

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093001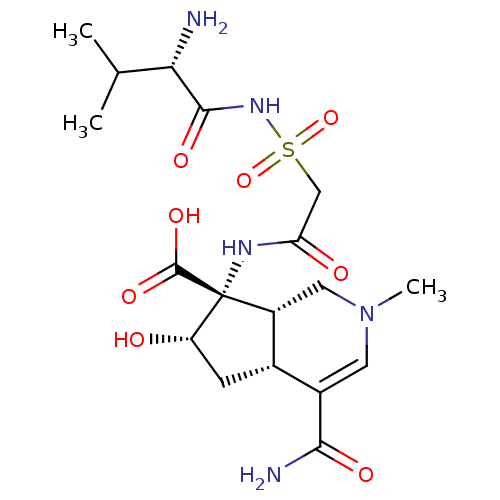 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093003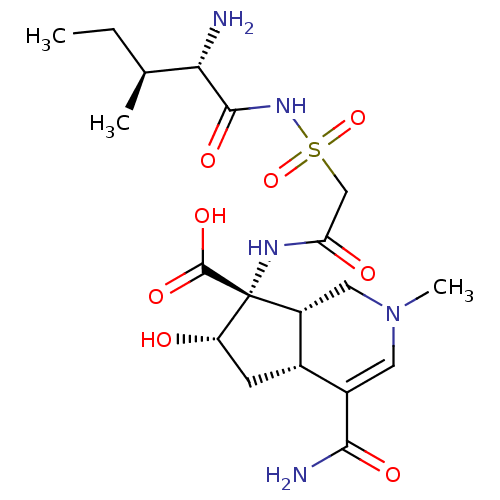 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50093005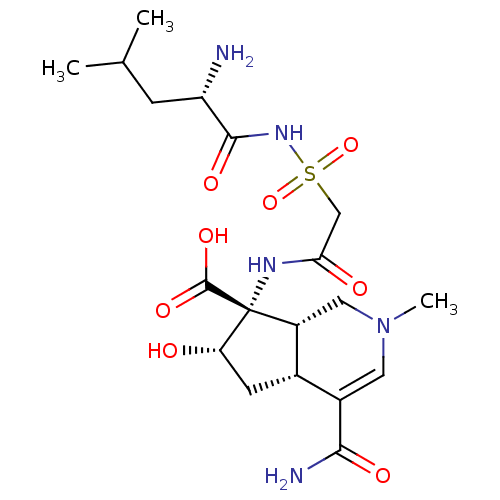 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093004 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093002 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Valine--tRNA ligase (Homo sapiens (Human)) | BDBM50093001 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093001 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093004 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093005 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093002 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Valine--tRNA ligase (Homo sapiens (Human)) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Valine--tRNA ligase (Homo sapiens (Human)) | BDBM50093005 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093006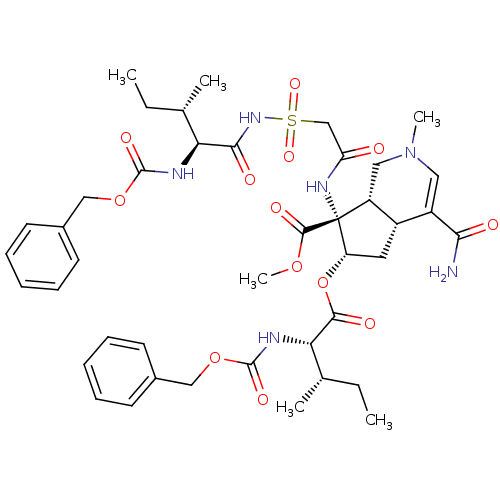 ((4aR,6S,7R,7aS)-6-((2S,3S)-2-Benzyloxycarbonylamin...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093005 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401749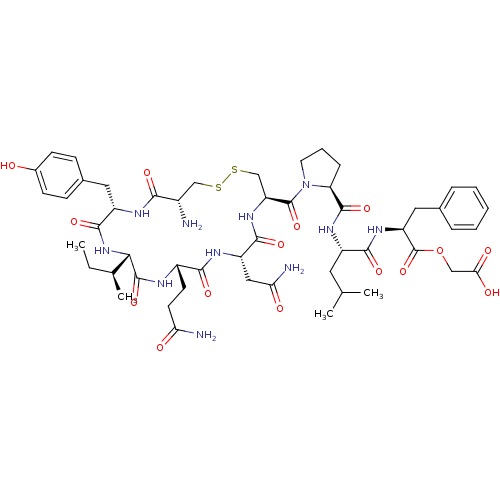 (CHEMBL2207130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50093001 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401751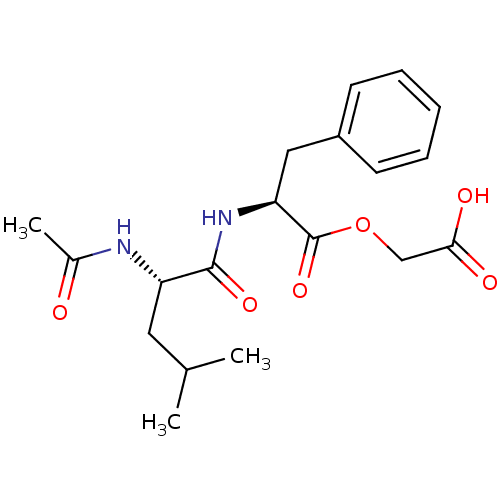 (CHEMBL2207128) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093008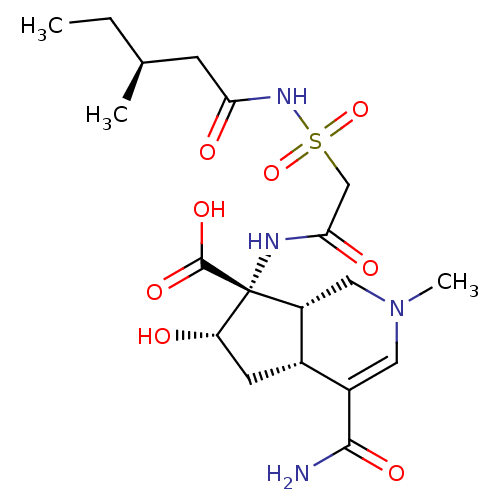 ((4aR,6S,7R,7aS)-4-Carbamoyl-6-hydroxy-2-methyl-7-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401752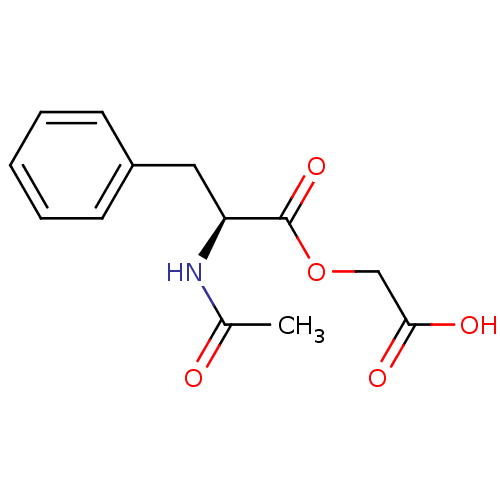 (CHEMBL2207127) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401750 (CHEMBL2207129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093007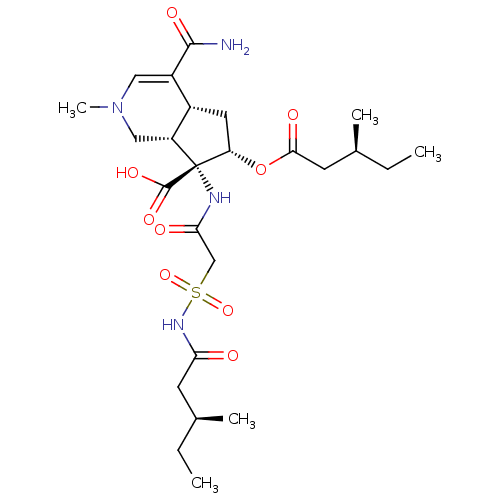 ((4aR,6S,7R,7aS)-4-Carbamoyl-2-methyl-6-((S)-3-meth...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401751 (CHEMBL2207128) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401752 (CHEMBL2207127) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401756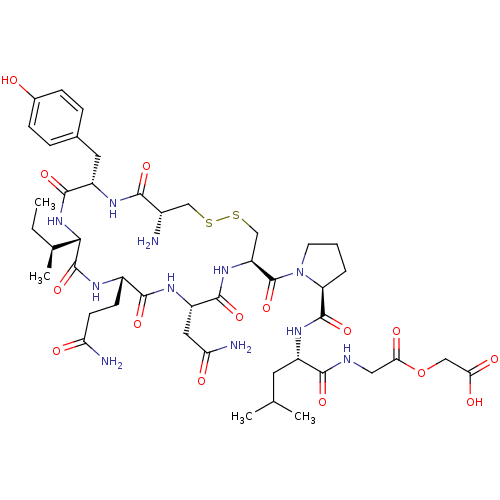 (CHEMBL2207143) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093007 ((4aR,6S,7R,7aS)-4-Carbamoyl-2-methyl-6-((S)-3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093008 ((4aR,6S,7R,7aS)-4-Carbamoyl-6-hydroxy-2-methyl-7-[...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401749 (CHEMBL2207130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401750 (CHEMBL2207129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401756 (CHEMBL2207143) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401757 (CHEMBL2207142) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401758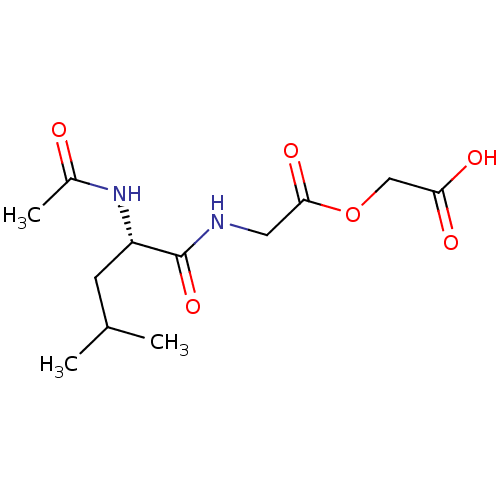 (CHEMBL2207141) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401746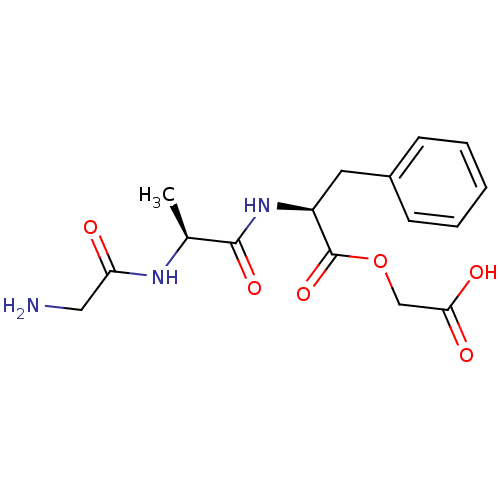 (CHEMBL2207133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401757 (CHEMBL2207142) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50093004 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401758 (CHEMBL2207141) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401759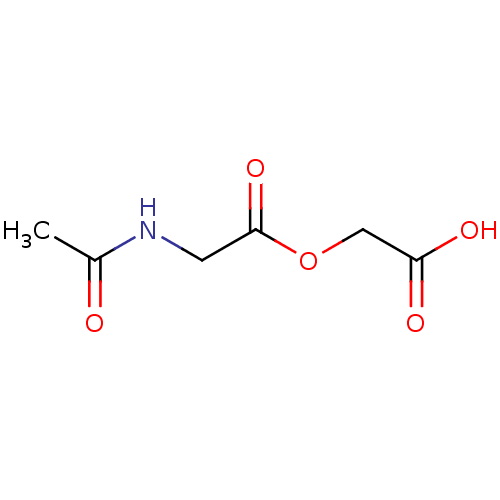 (CHEMBL2207140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401754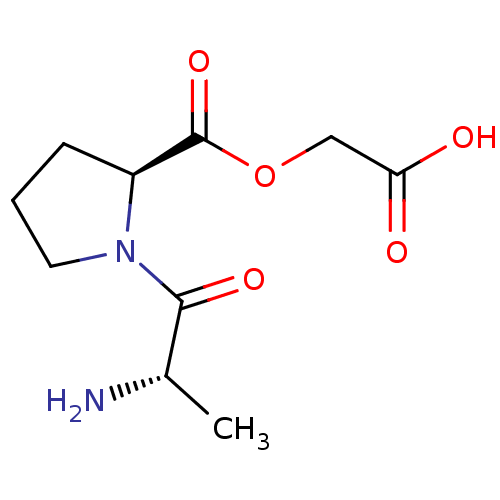 (CHEMBL2207145) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401747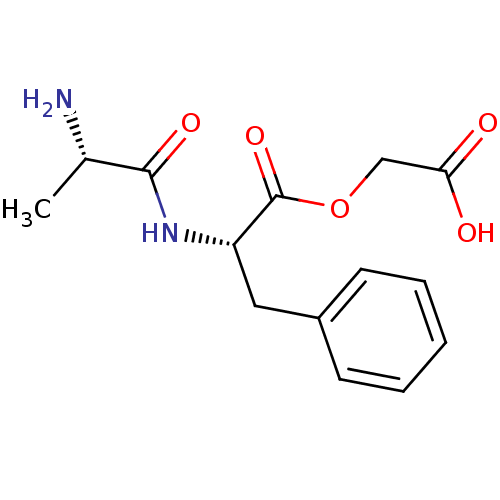 (CHEMBL2207132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401747 (CHEMBL2207132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401754 (CHEMBL2207145) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401755 (CHEMBL2207144) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401746 (CHEMBL2207133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401755 (CHEMBL2207144) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401753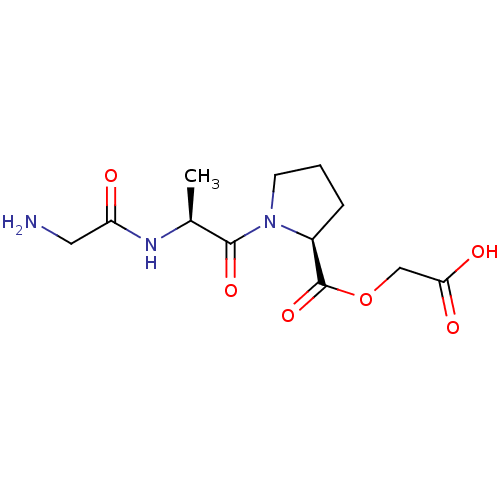 (CHEMBL2207146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM82195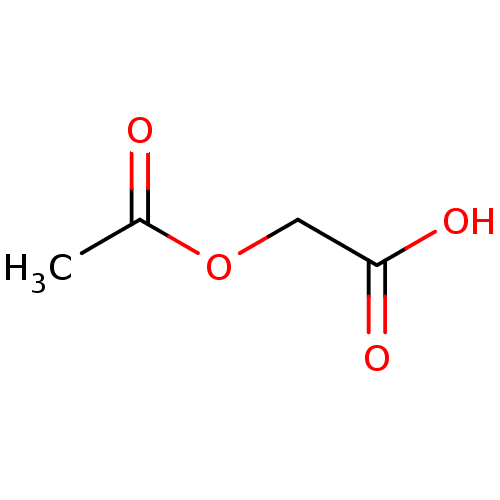 (Substrate analogue, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM82195 (Substrate analogue, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401748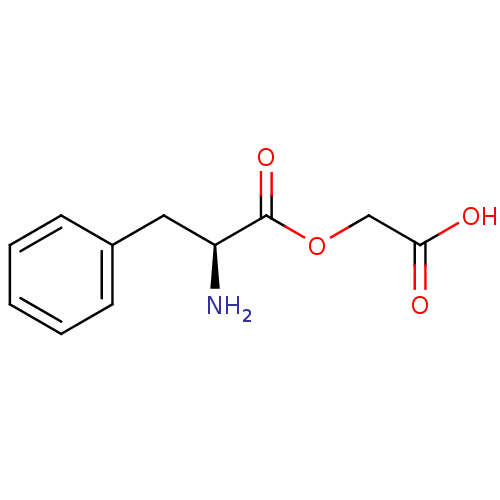 (CHEMBL2207131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of PAM in human DMS53 cells using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-glycine alpha-amidating monooxygenase (Homo sapiens (Human)) | BDBM50401753 (CHEMBL2207146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Competitive inhibition of secreted PAM in human DMS53 cell culture medium using as(R)-Tyr-(S)-Val-Gly as substrate after 2 hrs by HPLC analysis | Bioorg Med Chem Lett 22: 7015-8 (2012) Article DOI: 10.1016/j.bmcl.2012.10.004 BindingDB Entry DOI: 10.7270/Q2VT1T73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |