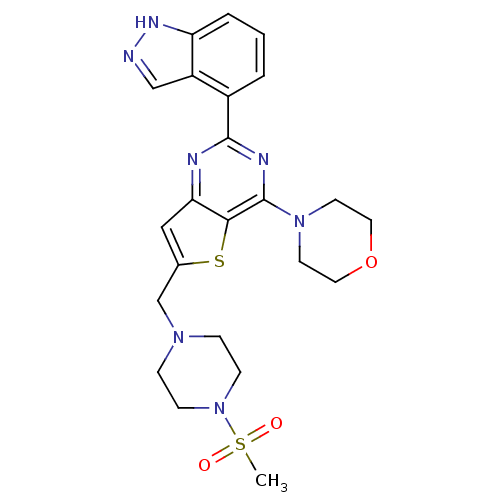
(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kgamma expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50433542
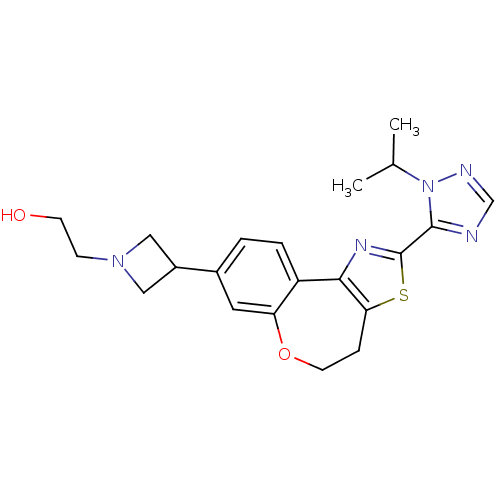
(CHEMBL2381274)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)C2CN(CCO)C2)s1 Show InChI InChI=1S/C21H25N5O2S/c1-13(2)26-20(22-12-23-26)21-24-19-16-4-3-14(15-10-25(11-15)6-7-27)9-17(16)28-8-5-18(19)29-21/h3-4,9,12-13,15,27H,5-8,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kalpha expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434815
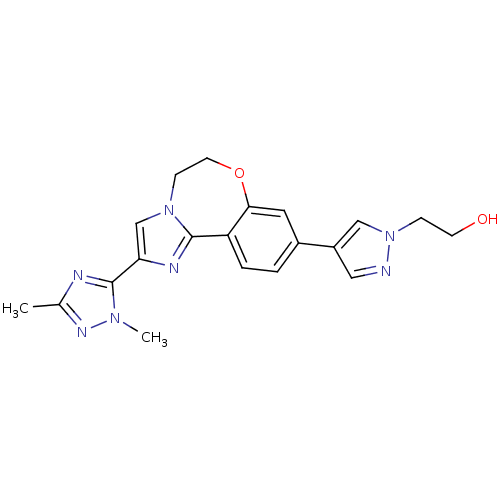
(CHEMBL2387085)Show SMILES Cc1nc(-c2cn3CCOc4cc(ccc4-c3n2)-c2cnn(CCO)c2)n(C)n1 Show InChI InChI=1S/C20H21N7O2/c1-13-22-20(25(2)24-13)17-12-26-6-8-29-18-9-14(3-4-16(18)19(26)23-17)15-10-21-27(11-15)5-7-28/h3-4,9-12,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50433536

(CHEMBL2381373)Show SMILES CC(C)n1ncnc1-c1nc-2c(CCOc3cc(ccc-23)-c2cnn(CCO)c2)s1 Show InChI InChI=1S/C21H22N6O2S/c1-13(2)27-20(22-12-24-27)21-25-19-16-4-3-14(15-10-23-26(11-15)6-7-28)9-17(16)29-8-5-18(19)30-21/h3-4,9-13,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kbeta expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presenc... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434816
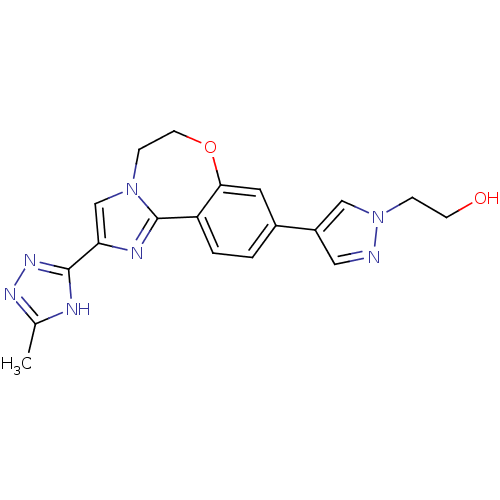
(CHEMBL2387084)Show SMILES Cc1nnc([nH]1)-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C19H19N7O2/c1-12-21-18(24-23-12)16-11-25-5-7-28-17-8-13(2-3-15(17)19(25)22-16)14-9-20-26(10-14)4-6-27/h2-3,8-11,27H,4-7H2,1H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-fused human recombinant PI3Kdelta expressed in baculovirus infected SF9 cells after 1 hr by scintillation proximity assay in presen... |
Bioorg Med Chem Lett 23: 2606-13 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.102
BindingDB Entry DOI: 10.7270/Q2W95BKF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50347563

(CHEMBL1801740)Show SMILES CCc1nc2c(C)cc(CC(C)C)nc2n1[C@H]1CCc2cc(ccc12)-c1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C29H31N7/c1-5-26-31-27-18(4)15-21(14-17(2)3)30-29(27)36(26)25-13-11-20-16-19(10-12-23(20)25)22-8-6-7-9-24(22)28-32-34-35-33-28/h6-10,12,15-17,25H,5,11,13-14H2,1-4H3,(H,32,33,34,35)/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human Angiotensin receptor 2 |
J Med Chem 54: 4219-33 (2011)
Article DOI: 10.1021/jm200409s
BindingDB Entry DOI: 10.7270/Q2SB463J |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266775

((2R,4S)-4-(2-Chlorophenyl)-N1-(4-chlorophenyl)-4-h...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccccc1Cl |r| Show InChI InChI=1S/C28H23Cl2N5O4/c29-18-8-10-19(11-9-18)32-27(38)35-17-28(39,21-5-1-2-6-22(21)30)15-23(35)26(37)33-24-13-12-20(16-31-24)34-14-4-3-7-25(34)36/h1-14,16,23,39H,15,17H2,(H,32,38)(H,31,33,37)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266920

((2R,4R)-N1-(4-Chlorophenyl)-4-ethoxy-4-ethyl-N2-(2...)Show SMILES CCO[C@]1(CC)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O |r| Show InChI InChI=1S/C27H28ClFN4O4/c1-3-27(37-4-2)16-23(33(17-27)26(36)30-19-10-8-18(28)9-11-19)25(35)31-22-13-12-20(15-21(22)29)32-14-6-5-7-24(32)34/h5-15,23H,3-4,16-17H2,1-2H3,(H,30,36)(H,31,35)/t23-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266921
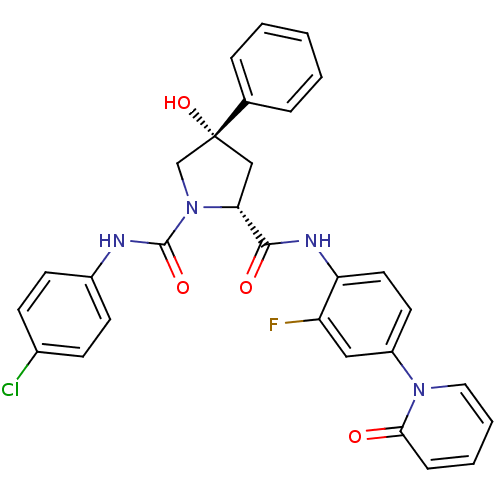
((2R,4S)-N1-(4-Chlorophenyl)-N2-(2-fluoro-4-(2-oxop...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-n1ccccc1=O)c1ccccc1 |r| Show InChI InChI=1S/C29H24ClFN4O4/c30-20-9-11-21(12-10-20)32-28(38)35-18-29(39,19-6-2-1-3-7-19)17-25(35)27(37)33-24-14-13-22(16-23(24)31)34-15-5-4-8-26(34)36/h1-16,25,39H,17-18H2,(H,32,38)(H,33,37)/t25-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266923
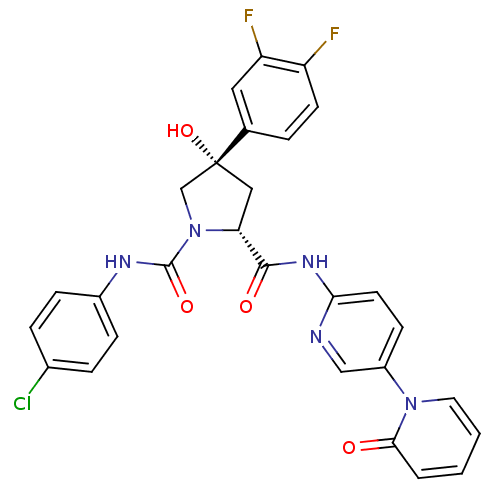
((2R,4S)-N1-(4-Chlorophenyl)-4-(3,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-18-5-7-19(8-6-18)33-27(39)36-16-28(40,17-4-10-21(30)22(31)13-17)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266924
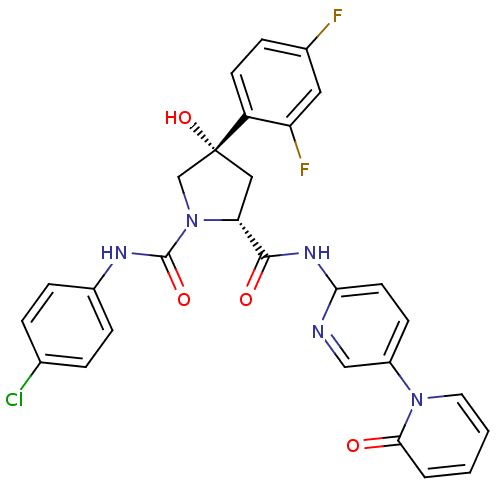
((2R,4S)-N1-(4-Chlorophenyl)-4-(2,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-17-4-7-19(8-5-17)33-27(39)36-16-28(40,21-10-6-18(30)13-22(21)31)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM50266924

((2R,4S)-N1-(4-Chlorophenyl)-4-(2,4-difluorophenyl)...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C28H22ClF2N5O4/c29-17-4-7-19(8-5-17)33-27(39)36-16-28(40,21-10-6-18(30)13-22(21)31)14-23(36)26(38)34-24-11-9-20(15-32-24)35-12-2-1-3-25(35)37/h1-13,15,23,40H,14,16H2,(H,33,39)(H,32,34,38)/t23-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.108 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit F10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266773

((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES Cc1ccccc1[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H26ClN5O4/c1-19-6-2-3-7-23(19)29(39)16-24(35(18-29)28(38)32-21-11-9-20(30)10-12-21)27(37)33-25-14-13-22(17-31-25)34-15-5-4-8-26(34)36/h2-15,17,24,39H,16,18H2,1H3,(H,32,38)(H,31,33,37)/t24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50328726
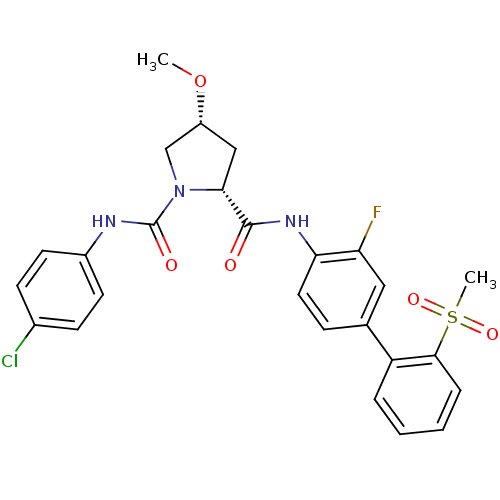
((2R,4R)-N1-(4-chlorophenyl)-N2-(3-fluoro-2'-(methy...)Show SMILES CO[C@@H]1C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cc1F)-c1ccccc1S(C)(=O)=O |r| Show InChI InChI=1S/C26H25ClFN3O5S/c1-36-19-14-23(31(15-19)26(33)29-18-10-8-17(27)9-11-18)25(32)30-22-12-7-16(13-21(22)28)20-5-3-4-6-24(20)37(2,34)35/h3-13,19,23H,14-15H2,1-2H3,(H,29,33)(H,30,32)/t19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development,
| Assay Description
FXa inhibition were determined by using an inhibition assay. |
Chem Biol Drug Des 69: 444-50 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00520.x
BindingDB Entry DOI: 10.7270/Q2PZ5799 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266922
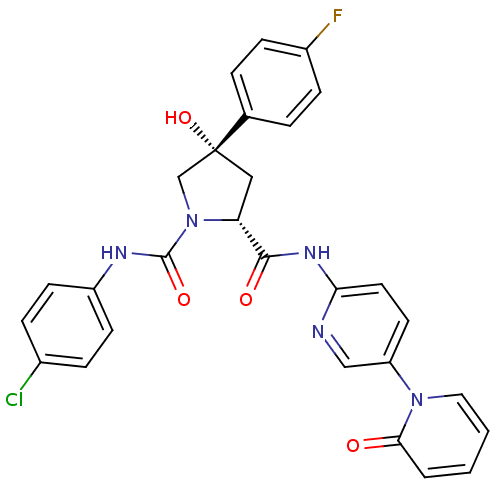
((2R,4S)-N1-(4-Chlorophenyl)-4-(4-fluorophenyl)-4-h...)Show SMILES O[C@@]1(C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C28H23ClFN5O4/c29-19-6-10-21(11-7-19)32-27(38)35-17-28(39,18-4-8-20(30)9-5-18)15-23(35)26(37)33-24-13-12-22(16-31-24)34-14-2-1-3-25(34)36/h1-14,16,23,39H,15,17H2,(H,32,38)(H,31,33,37)/t23-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50266743
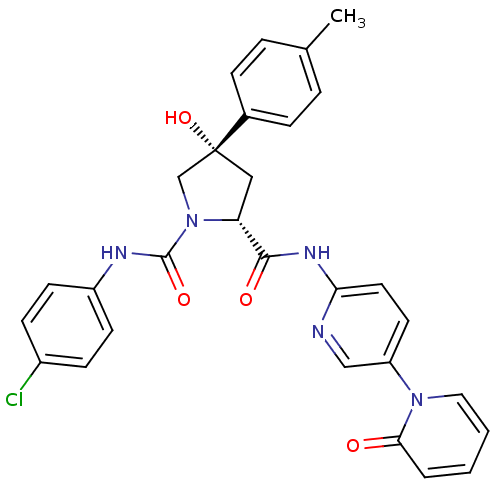
((2R,4S)-N1-(4-Chlorophenyl)-4-hydroxy-N2-(5-(2-oxo...)Show SMILES Cc1ccc(cc1)[C@@]1(O)C[C@@H](N(C1)C(=O)Nc1ccc(Cl)cc1)C(=O)Nc1ccc(cn1)-n1ccccc1=O |r| Show InChI InChI=1S/C29H26ClN5O4/c1-19-5-7-20(8-6-19)29(39)16-24(35(18-29)28(38)32-22-11-9-21(30)10-12-22)27(37)33-25-14-13-23(17-31-25)34-15-3-2-4-26(34)36/h2-15,17,24,39H,16,18H2,1H3,(H,32,38)(H,31,33,37)/t24-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Factor-10a |
Bioorg Med Chem 17: 2501-11 (2009)
Article DOI: 10.1016/j.bmc.2009.01.063
BindingDB Entry DOI: 10.7270/Q2RX9BXD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data