 Found 182 hits with Last Name = 'dunsdon' and Initial = 'rm'
Found 182 hits with Last Name = 'dunsdon' and Initial = 'rm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Interleukin-8
(Homo sapiens (Human)) | BDBM50291126
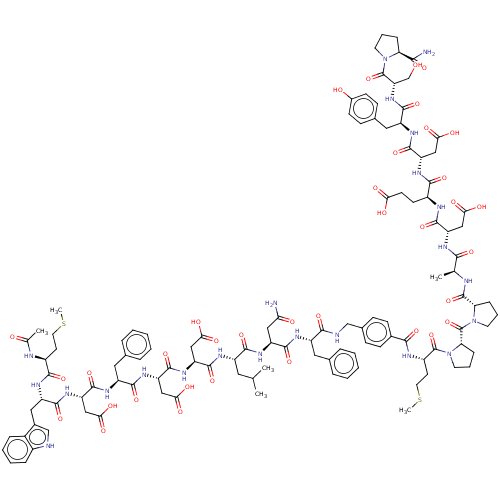
(CHEMBL265735 | CHEMBL3856136 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1ccc(cc1)C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C114H147N23O36S2/c1-58(2)44-73(124-107(166)81(52-92(148)149)133-110(169)83(54-94(152)153)131-102(161)75(46-62-20-11-8-12-21-62)126-109(168)82(53-93(150)151)132-104(163)77(48-66-56-117-69-23-14-13-22-68(66)69)128-100(159)71(37-42-174-5)120-60(4)139)101(160)129-78(49-88(115)141)105(164)125-74(45-61-18-9-7-10-19-61)98(157)118-55-64-27-31-65(32-28-64)97(156)122-72(38-43-175-6)112(171)137-41-17-26-87(137)114(173)136-40-16-25-86(136)111(170)119-59(3)96(155)123-79(50-90(144)145)106(165)121-70(35-36-89(142)143)99(158)130-80(51-91(146)147)108(167)127-76(47-63-29-33-67(140)34-30-63)103(162)134-84(57-138)113(172)135-39-15-24-85(135)95(116)154/h7-14,18-23,27-34,56,58-59,70-87,117,138,140H,15-17,24-26,35-55,57H2,1-6H3,(H2,115,141)(H2,116,154)(H,118,157)(H,119,170)(H,120,139)(H,121,165)(H,122,156)(H,123,155)(H,124,166)(H,125,164)(H,126,168)(H,127,167)(H,128,159)(H,129,160)(H,130,158)(H,131,161)(H,132,163)(H,133,169)(H,134,162)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,150,151)(H,152,153)/t59-,70+,71-,72-,73-,74-,75-,76+,77-,78-,79+,80+,81-,82-,83-,84+,85+,86+,87+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291119
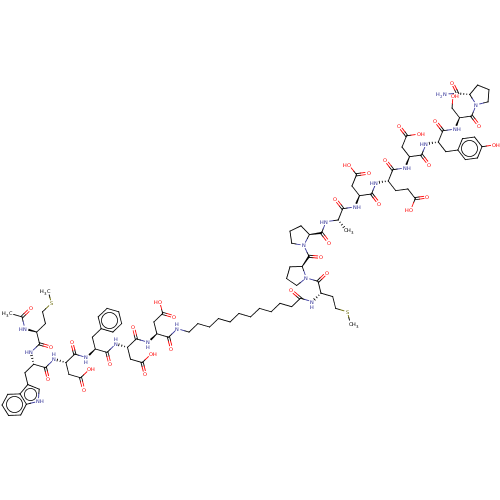
(CHEMBL267510 | CHEMBL3856125 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)NCCCCCCCCCCCC(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C95H129N19O32S2/c1-50(81(132)103-65(44-76(123)124)88(139)102-58(30-31-74(119)120)83(134)108-66(45-77(125)126)89(140)105-62(41-53-26-28-55(117)29-27-53)86(137)111-69(49-115)94(145)112-35-15-22-70(112)80(96)131)99-92(143)71-23-16-36-113(71)95(146)72-24-17-37-114(72)93(144)60(33-39-148-4)101-73(118)25-11-6-5-7-14-34-97-82(133)64(43-75(121)122)107-91(142)68(47-79(129)130)109-85(136)61(40-52-18-9-8-10-19-52)104-90(141)67(46-78(127)128)110-87(138)63(42-54-48-98-57-21-13-12-20-56(54)57)106-84(135)59(32-38-147-3)100-51(2)116/h8-10,12-13,18-21,26-29,48,50,58-72,98,115,117H,5-7,11,14-17,22-25,30-47,49H2,1-4H3,(H2,96,131)(H,97,133)(H,99,143)(H,100,116)(H,101,118)(H,102,139)(H,103,132)(H,104,141)(H,105,140)(H,106,135)(H,107,142)(H,108,134)(H,109,136)(H,110,138)(H,111,137)(H,119,120)(H,121,122)(H,123,124)(H,125,126)(H,127,128)(H,129,130)/t50-,58-,59-,60+,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291124
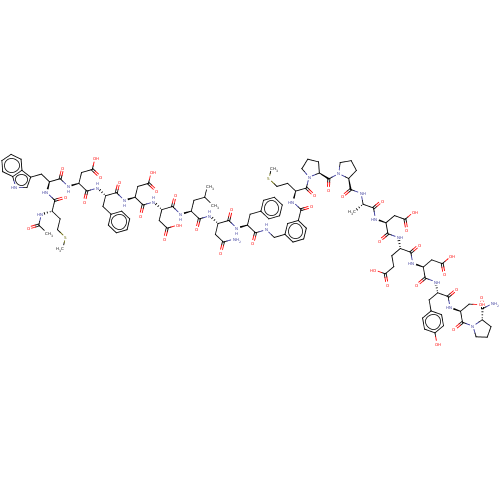
(CHEMBL3856133 | CHEMBL411572 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1cccc(c1)C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C114H147N23O36S2/c1-58(2)43-73(124-107(166)81(52-92(148)149)133-110(169)83(54-94(152)153)131-102(161)75(46-62-21-11-8-12-22-62)126-109(168)82(53-93(150)151)132-104(163)77(48-66-56-117-69-26-14-13-25-68(66)69)128-100(159)71(36-41-174-5)120-60(4)139)101(160)129-78(49-88(115)141)105(164)125-74(45-61-19-9-7-10-20-61)98(157)118-55-64-23-15-24-65(44-64)97(156)122-72(37-42-175-6)112(171)137-40-18-29-87(137)114(173)136-39-17-28-86(136)111(170)119-59(3)96(155)123-79(50-90(144)145)106(165)121-70(34-35-89(142)143)99(158)130-80(51-91(146)147)108(167)127-76(47-63-30-32-67(140)33-31-63)103(162)134-84(57-138)113(172)135-38-16-27-85(135)95(116)154/h7-15,19-26,30-33,44,56,58-59,70-87,117,138,140H,16-18,27-29,34-43,45-55,57H2,1-6H3,(H2,115,141)(H2,116,154)(H,118,157)(H,119,170)(H,120,139)(H,121,165)(H,122,156)(H,123,155)(H,124,166)(H,125,164)(H,126,168)(H,127,167)(H,128,159)(H,129,160)(H,130,158)(H,131,161)(H,132,163)(H,133,169)(H,134,162)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,150,151)(H,152,153)/t59-,70+,71-,72-,73-,74-,75-,76+,77-,78-,79+,80+,81-,82-,83-,84+,85+,86+,87+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291118
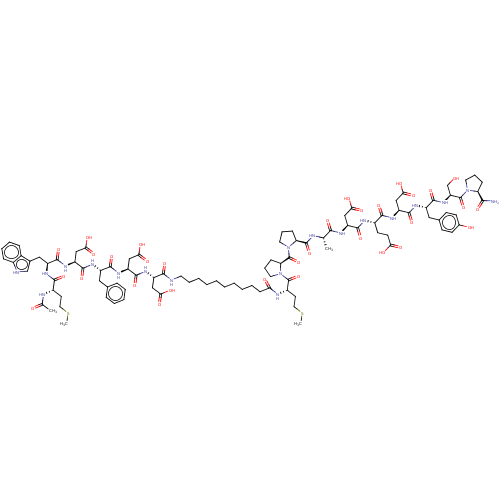
(CHEMBL3856131 | CHEMBL438589 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)NCCCCCCCCCCC(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C98H135N19O32S2/c1-53(84(135)106-68(47-79(126)127)91(142)105-61(33-34-77(122)123)86(137)111-69(48-80(128)129)92(143)108-65(44-56-29-31-58(120)32-30-56)89(140)114-72(52-118)97(148)115-38-18-25-73(115)83(99)134)102-95(146)74-26-19-39-116(74)98(149)75-27-20-40-117(75)96(147)63(36-42-151-4)104-76(121)28-14-9-7-5-6-8-10-17-37-100-85(136)67(46-78(124)125)110-94(145)71(50-82(132)133)112-88(139)64(43-55-21-12-11-13-22-55)107-93(144)70(49-81(130)131)113-90(141)66(45-57-51-101-60-24-16-15-23-59(57)60)109-87(138)62(35-41-150-3)103-54(2)119/h11-13,15-16,21-24,29-32,51,53,61-75,101,118,120H,5-10,14,17-20,25-28,33-50,52H2,1-4H3,(H2,99,134)(H,100,136)(H,102,146)(H,103,119)(H,104,121)(H,105,142)(H,106,135)(H,107,144)(H,108,143)(H,109,138)(H,110,145)(H,111,137)(H,112,139)(H,113,141)(H,114,140)(H,122,123)(H,124,125)(H,126,127)(H,128,129)(H,130,131)(H,132,133)/t53-,61-,62-,63+,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291131
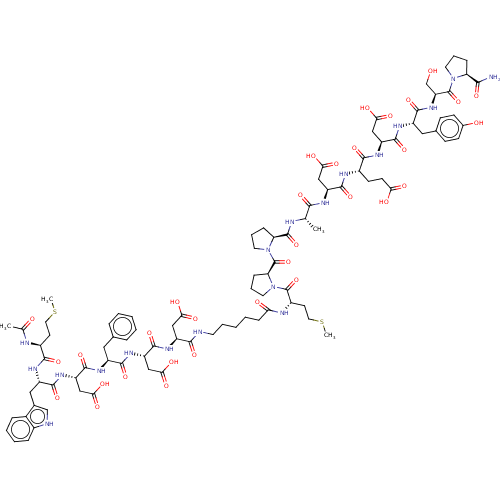
(CHEMBL3856127 | CHEMBL413247 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)NCCCCCC(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C93H125N19O32S2/c1-48(79(130)101-63(42-74(121)122)86(137)100-56(28-29-72(117)118)81(132)106-64(43-75(123)124)87(138)103-60(39-51-24-26-53(115)27-25-51)84(135)109-67(47-113)92(143)110-33-13-20-68(110)78(94)129)97-90(141)69-21-14-34-111(69)93(144)70-22-15-35-112(70)91(142)58(31-37-146-4)99-71(116)23-9-6-12-32-95-80(131)62(41-73(119)120)105-89(140)66(45-77(127)128)107-83(134)59(38-50-16-7-5-8-17-50)102-88(139)65(44-76(125)126)108-85(136)61(40-52-46-96-55-19-11-10-18-54(52)55)104-82(133)57(30-36-145-3)98-49(2)114/h5,7-8,10-11,16-19,24-27,46,48,56-70,96,113,115H,6,9,12-15,20-23,28-45,47H2,1-4H3,(H2,94,129)(H,95,131)(H,97,141)(H,98,114)(H,99,116)(H,100,137)(H,101,130)(H,102,139)(H,103,138)(H,104,133)(H,105,140)(H,106,132)(H,107,134)(H,108,136)(H,109,135)(H,117,118)(H,119,120)(H,121,122)(H,123,124)(H,125,126)(H,127,128)/t48-,56-,57-,58+,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291125
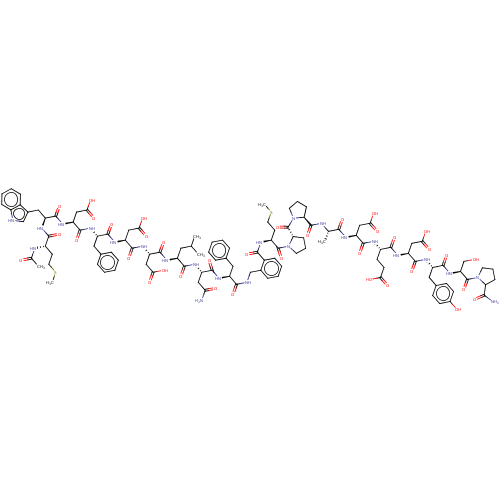
(CHEMBL3856135 | CHEMBL411548 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCc1ccccc1C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCCC1C(N)=O |r| Show InChI InChI=1S/C114H147N23O36S2/c1-58(2)44-73(124-107(166)81(52-92(148)149)133-110(169)83(54-94(152)153)131-102(161)75(46-62-22-11-8-12-23-62)126-109(168)82(53-93(150)151)132-104(163)77(48-65-56-117-69-27-16-15-25-67(65)69)128-100(159)71(37-42-174-5)120-60(4)139)101(160)129-78(49-88(115)141)105(164)125-74(45-61-20-9-7-10-21-61)98(157)118-55-64-24-13-14-26-68(64)97(156)122-72(38-43-175-6)112(171)137-41-19-30-87(137)114(173)136-40-18-29-86(136)111(170)119-59(3)96(155)123-79(50-90(144)145)106(165)121-70(35-36-89(142)143)99(158)130-80(51-91(146)147)108(167)127-76(47-63-31-33-66(140)34-32-63)103(162)134-84(57-138)113(172)135-39-17-28-85(135)95(116)154/h7-16,20-27,31-34,56,58-59,70-87,117,138,140H,17-19,28-30,35-55,57H2,1-6H3,(H2,115,141)(H2,116,154)(H,118,157)(H,119,170)(H,120,139)(H,121,165)(H,122,156)(H,123,155)(H,124,166)(H,125,164)(H,126,168)(H,127,167)(H,128,159)(H,129,160)(H,130,158)(H,131,161)(H,132,163)(H,133,169)(H,134,162)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,150,151)(H,152,153)/t59-,70+,71-,72-,73-,74-,75-,76+,77-,78-,79+,80+,81-,82-,83-,84+,85+,86+,87+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291120
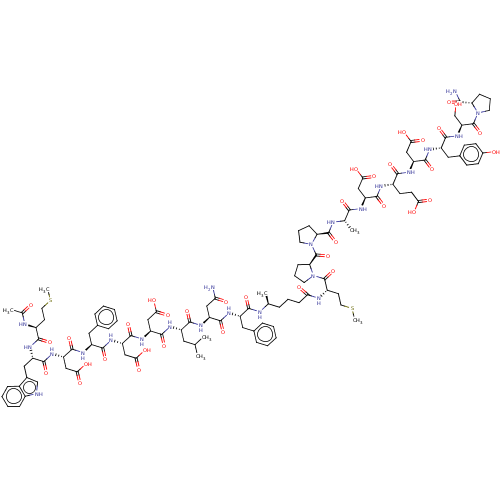
(CHEMBL3856128 | CHEMBL410171 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C)CCCC(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C112H151N23O36S2/c1-57(2)44-71(122-105(164)79(52-91(147)148)131-108(167)81(54-93(151)152)129-100(159)73(46-62-23-12-9-13-24-62)124-107(166)80(53-92(149)150)130-102(161)75(48-64-55-115-67-26-15-14-25-66(64)67)126-97(156)69(37-42-172-6)118-60(5)137)99(158)127-76(49-86(113)139)103(162)123-72(45-61-21-10-8-11-22-61)98(157)116-58(3)20-16-30-87(140)119-70(38-43-173-7)110(169)135-41-19-29-85(135)112(171)134-40-18-28-84(134)109(168)117-59(4)95(154)121-77(50-89(143)144)104(163)120-68(35-36-88(141)142)96(155)128-78(51-90(145)146)106(165)125-74(47-63-31-33-65(138)34-32-63)101(160)132-82(56-136)111(170)133-39-17-27-83(133)94(114)153/h8-15,21-26,31-34,55,57-59,68-85,115,136,138H,16-20,27-30,35-54,56H2,1-7H3,(H2,113,139)(H2,114,153)(H,116,157)(H,117,168)(H,118,137)(H,119,140)(H,120,163)(H,121,154)(H,122,164)(H,123,162)(H,124,166)(H,125,165)(H,126,156)(H,127,158)(H,128,155)(H,129,159)(H,130,161)(H,131,167)(H,132,160)(H,141,142)(H,143,144)(H,145,146)(H,147,148)(H,149,150)(H,151,152)/t58-,59-,68+,69-,70+,71-,72-,73-,74+,75-,76-,77+,78+,79-,80-,81-,82+,83+,84+,85+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291122
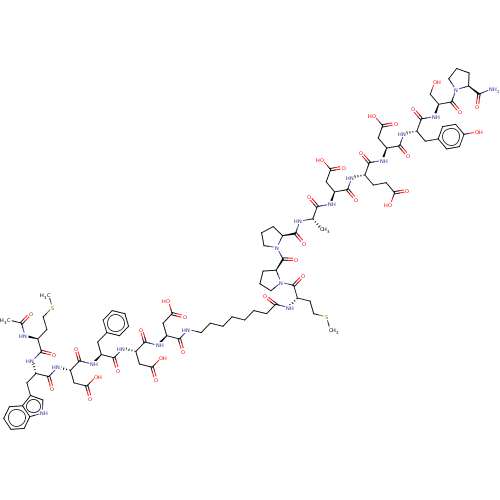
(CHEMBL3856126 | CHEMBL428324 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)NCCCCCCCC(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C99H137N19O32S2/c1-54(85(136)107-69(48-80(127)128)92(143)106-62(34-35-78(123)124)87(138)112-70(49-81(129)130)93(144)109-66(45-57-30-32-59(121)33-31-57)90(141)115-73(53-119)98(149)116-39-19-26-74(116)84(100)135)103-96(147)75-27-20-40-117(75)99(150)76-28-21-41-118(76)97(148)64(37-43-152-4)105-77(122)29-15-10-8-6-5-7-9-11-18-38-101-86(137)68(47-79(125)126)111-95(146)72(51-83(133)134)113-89(140)65(44-56-22-13-12-14-23-56)108-94(145)71(50-82(131)132)114-91(142)67(46-58-52-102-61-25-17-16-24-60(58)61)110-88(139)63(36-42-151-3)104-55(2)120/h12-14,16-17,22-25,30-33,52,54,62-76,102,119,121H,5-11,15,18-21,26-29,34-51,53H2,1-4H3,(H2,100,135)(H,101,137)(H,103,147)(H,104,120)(H,105,122)(H,106,143)(H,107,136)(H,108,145)(H,109,144)(H,110,139)(H,111,146)(H,112,138)(H,113,140)(H,114,142)(H,115,141)(H,123,124)(H,125,126)(H,127,128)(H,129,130)(H,131,132)(H,133,134)/t54-,62-,63-,64+,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291128
(CHEMBL267749 | CHEMBL3856129 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCCCCC(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C111H149N23O36S2/c1-57(2)44-70(121-104(163)78(52-90(146)147)130-107(166)80(54-92(150)151)128-99(158)72(46-61-22-11-8-12-23-61)123-106(165)79(53-91(148)149)129-101(160)74(48-63-55-115-66-25-14-13-24-65(63)66)125-97(156)68(36-42-171-5)117-59(4)136)98(157)126-75(49-85(112)138)102(161)122-71(45-60-20-9-7-10-21-60)95(154)114-38-16-15-29-86(139)118-69(37-43-172-6)109(168)134-41-19-28-84(134)111(170)133-40-18-27-83(133)108(167)116-58(3)94(153)120-76(50-88(142)143)103(162)119-67(34-35-87(140)141)96(155)127-77(51-89(144)145)105(164)124-73(47-62-30-32-64(137)33-31-62)100(159)131-81(56-135)110(169)132-39-17-26-82(132)93(113)152/h7-14,20-25,30-33,55,57-58,67-84,115,135,137H,15-19,26-29,34-54,56H2,1-6H3,(H2,112,138)(H2,113,152)(H,114,154)(H,116,167)(H,117,136)(H,118,139)(H,119,162)(H,120,153)(H,121,163)(H,122,161)(H,123,165)(H,124,164)(H,125,156)(H,126,157)(H,127,155)(H,128,158)(H,129,160)(H,130,166)(H,131,159)(H,140,141)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,150,151)/t58-,67+,68-,69+,70-,71-,72-,73+,74-,75-,76+,77+,78-,79-,80-,81+,82+,83+,84+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50213962
(CHEMBL410765)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C112H150N24O38S2/c1-55(2)41-69(122-103(165)77(49-89(149)150)131-106(168)79(51-91(153)154)129-98(160)70(42-59-19-10-8-11-20-59)123-105(167)78(50-90(151)152)130-100(162)73(45-62-52-115-65-24-15-14-23-64(62)65)126-96(158)67(34-39-175-6)118-58(5)139)97(159)127-74(46-84(113)141)101(163)125-72(43-60-21-12-9-13-22-60)107(169)133-92(57(4)138)109(171)116-53-85(142)119-68(35-40-176-7)110(172)136-38-18-27-83(136)112(174)135-37-17-26-82(135)108(170)117-56(3)94(156)121-75(47-87(145)146)102(164)120-66(32-33-86(143)144)95(157)128-76(48-88(147)148)104(166)124-71(44-61-28-30-63(140)31-29-61)99(161)132-80(54-137)111(173)134-36-16-25-81(134)93(114)155/h8-15,19-24,28-31,52,55-57,66-83,92,115,137-138,140H,16-18,25-27,32-51,53-54H2,1-7H3,(H2,113,141)(H2,114,155)(H,116,171)(H,117,170)(H,118,139)(H,119,142)(H,120,164)(H,121,156)(H,122,165)(H,123,167)(H,124,166)(H,125,163)(H,126,158)(H,127,159)(H,128,157)(H,129,160)(H,130,162)(H,131,168)(H,132,161)(H,133,169)(H,143,144)(H,145,146)(H,147,148)(H,149,150)(H,151,152)(H,153,154)/t56-,57+,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,92-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291130
(CHEMBL3856130 | CHEMBL405300 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)NCCCC(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C91H121N19O32S2/c1-46(77(128)99-61(40-72(119)120)84(135)98-54(26-27-70(115)116)79(130)104-62(41-73(121)122)85(136)101-58(37-49-22-24-51(113)25-23-49)82(133)107-65(45-111)90(141)108-31-11-18-66(108)76(92)127)95-88(139)67-19-12-32-109(67)91(142)68-20-13-33-110(68)89(140)56(29-35-144-4)97-69(114)21-10-30-93-78(129)60(39-71(117)118)103-87(138)64(43-75(125)126)105-81(132)57(36-48-14-6-5-7-15-48)100-86(137)63(42-74(123)124)106-83(134)59(38-50-44-94-53-17-9-8-16-52(50)53)102-80(131)55(28-34-143-3)96-47(2)112/h5-9,14-17,22-25,44,46,54-68,94,111,113H,10-13,18-21,26-43,45H2,1-4H3,(H2,92,127)(H,93,129)(H,95,139)(H,96,112)(H,97,114)(H,98,135)(H,99,128)(H,100,137)(H,101,136)(H,102,131)(H,103,138)(H,104,130)(H,105,132)(H,106,134)(H,107,133)(H,115,116)(H,117,118)(H,119,120)(H,121,122)(H,123,124)(H,125,126)/t46-,54-,55-,56+,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291123
(CHEMBL3856132 | CHEMBL410211 | Interleukin-8 inhib...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C89H117N19O32S2/c1-44(75(126)97-59(37-70(117)118)82(133)96-52(24-25-68(113)114)77(128)102-60(38-71(119)120)83(134)99-56(34-47-20-22-49(111)23-21-47)80(131)105-63(43-109)88(139)106-28-10-17-64(106)74(90)125)93-86(137)65-18-11-29-107(65)89(140)66-19-12-30-108(66)87(138)54(27-32-142-4)95-67(112)42-92-76(127)58(36-69(115)116)101-85(136)62(40-73(123)124)103-79(130)55(33-46-13-6-5-7-14-46)98-84(135)61(39-72(121)122)104-81(132)57(35-48-41-91-51-16-9-8-15-50(48)51)100-78(129)53(26-31-141-3)94-45(2)110/h5-9,13-16,20-23,41,44,52-66,91,109,111H,10-12,17-19,24-40,42-43H2,1-4H3,(H2,90,125)(H,92,127)(H,93,137)(H,94,110)(H,95,112)(H,96,133)(H,97,126)(H,98,135)(H,99,134)(H,100,129)(H,101,136)(H,102,128)(H,103,130)(H,104,132)(H,105,131)(H,113,114)(H,115,116)(H,117,118)(H,119,120)(H,121,122)(H,123,124)/t44-,52-,53-,54+,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291121
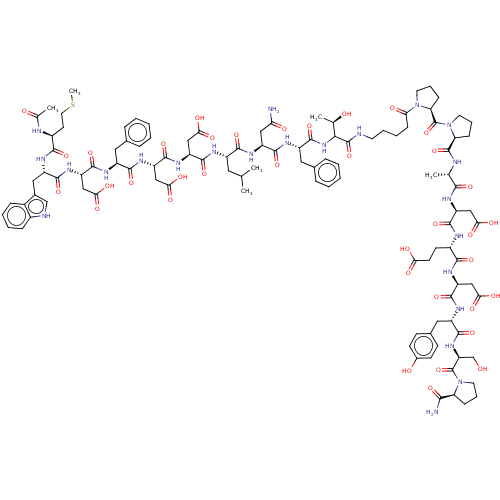
(CHEMBL2370828 | CHEMBL3856124 | Interleukin-8 inhi...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC([C@@H](C)O)C(=O)NCCCCC(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C110H147N23O37S/c1-55(2)42-68(119-102(162)76(50-88(146)147)128-105(165)78(52-90(150)151)126-97(157)69(43-59-20-9-7-10-21-59)120-104(164)77(51-89(148)149)127-99(159)72(46-62-53-114-65-25-14-13-24-64(62)65)123-95(155)67(36-41-171-6)116-58(5)136)96(156)124-73(47-83(111)138)100(160)122-71(44-60-22-11-8-12-23-60)106(166)130-91(57(4)135)108(168)113-37-16-15-29-84(139)131-38-19-28-82(131)110(170)133-40-18-27-81(133)107(167)115-56(3)93(153)118-74(48-86(142)143)101(161)117-66(34-35-85(140)141)94(154)125-75(49-87(144)145)103(163)121-70(45-61-30-32-63(137)33-31-61)98(158)129-79(54-134)109(169)132-39-17-26-80(132)92(112)152/h7-14,20-25,30-33,53,55-57,66-82,91,114,134-135,137H,15-19,26-29,34-52,54H2,1-6H3,(H2,111,138)(H2,112,152)(H,113,168)(H,115,167)(H,116,136)(H,117,161)(H,118,153)(H,119,162)(H,120,164)(H,121,163)(H,122,160)(H,123,155)(H,124,156)(H,125,154)(H,126,157)(H,127,159)(H,128,165)(H,129,158)(H,130,166)(H,140,141)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,150,151)/t56-,57+,66+,67-,68-,69-,70+,71-,72-,73-,74+,75+,76-,77-,78-,79+,80+,81+,82+,91-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Interleukin-8
(Homo sapiens (Human)) | BDBM50291129
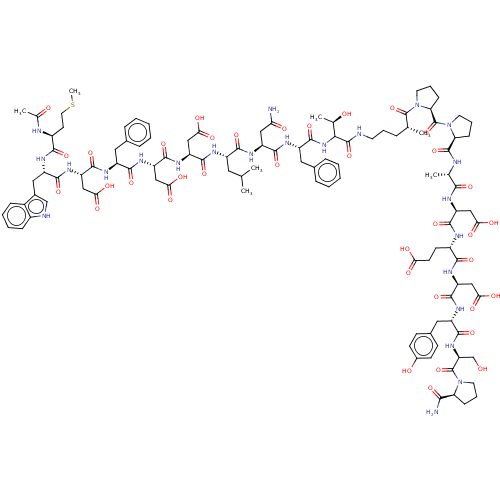
(CHEMBL2370827 | CHEMBL3856134 | Interleukin-8 inhi...)Show SMILES CSCC[C@H](NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC([C@@H](C)O)C(=O)NCCC[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O Show InChI InChI=1S/C111H149N23O37S/c1-55(2)42-69(120-102(162)77(50-88(146)147)129-105(165)79(52-90(150)151)127-97(157)70(43-60-21-10-8-11-22-60)121-104(164)78(51-89(148)149)128-99(159)73(46-63-53-115-66-26-15-14-25-65(63)66)124-95(155)68(36-41-172-7)117-59(6)137)96(156)125-74(47-84(112)139)100(160)123-72(44-61-23-12-9-13-24-61)106(166)131-91(58(5)136)108(168)114-37-16-20-56(3)109(169)134-40-19-29-83(134)111(171)133-39-18-28-82(133)107(167)116-57(4)93(153)119-75(48-86(142)143)101(161)118-67(34-35-85(140)141)94(154)126-76(49-87(144)145)103(163)122-71(45-62-30-32-64(138)33-31-62)98(158)130-80(54-135)110(170)132-38-17-27-81(132)92(113)152/h8-15,21-26,30-33,53,55-58,67-83,91,115,135-136,138H,16-20,27-29,34-52,54H2,1-7H3,(H2,112,139)(H2,113,152)(H,114,168)(H,116,167)(H,117,137)(H,118,161)(H,119,153)(H,120,162)(H,121,164)(H,122,163)(H,123,160)(H,124,155)(H,125,156)(H,126,154)(H,127,157)(H,128,159)(H,129,165)(H,130,158)(H,131,166)(H,140,141)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,150,151)/t56-,57+,58-,67-,68+,69+,70+,71-,72+,73+,74+,75-,76-,77+,78+,79+,80-,81-,82-,83-,91+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Interleukin-8 (IL-8) |
Bioorg Med Chem Lett 7: 429-432 (1997)
Article DOI: 10.1016/S0960-894X(97)00036-X
BindingDB Entry DOI: 10.7270/Q2NK3F2S |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096734

((S)-4-[(S)-3-Carboxy-2-(3-carboxy-propionylamino)-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)NCc1ccc(O)cc1 Show InChI InChI=1S/C47H62F3N7O15/c1-24(2)19-30(41(68)56-33(22-47(48,49)50)38(66)44(71)51-23-26-11-13-28(58)14-12-26)55-45(72)39(46(4,5)6)57-43(70)31(20-27-10-8-7-9-25(27)3)54-40(67)29(15-17-35(60)61)53-42(69)32(21-37(64)65)52-34(59)16-18-36(62)63/h7-14,24,29-33,39,58H,15-23H2,1-6H3,(H,51,71)(H,52,59)(H,53,69)(H,54,67)(H,55,72)(H,56,68)(H,57,70)(H,60,61)(H,62,63)(H,64,65)/t29-,30-,31-,32-,33+,39+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096726
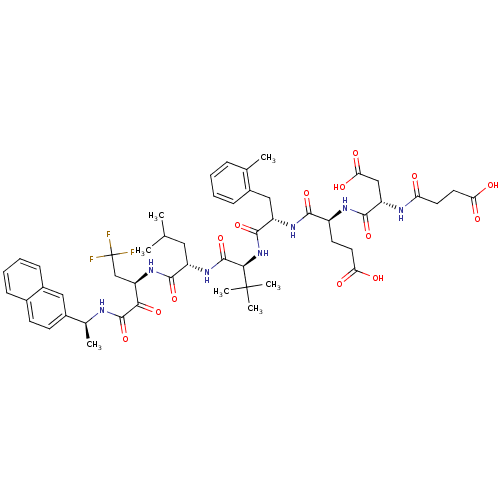
(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)N[C@@H](C)c1ccc2ccccc2c1 Show InChI InChI=1S/C52H66F3N7O14/c1-27(2)22-35(46(72)61-38(26-52(53,54)55)43(70)49(75)56-29(4)32-17-16-30-13-10-11-15-33(30)23-32)60-50(76)44(51(5,6)7)62-48(74)36(24-31-14-9-8-12-28(31)3)59-45(71)34(18-20-40(64)65)58-47(73)37(25-42(68)69)57-39(63)19-21-41(66)67/h8-17,23,27,29,34-38,44H,18-22,24-26H2,1-7H3,(H,56,75)(H,57,63)(H,58,73)(H,59,71)(H,60,76)(H,61,72)(H,62,74)(H,64,65)(H,66,67)(H,68,69)/t29-,34-,35-,36-,37-,38+,44+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096729

((S)-4-((S)-1-{(S)-1-[(S)-1-((R)-1-Benzylaminooxaly...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C47H62F3N7O14/c1-25(2)20-30(41(67)56-33(23-47(48,49)50)38(65)44(70)51-24-27-13-8-7-9-14-27)55-45(71)39(46(4,5)6)57-43(69)31(21-28-15-11-10-12-26(28)3)54-40(66)29(16-18-35(59)60)53-42(68)32(22-37(63)64)52-34(58)17-19-36(61)62/h7-15,25,29-33,39H,16-24H2,1-6H3,(H,51,70)(H,52,58)(H,53,68)(H,54,66)(H,55,71)(H,56,67)(H,57,69)(H,59,60)(H,61,62)(H,63,64)/t29-,30-,31-,32-,33+,39+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096730

(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)N[C@@H](C)c1ccccc1 Show InChI InChI=1S/C48H64F3N7O14/c1-25(2)21-31(42(68)57-34(24-48(49,50)51)39(66)45(71)52-27(4)28-14-9-8-10-15-28)56-46(72)40(47(5,6)7)58-44(70)32(22-29-16-12-11-13-26(29)3)55-41(67)30(17-19-36(60)61)54-43(69)33(23-38(64)65)53-35(59)18-20-37(62)63/h8-16,25,27,30-34,40H,17-24H2,1-7H3,(H,52,71)(H,53,59)(H,54,69)(H,55,67)(H,56,72)(H,57,68)(H,58,70)(H,60,61)(H,62,63)(H,64,65)/t27-,30-,31-,32-,33-,34+,40+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096727
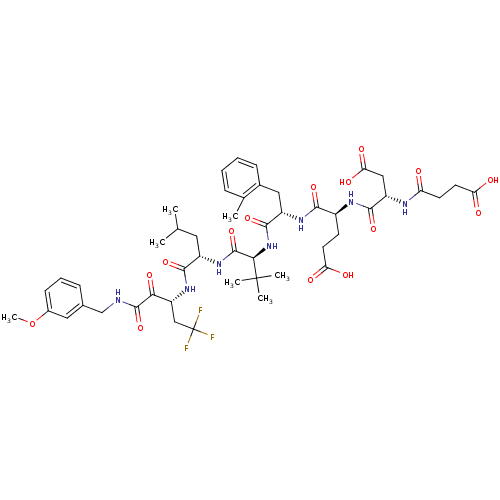
((S)-4-[(S)-3-Carboxy-2-(3-carboxy-propionylamino)-...)Show SMILES COc1cccc(CNC(=O)C(=O)[C@@H](CC(F)(F)F)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc2ccccc2C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)c1 Show InChI InChI=1S/C48H64F3N7O15/c1-25(2)19-31(42(68)57-34(23-48(49,50)51)39(66)45(71)52-24-27-12-10-14-29(20-27)73-7)56-46(72)40(47(4,5)6)58-44(70)32(21-28-13-9-8-11-26(28)3)55-41(67)30(15-17-36(60)61)54-43(69)33(22-38(64)65)53-35(59)16-18-37(62)63/h8-14,20,25,30-34,40H,15-19,21-24H2,1-7H3,(H,52,71)(H,53,59)(H,54,69)(H,55,67)(H,56,72)(H,57,68)(H,58,70)(H,60,61)(H,62,63)(H,64,65)/t30-,31-,32-,33-,34+,40+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096728

((S)-4-((S)-1-{(S)-1-[(S)-1-((S)-1-Amino-2-carbamoy...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](N)C(=O)C(N)=O Show InChI InChI=1S/C38H56N8O14/c1-18(2)15-22(36(59)46-31(39)29(54)32(40)55)44-37(60)30(38(4,5)6)45-35(58)23(16-20-10-8-7-9-19(20)3)43-33(56)21(11-13-26(48)49)42-34(57)24(17-28(52)53)41-25(47)12-14-27(50)51/h7-10,18,21-24,30-31H,11-17,39H2,1-6H3,(H2,40,55)(H,41,47)(H,42,57)(H,43,56)(H,44,60)(H,45,58)(H,46,59)(H,48,49)(H,50,51)(H,52,53)/t21-,22-,23-,24-,30+,31-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096733
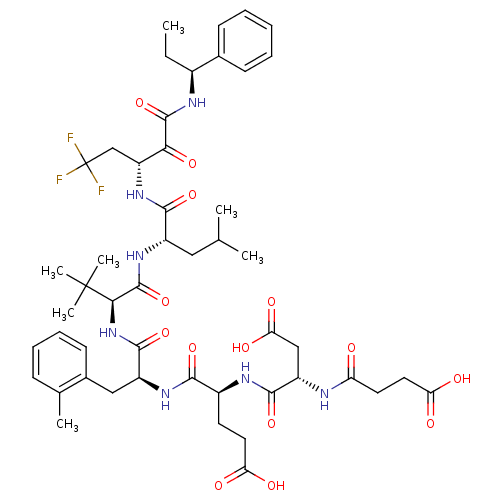
(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC[C@H](NC(=O)C(=O)[C@@H](CC(F)(F)F)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C49H66F3N7O14/c1-8-30(28-15-10-9-11-16-28)54-46(72)40(67)35(25-49(50,51)52)58-43(69)32(22-26(2)3)57-47(73)41(48(5,6)7)59-45(71)33(23-29-17-13-12-14-27(29)4)56-42(68)31(18-20-37(61)62)55-44(70)34(24-39(65)66)53-36(60)19-21-38(63)64/h9-17,26,30-35,41H,8,18-25H2,1-7H3,(H,53,60)(H,54,72)(H,55,70)(H,56,68)(H,57,73)(H,58,69)(H,59,71)(H,61,62)(H,63,64)(H,65,66)/t30-,31-,32-,33-,34-,35+,41+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Binding affinity towards 5-hydroxytryptamine 3 receptor |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096725
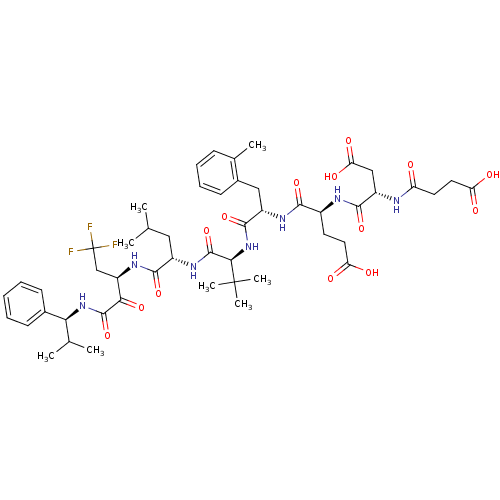
(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)N[C@@H](C(C)C)c1ccccc1 Show InChI InChI=1S/C50H68F3N7O14/c1-26(2)22-32(44(70)58-35(25-50(51,52)53)41(68)47(73)59-40(27(3)4)29-15-10-9-11-16-29)57-48(74)42(49(6,7)8)60-46(72)33(23-30-17-13-12-14-28(30)5)56-43(69)31(18-20-37(62)63)55-45(71)34(24-39(66)67)54-36(61)19-21-38(64)65/h9-17,26-27,31-35,40,42H,18-25H2,1-8H3,(H,54,61)(H,55,71)(H,56,69)(H,57,74)(H,58,70)(H,59,73)(H,60,72)(H,62,63)(H,64,65)(H,66,67)/t31-,32-,33-,34-,35+,40-,42+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against delta receptor of (endomorphin 2) in mouse vas deferens was determined |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096732

(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC[C@H](NC(=O)C(=O)[C@@H](CC(F)(F)F)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)c1ccc2ccccc2c1 Show InChI InChI=1S/C53H68F3N7O14/c1-8-34(33-18-17-30-14-11-12-16-32(30)24-33)58-50(76)44(71)39(27-53(54,55)56)62-47(73)36(23-28(2)3)61-51(77)45(52(5,6)7)63-49(75)37(25-31-15-10-9-13-29(31)4)60-46(72)35(19-21-41(65)66)59-48(74)38(26-43(69)70)57-40(64)20-22-42(67)68/h9-18,24,28,34-39,45H,8,19-23,25-27H2,1-7H3,(H,57,64)(H,58,76)(H,59,74)(H,60,72)(H,61,77)(H,62,73)(H,63,75)(H,65,66)(H,67,68)(H,69,70)/t34-,35-,36-,37-,38-,39+,45+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096724
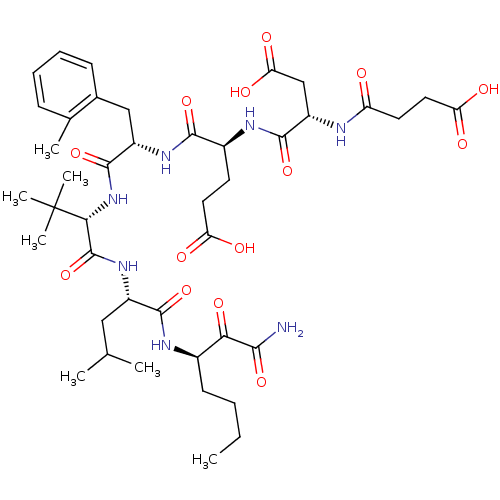
((S)-4-((S)-1-{(S)-1-[(S)-1-((R)-1-Aminooxalyl-pent...)Show SMILES CCCC[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)C(N)=O Show InChI InChI=1S/C42H63N7O14/c1-8-9-14-25(34(57)36(43)58)45-38(60)27(19-22(2)3)48-41(63)35(42(5,6)7)49-40(62)28(20-24-13-11-10-12-23(24)4)47-37(59)26(15-17-31(51)52)46-39(61)29(21-33(55)56)44-30(50)16-18-32(53)54/h10-13,22,25-29,35H,8-9,14-21H2,1-7H3,(H2,43,58)(H,44,50)(H,45,60)(H,46,61)(H,47,59)(H,48,63)(H,49,62)(H,51,52)(H,53,54)(H,55,56)/t25-,26+,27+,28+,29+,35-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50096724

((S)-4-((S)-1-{(S)-1-[(S)-1-((R)-1-Aminooxalyl-pent...)Show SMILES CCCC[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)C(N)=O Show InChI InChI=1S/C42H63N7O14/c1-8-9-14-25(34(57)36(43)58)45-38(60)27(19-22(2)3)48-41(63)35(42(5,6)7)49-40(62)28(20-24-13-11-10-12-23(24)4)47-37(59)26(15-17-31(51)52)46-39(61)29(21-33(55)56)44-30(50)16-18-32(53)54/h10-13,22,25-29,35H,8-9,14-21H2,1-7H3,(H2,43,58)(H,44,50)(H,45,60)(H,46,61)(H,47,59)(H,48,63)(H,49,62)(H,51,52)(H,53,54)(H,55,56)/t25-,26+,27+,28+,29+,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of Serine protease chymotrypsin |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50096731

(4-[3-Carboxy-2-(3-carboxy-propionylamino)-propiony...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CCC(O)=O)C(C)(C)C)C(=O)N[C@H](CC(F)(F)F)C(=O)C(=O)N[C@H](C)c1ccccc1 Show InChI InChI=1S/C48H64F3N7O14/c1-25(2)21-31(42(68)57-34(24-48(49,50)51)39(66)45(71)52-27(4)28-14-9-8-10-15-28)56-46(72)40(47(5,6)7)58-44(70)32(22-29-16-12-11-13-26(29)3)55-41(67)30(17-19-36(60)61)54-43(69)33(23-38(64)65)53-35(59)18-20-37(62)63/h8-16,25,27,30-34,40H,17-24H2,1-7H3,(H,52,71)(H,53,59)(H,54,69)(H,55,67)(H,56,72)(H,57,68)(H,58,70)(H,60,61)(H,62,63)(H,64,65)/t27-,30+,31+,32+,33+,34-,40-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discover Welwyn
Curated by ChEMBL
| Assay Description
Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... |
Bioorg Med Chem Lett 11: 355-7 (2001)
BindingDB Entry DOI: 10.7270/Q2WQ0322 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50382046

(CHEMBL2022737)Show SMILES Fc1ccccc1C(=O)Nc1ccc(-c2nnc(NCCCCN3CCCCC3)o2)c(Cl)c1 Show InChI InChI=1S/C24H27ClFN5O2/c25-20-16-17(28-22(32)19-8-2-3-9-21(19)26)10-11-18(20)23-29-30-24(33-23)27-12-4-7-15-31-13-5-1-6-14-31/h2-3,8-11,16H,1,4-7,12-15H2,(H,27,30)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3531-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.062
BindingDB Entry DOI: 10.7270/Q2SB46SH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50135663
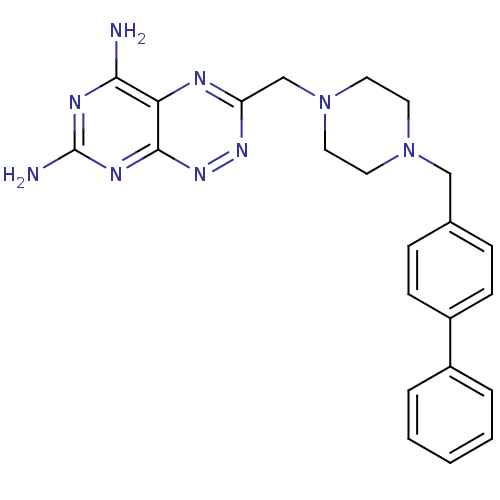
(3-(4-Biphenyl-4-ylmethyl-piperazin-1-ylmethyl)-pyr...)Show SMILES Nc1nc(N)c2nc(CN3CCN(Cc4ccc(cc4)-c4ccccc4)CC3)nnc2n1 Show InChI InChI=1S/C23H25N9/c24-21-20-22(28-23(25)27-21)30-29-19(26-20)15-32-12-10-31(11-13-32)14-16-6-8-18(9-7-16)17-4-2-1-3-5-17/h1-9H,10-15H2,(H4,24,25,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Protein-tyrosine phosphatase 1B in 300 nM DTT |
Bioorg Med Chem Lett 13: 2895-8 (2003)
BindingDB Entry DOI: 10.7270/Q21V5DDS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50135663

(3-(4-Biphenyl-4-ylmethyl-piperazin-1-ylmethyl)-pyr...)Show SMILES Nc1nc(N)c2nc(CN3CCN(Cc4ccc(cc4)-c4ccccc4)CC3)nnc2n1 Show InChI InChI=1S/C23H25N9/c24-21-20-22(28-23(25)27-21)30-29-19(26-20)15-32-12-10-31(11-13-32)14-16-6-8-18(9-7-16)17-4-2-1-3-5-17/h1-9H,10-15H2,(H4,24,25,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Protein-tyrosine phosphatase 1B in 300 nM DTT |
Bioorg Med Chem Lett 13: 2895-8 (2003)
BindingDB Entry DOI: 10.7270/Q21V5DDS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50135667

(3-(4-Naphthalen-2-ylmethyl-piperazin-1-ylmethyl)-p...)Show SMILES Nc1nc(N)c2nc(CN3CCN(Cc4ccc5ccccc5c4)CC3)nnc2n1 Show InChI InChI=1S/C21H23N9/c22-19-18-20(26-21(23)25-19)28-27-17(24-18)13-30-9-7-29(8-10-30)12-14-5-6-15-3-1-2-4-16(15)11-14/h1-6,11H,7-10,12-13H2,(H4,22,23,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Protein-tyrosine phosphatase 1B in 300 nM DTT |
Bioorg Med Chem Lett 13: 2895-8 (2003)
BindingDB Entry DOI: 10.7270/Q21V5DDS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381979
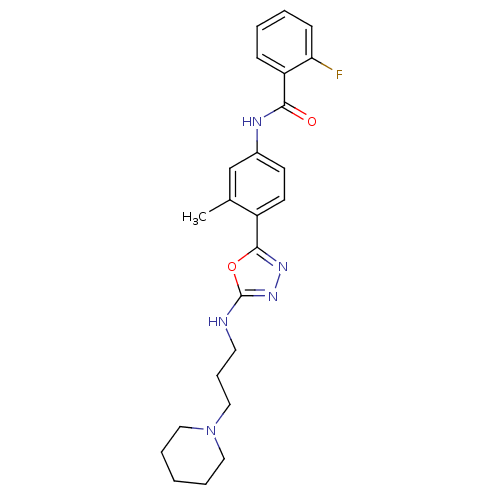
(CHEMBL2023925)Show SMILES Cc1cc(NC(=O)c2ccccc2F)ccc1-c1nnc(NCCCN2CCCCC2)o1 Show InChI InChI=1S/C24H28FN5O2/c1-17-16-18(27-22(31)20-8-3-4-9-21(20)25)10-11-19(17)23-28-29-24(32-23)26-12-7-15-30-13-5-2-6-14-30/h3-4,8-11,16H,2,5-7,12-15H2,1H3,(H,26,29)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381984
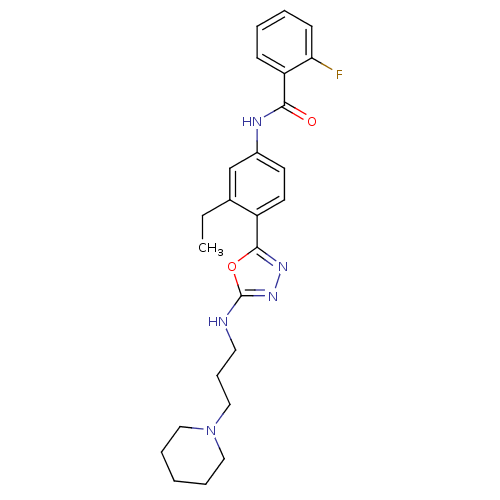
(CHEMBL2023930)Show SMILES CCc1cc(NC(=O)c2ccccc2F)ccc1-c1nnc(NCCCN2CCCCC2)o1 Show InChI InChI=1S/C25H30FN5O2/c1-2-18-17-19(28-23(32)21-9-4-5-10-22(21)26)11-12-20(18)24-29-30-25(33-24)27-13-8-16-31-14-6-3-7-15-31/h4-5,9-12,17H,2-3,6-8,13-16H2,1H3,(H,27,30)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(Homo sapiens (Human)) | BDBM50381980
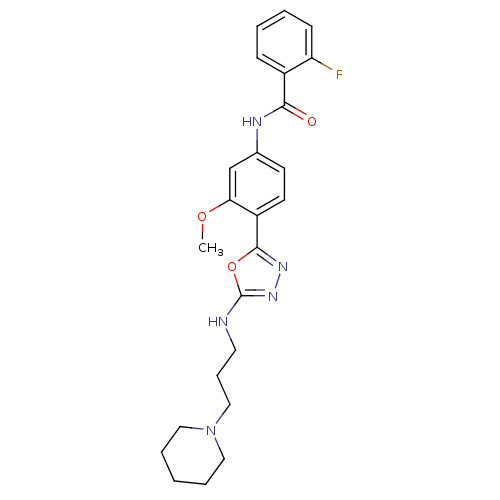
(CHEMBL2022495)Show SMILES COc1cc(NC(=O)c2ccccc2F)ccc1-c1nnc(NCCCN2CCCCC2)o1 Show InChI InChI=1S/C24H28FN5O3/c1-32-21-16-17(27-22(31)18-8-3-4-9-20(18)25)10-11-19(21)23-28-29-24(33-23)26-12-7-15-30-13-5-2-6-14-30/h3-4,8-11,16H,2,5-7,12-15H2,1H3,(H,26,29)(H,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha1 nAChR |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381988
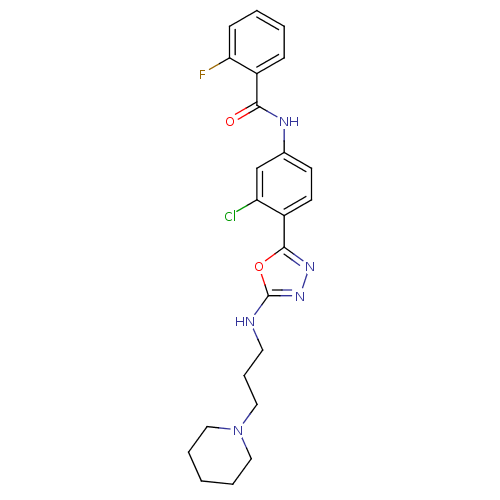
(CHEMBL2023924)Show SMILES Fc1ccccc1C(=O)Nc1ccc(-c2nnc(NCCCN3CCCCC3)o2)c(Cl)c1 Show InChI InChI=1S/C23H25ClFN5O2/c24-19-15-16(27-21(31)18-7-2-3-8-20(18)25)9-10-17(19)22-28-29-23(32-22)26-11-6-14-30-12-4-1-5-13-30/h2-3,7-10,15H,1,4-6,11-14H2,(H,26,29)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50135670
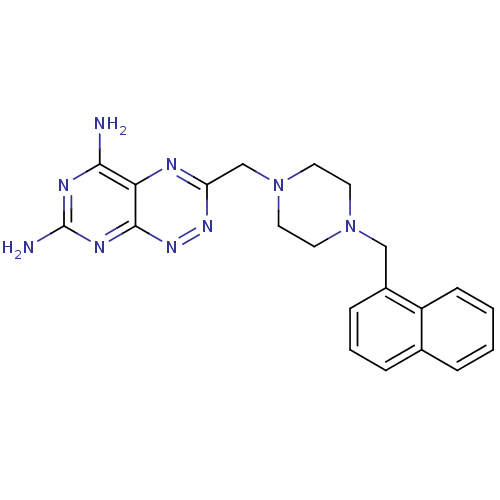
(3-(4-Naphthalen-1-ylmethyl-piperazin-1-ylmethyl)-p...)Show SMILES Nc1nc(N)c2nc(CN3CCN(Cc4cccc5ccccc45)CC3)nnc2n1 Show InChI InChI=1S/C21H23N9/c22-19-18-20(26-21(23)25-19)28-27-17(24-18)13-30-10-8-29(9-11-30)12-15-6-3-5-14-4-1-2-7-16(14)15/h1-7H,8-13H2,(H4,22,23,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Protein-tyrosine phosphatase 1B in 300 nM DTT |
Bioorg Med Chem Lett 13: 2895-8 (2003)
BindingDB Entry DOI: 10.7270/Q21V5DDS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50135663

(3-(4-Biphenyl-4-ylmethyl-piperazin-1-ylmethyl)-pyr...)Show SMILES Nc1nc(N)c2nc(CN3CCN(Cc4ccc(cc4)-c4ccccc4)CC3)nnc2n1 Show InChI InChI=1S/C23H25N9/c24-21-20-22(28-23(25)27-21)30-29-19(26-20)15-32-12-10-31(11-13-32)14-16-6-8-18(9-7-16)17-4-2-1-3-5-17/h1-9H,10-15H2,(H4,24,25,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration required against Tyrosine phosphatase SHP-2 in the presence of 300 nM DTT |
Bioorg Med Chem Lett 13: 2895-8 (2003)
BindingDB Entry DOI: 10.7270/Q21V5DDS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381980

(CHEMBL2022495)Show SMILES COc1cc(NC(=O)c2ccccc2F)ccc1-c1nnc(NCCCN2CCCCC2)o1 Show InChI InChI=1S/C24H28FN5O3/c1-32-21-16-17(27-22(31)18-8-3-4-9-20(18)25)10-11-19(21)23-28-29-24(33-23)26-12-7-15-30-13-5-2-6-14-30/h3-4,8-11,16H,2,5-7,12-15H2,1H3,(H,26,29)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50381980

(CHEMBL2022495)Show SMILES COc1cc(NC(=O)c2ccccc2F)ccc1-c1nnc(NCCCN2CCCCC2)o1 Show InChI InChI=1S/C24H28FN5O3/c1-32-21-16-17(27-22(31)18-8-3-4-9-20(18)25)10-11-19(21)23-28-29-24(33-23)26-12-7-15-30-13-5-2-6-14-30/h3-4,8-11,16H,2,5-7,12-15H2,1H3,(H,26,29)(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha4beta2 nAChR |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381980

(CHEMBL2022495)Show SMILES COc1cc(NC(=O)c2ccccc2F)ccc1-c1nnc(NCCCN2CCCCC2)o1 Show InChI InChI=1S/C24H28FN5O3/c1-32-21-16-17(27-22(31)18-8-3-4-9-20(18)25)10-11-19(21)23-28-29-24(33-23)26-12-7-15-30-13-5-2-6-14-30/h3-4,8-11,16H,2,5-7,12-15H2,1H3,(H,26,29)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 22: 3531-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.062
BindingDB Entry DOI: 10.7270/Q2SB46SH |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381983

(CHEMBL2023929)Show SMILES Fc1ccccc1C(=O)Nc1ccc(-c2nnc(NCCCN3CCCCC3)o2)c(c1)C(F)(F)F Show InChI InChI=1S/C24H25F4N5O2/c25-20-8-3-2-7-18(20)21(34)30-16-9-10-17(19(15-16)24(26,27)28)22-31-32-23(35-22)29-11-6-14-33-12-4-1-5-13-33/h2-3,7-10,15H,1,4-6,11-14H2,(H,29,32)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50135668
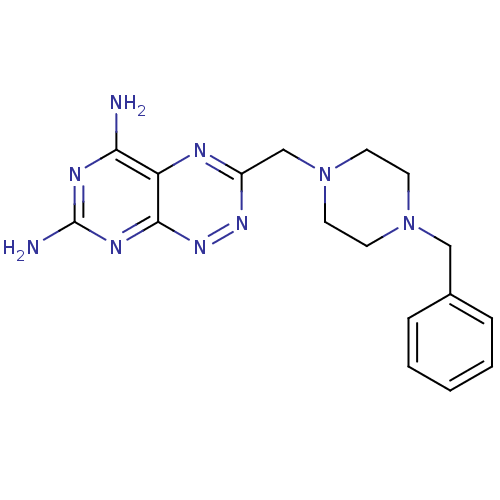
(3-(4-Benzyl-piperazin-1-ylmethyl)-pyrimido[5,4-e][...)Show InChI InChI=1S/C17H21N9/c18-15-14-16(22-17(19)21-15)24-23-13(20-14)11-26-8-6-25(7-9-26)10-12-4-2-1-3-5-12/h1-5H,6-11H2,(H4,18,19,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Protein-tyrosine phosphatase 1B in 300 nM DTT |
Bioorg Med Chem Lett 13: 2895-8 (2003)
BindingDB Entry DOI: 10.7270/Q21V5DDS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50135668

(3-(4-Benzyl-piperazin-1-ylmethyl)-pyrimido[5,4-e][...)Show InChI InChI=1S/C17H21N9/c18-15-14-16(22-17(19)21-15)24-23-13(20-14)11-26-8-6-25(7-9-26)10-12-4-2-1-3-5-12/h1-5H,6-11H2,(H4,18,19,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of Protein-tyrosine phosphatase 1B in 300 nM DTT |
Bioorg Med Chem Lett 13: 2895-8 (2003)
BindingDB Entry DOI: 10.7270/Q21V5DDS |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50382039
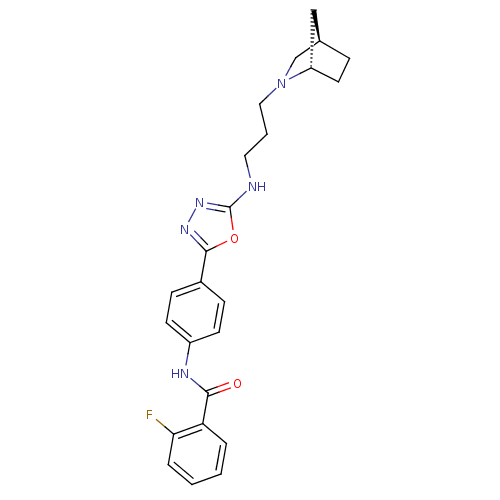
(CHEMBL2022504)Show SMILES Fc1ccccc1C(=O)Nc1ccc(cc1)-c1nnc(NCCCN2C[C@@H]3CC[C@H]2C3)o1 |r| Show InChI InChI=1S/C24H26FN5O2/c25-21-5-2-1-4-20(21)22(31)27-18-9-7-17(8-10-18)23-28-29-24(32-23)26-12-3-13-30-15-16-6-11-19(30)14-16/h1-2,4-5,7-10,16,19H,3,6,11-15H2,(H,26,29)(H,27,31)/t16-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3531-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.062
BindingDB Entry DOI: 10.7270/Q2SB46SH |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(Homo sapiens (Human)) | BDBM50382048
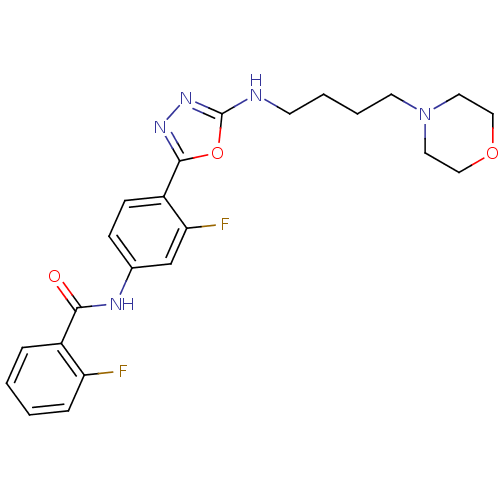
(CHEMBL2022739)Show SMILES Fc1ccccc1C(=O)Nc1ccc(-c2nnc(NCCCCN3CCOCC3)o2)c(F)c1 Show InChI InChI=1S/C23H25F2N5O3/c24-19-6-2-1-5-17(19)21(31)27-16-7-8-18(20(25)15-16)22-28-29-23(33-22)26-9-3-4-10-30-11-13-32-14-12-30/h1-2,5-8,15H,3-4,9-14H2,(H,26,29)(H,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at alpha1 nAChR |
Bioorg Med Chem Lett 22: 3531-4 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.062
BindingDB Entry DOI: 10.7270/Q2SB46SH |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381976

(CHEMBL2023921)Show SMILES Fc1cc(ccc1NC(=O)c1ccccc1F)-c1nnc(NCCCN2CCCCC2)o1 Show InChI InChI=1S/C23H25F2N5O2/c24-18-8-3-2-7-17(18)21(31)27-20-10-9-16(15-19(20)25)22-28-29-23(32-22)26-11-6-14-30-12-4-1-5-13-30/h2-3,7-10,15H,1,4-6,11-14H2,(H,26,29)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381968

(CHEMBL2022938)Show SMILES Fc1ccccc1C(=O)Nc1ccc(cc1)-c1nnc(NCCCN2CCCCCC2)o1 Show InChI InChI=1S/C24H28FN5O2/c25-21-9-4-3-8-20(21)22(31)27-19-12-10-18(11-13-19)23-28-29-24(32-23)26-14-7-17-30-15-5-1-2-6-16-30/h3-4,8-13H,1-2,5-7,14-17H2,(H,26,29)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381972
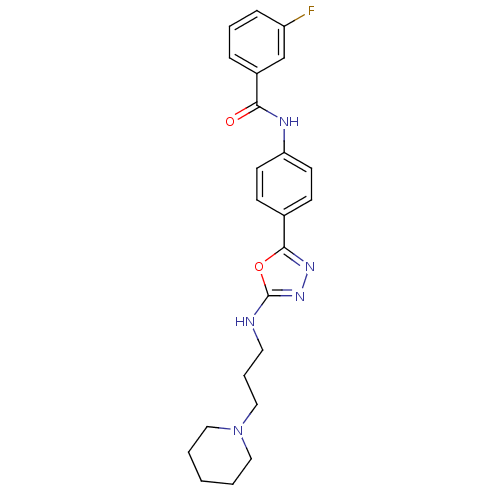
(CHEMBL2023916)Show SMILES Fc1cccc(c1)C(=O)Nc1ccc(cc1)-c1nnc(NCCCN2CCCCC2)o1 Show InChI InChI=1S/C23H26FN5O2/c24-19-7-4-6-18(16-19)21(30)26-20-10-8-17(9-11-20)22-27-28-23(31-22)25-12-5-15-29-13-2-1-3-14-29/h4,6-11,16H,1-3,5,12-15H2,(H,25,28)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381971

(CHEMBL2022941)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)-c1nnc(NCCCN2CCCCC2)o1 Show InChI InChI=1S/C24H29N5O2/c1-18-8-3-4-9-21(18)22(30)26-20-12-10-19(11-13-20)23-27-28-24(31-23)25-14-7-17-29-15-5-2-6-16-29/h3-4,8-13H,2,5-7,14-17H2,1H3,(H,25,28)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381967

(CHEMBL2022496)Show SMILES Fc1ccccc1C(=O)Nc1ccc(cc1)-c1nnc(NCCCN2CCCCC2)o1 Show InChI InChI=1S/C23H26FN5O2/c24-20-8-3-2-7-19(20)21(30)26-18-11-9-17(10-12-18)22-27-28-23(31-22)25-13-6-16-29-14-4-1-5-15-29/h2-3,7-12H,1,4-6,13-16H2,(H,25,28)(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50381966

(CHEMBL2022499)Show SMILES Fc1ccccc1C(=O)Nc1ccc(cc1)-c1nnc(NCCCN2CCCC2)o1 Show InChI InChI=1S/C22H24FN5O2/c23-19-7-2-1-6-18(19)20(29)25-17-10-8-16(9-11-17)21-26-27-22(30-21)24-12-5-15-28-13-3-4-14-28/h1-2,6-11H,3-5,12-15H2,(H,24,27)(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]Dofetilide from human ERG expressed in CHO-K1 cells by scintillation proximity assay |
Bioorg Med Chem Lett 22: 3560-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.040
BindingDB Entry DOI: 10.7270/Q21V5FZC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data