

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50404011 (CHEMBL36900) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50473991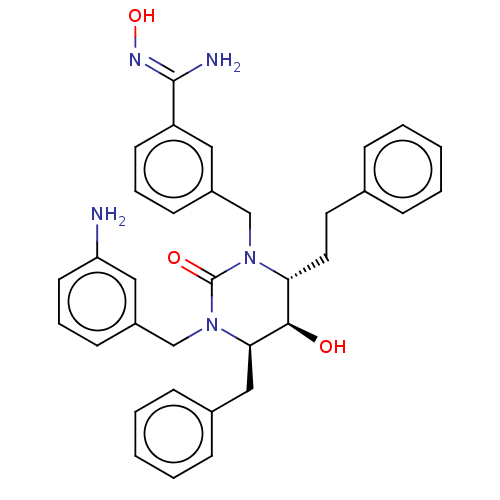 (CHEMBL416783) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50404007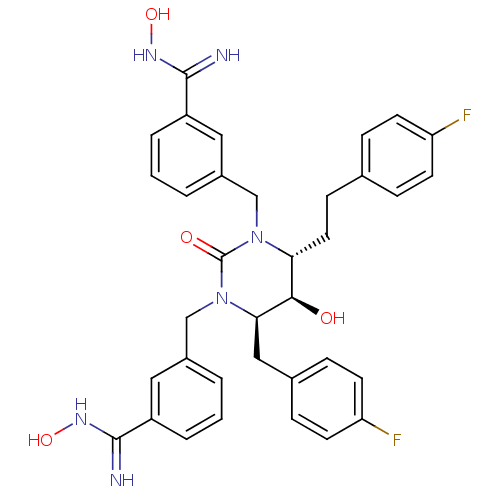 (CHEMBL116517) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM164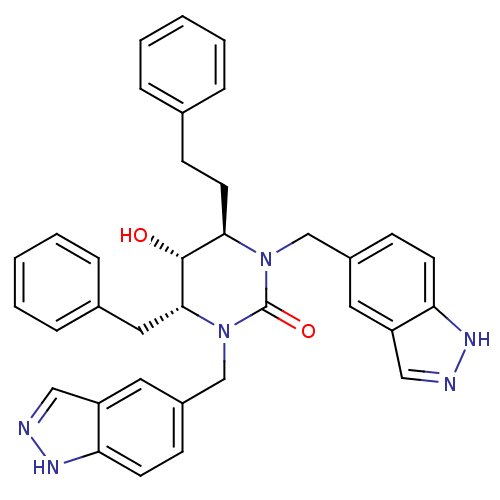 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis(1H-indazol-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50065089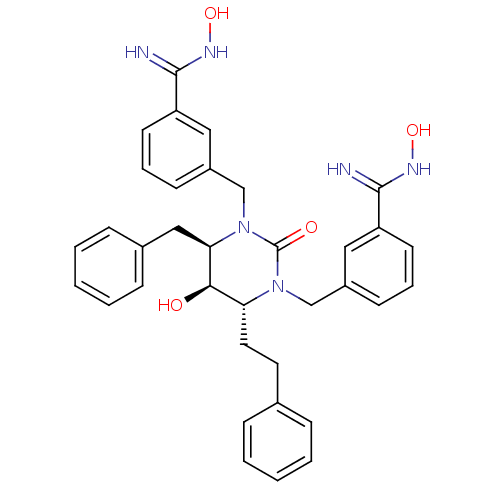 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-benzamide oxime)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50404010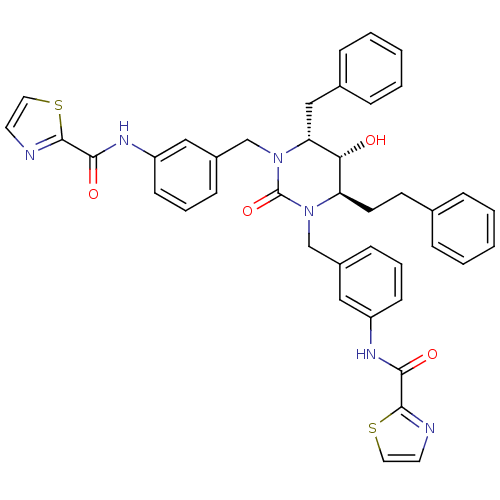 (CHEMBL324293) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50473996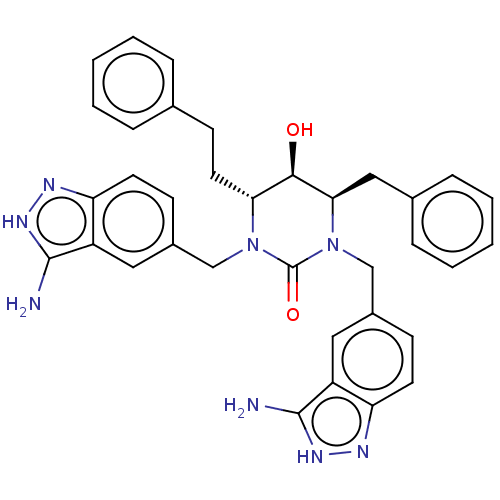 (CHEMBL291004) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50091753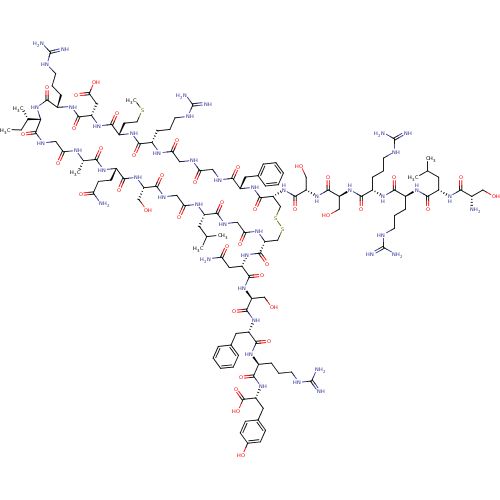 (CHEMBL405854 | H-Ser-Leu-Arg-Arg-Ser-Ser-cyclic(Cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50061031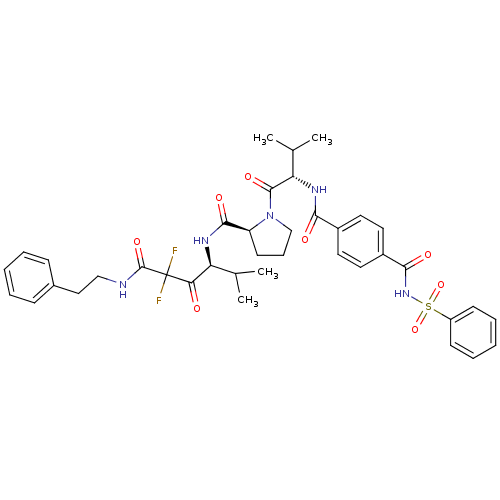 ((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for human leukocyte elastase | J Med Chem 40: 3173-81 (1997) Article DOI: 10.1021/jm970250z BindingDB Entry DOI: 10.7270/Q2GT5M85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1149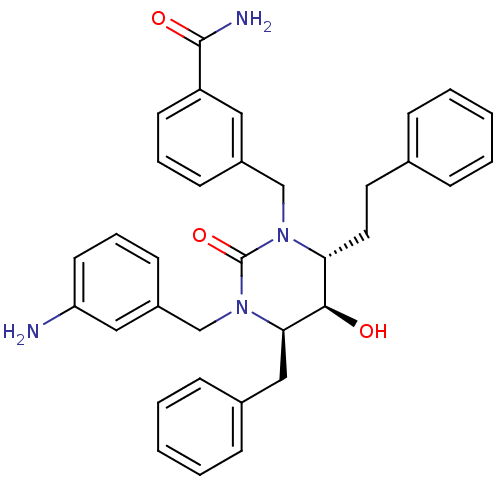 ((4R,5R,6R)-Tetrahydro-1-(3-aminophenyl-methyl)-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50473992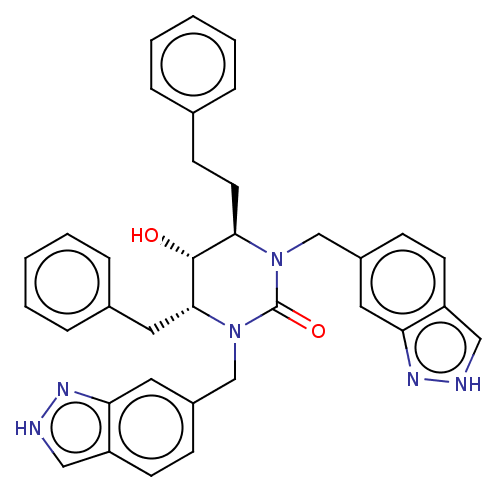 (CHEMBL416612) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50404006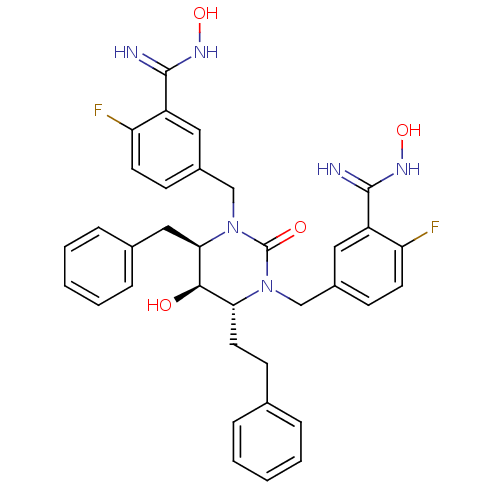 (CHEMBL366576) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527524 (CHEMBL4566742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527524 (CHEMBL4566742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50404005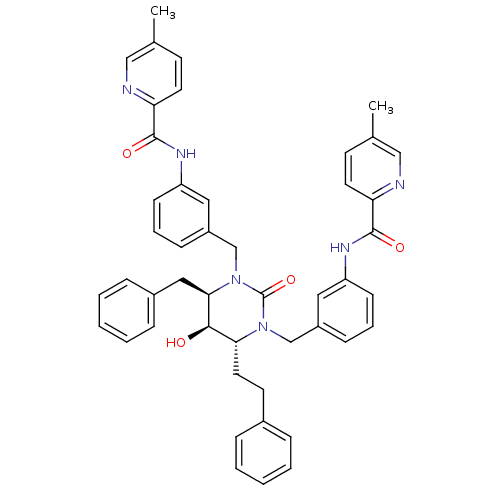 (CHEMBL113884) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1144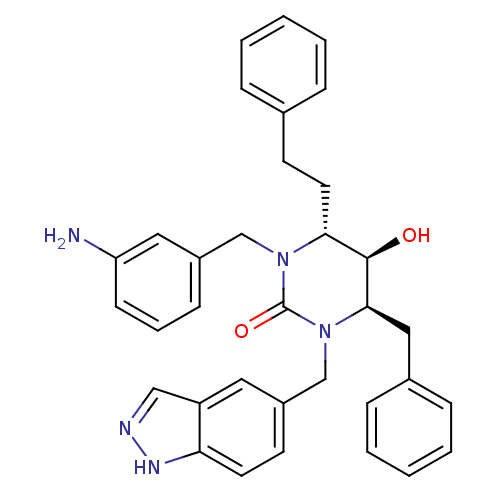 ((4R,5R,6R)-1-[(3-aminophenyl)methyl]-4-benzyl-5-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50146972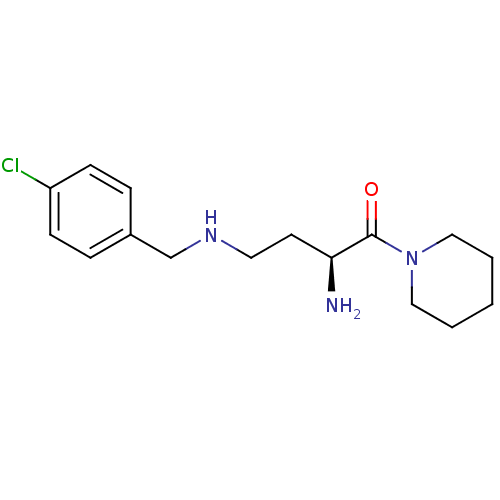 ((S)-4-(4-chlorobenzylamino)-2-amino-1-(piperidin-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP2 (unknown origin) | Bioorg Med Chem Lett 18: 4154-8 (2008) Article DOI: 10.1016/j.bmcl.2008.05.080 BindingDB Entry DOI: 10.7270/Q2G160N3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50146972 ((S)-4-(4-chlorobenzylamino)-2-amino-1-(piperidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry University of Antwerp Curated by ChEMBL | Assay Description Inhibition of DPP4 | Bioorg Med Chem Lett 16: 4777-9 (2006) Article DOI: 10.1016/j.bmcl.2006.06.082 BindingDB Entry DOI: 10.7270/Q2GM86XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1080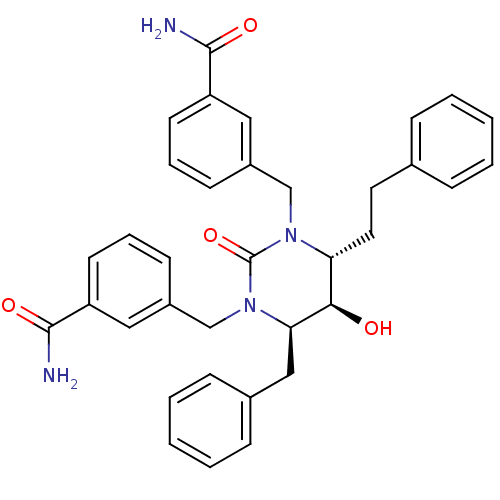 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-benzamido)methyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 3 (Homo sapiens (Human)) | BDBM50091751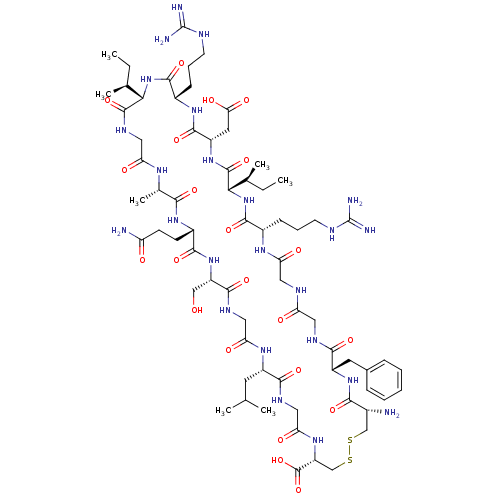 (CHEMBL411542 | Cyclic-(Cys-Phe-Gly-Gly-Arg-Ile-Asp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial Natriuretic Peptide Clearance Receptor. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atrial natriuretic peptide receptor 1 (Homo sapiens (Human)) | BDBM50091751 (CHEMBL411542 | Cyclic-(Cys-Phe-Gly-Gly-Arg-Ile-Asp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]- ANP from the Atrial natriuretic peptide receptor A. | Bioorg Med Chem Lett 10: 1949-52 (2001) BindingDB Entry DOI: 10.7270/Q2BK1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1107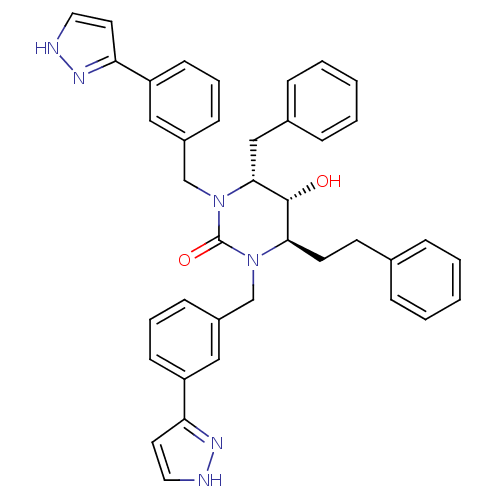 ((4R,5R,6R)-4-benzyl-5-hydroxy-6-(2-phenylethyl)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1148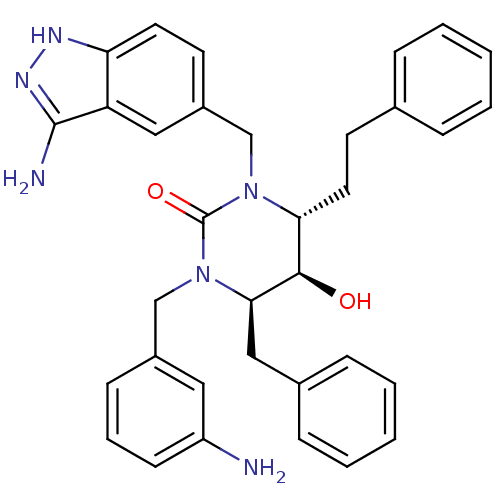 ((4R,5R,6R)-1-[(3-amino-1H-indazol-5-yl)methyl]-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1145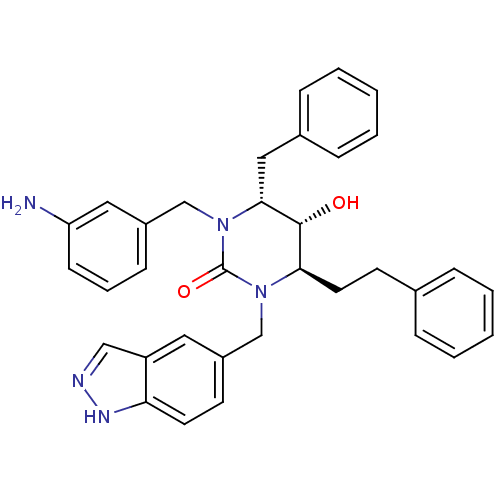 ((4R,5R,6R)-1-[(3-aminophenyl)methyl]-6-benzyl-5-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1109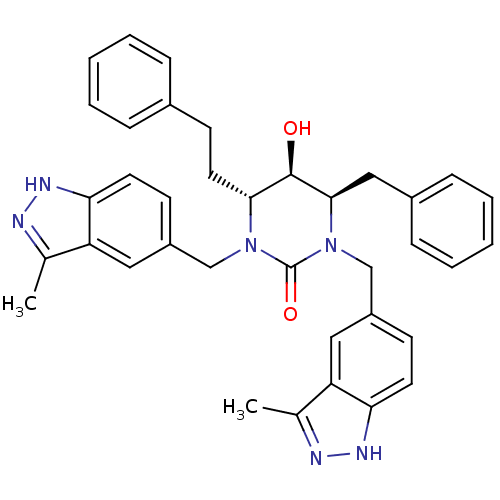 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis[(3-methyl-1H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527552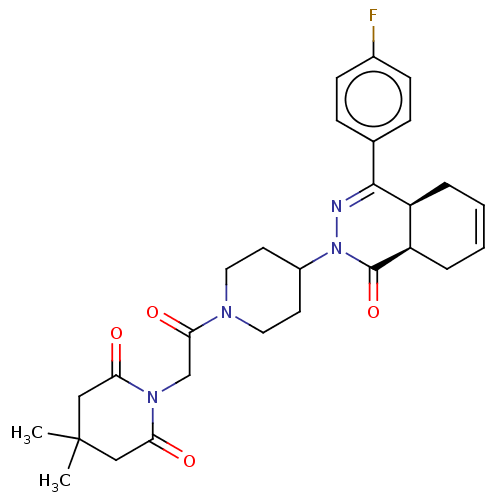 (CHEMBL4435111) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527552 (CHEMBL4435111) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527532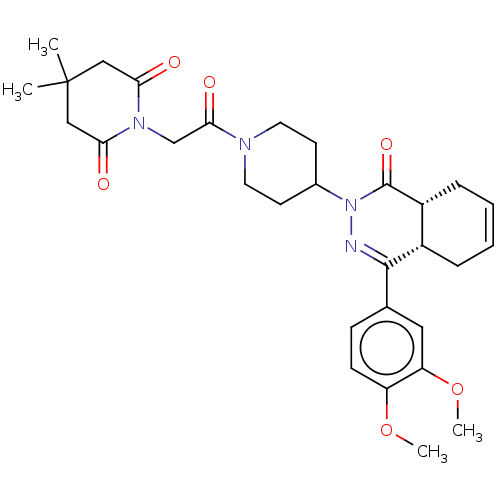 (CHEMBL4441748) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527532 (CHEMBL4441748) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1146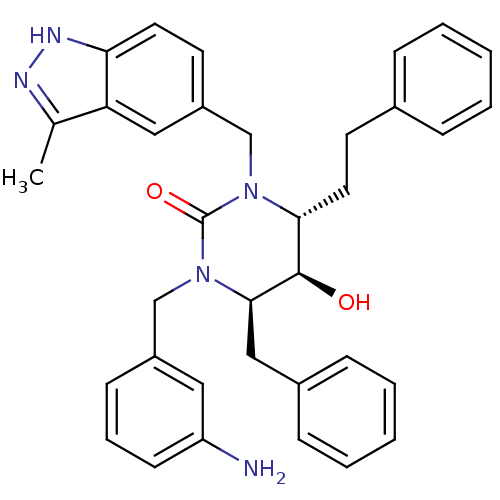 ((4R,5R,6R)-1-[(3-aminophenyl)methyl]-6-benzyl-5-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1102 ((4R,5R,6R)-Tetrahydro-1,3-bis[(3-carboxamidophenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527537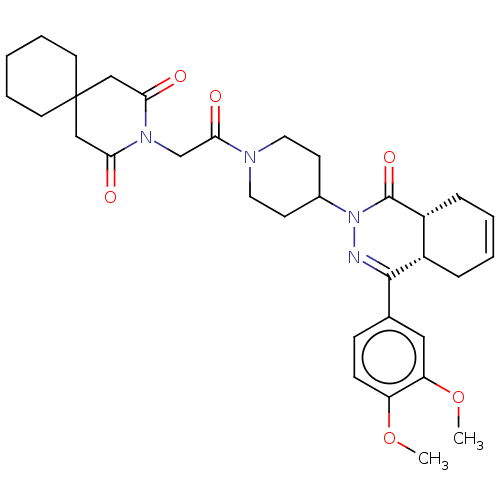 (CHEMBL4453005) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527531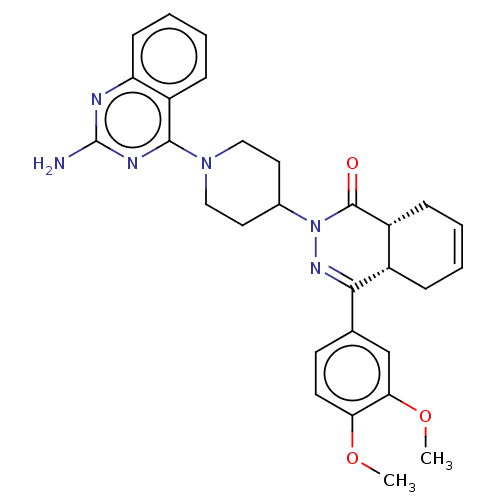 (CHEMBL4524402) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527531 (CHEMBL4524402) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527537 (CHEMBL4453005) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527528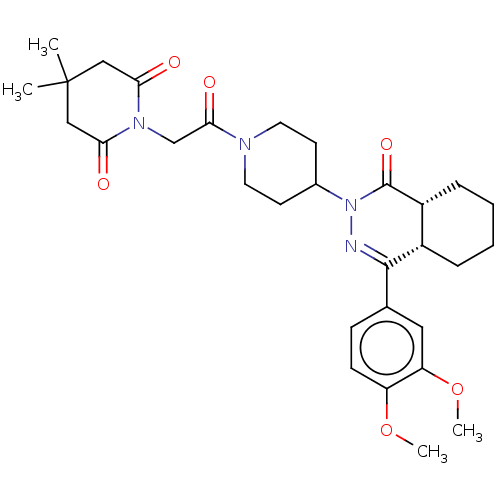 (CHEMBL4441871) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527528 (CHEMBL4441871) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527550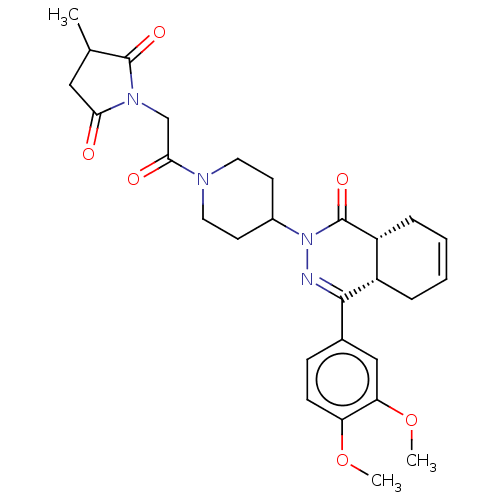 (CHEMBL4461456) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527550 (CHEMBL4461456) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50063266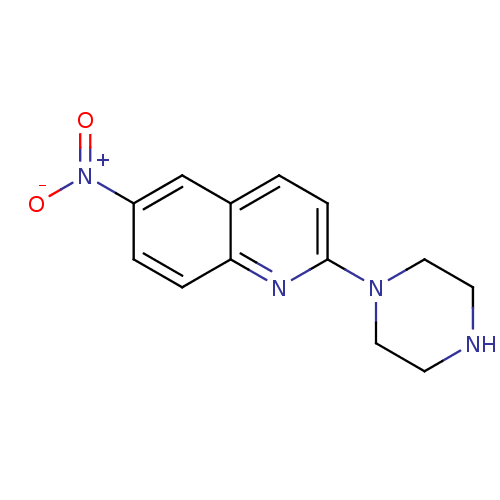 (6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Medicines Institute Curated by ChEMBL | Assay Description Displacement of [3H]-5HT from human SERT | Eur J Med Chem 49: 200-10 (2012) Article DOI: 10.1016/j.ejmech.2012.01.012 BindingDB Entry DOI: 10.7270/Q2K35V4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50372562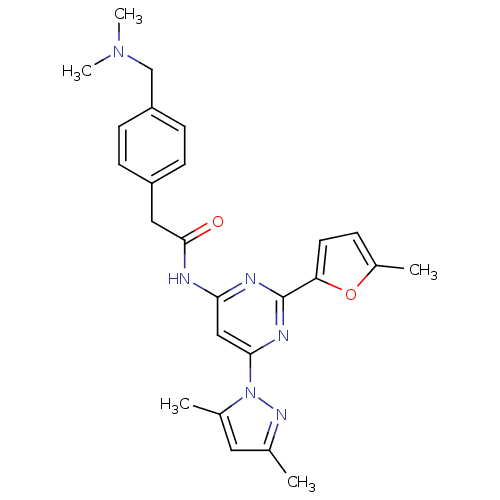 (CHEMBL255739) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human adenosine A2A receptor expressed in HEK293 cells | Bioorg Med Chem Lett 18: 1269-73 (2008) Article DOI: 10.1016/j.bmcl.2008.01.036 BindingDB Entry DOI: 10.7270/Q2K35VGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527535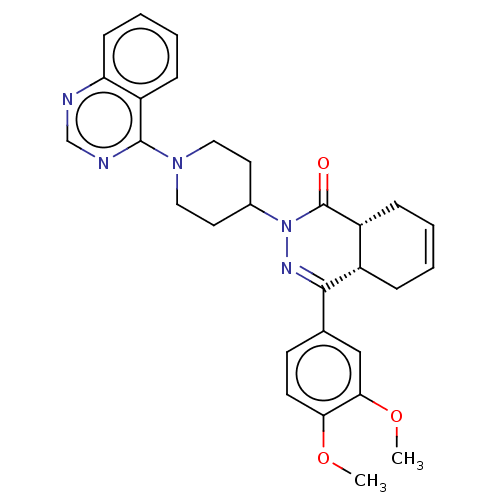 (CHEMBL4589927) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527535 (CHEMBL4589927) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527533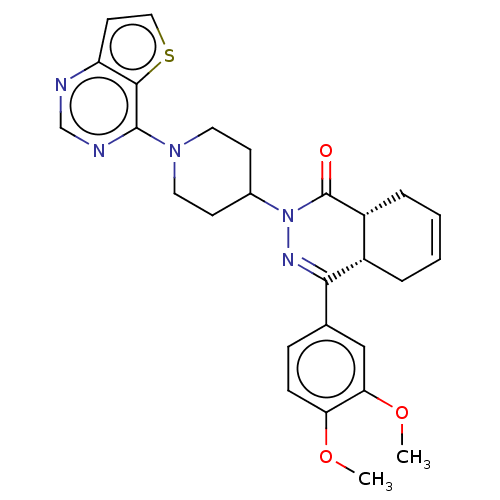 (CHEMBL4592553) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50527533 (CHEMBL4592553) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human PDE4D2 expressed in Escherichia coli BL21 (DE3) using cAMP as substrate by PDELight HTS cAMP phosphodiesterase Kit based | J Med Chem 63: 3485-3507 (2020) Article DOI: 10.1021/acs.jmedchem.9b00985 BindingDB Entry DOI: 10.7270/Q20005JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50473993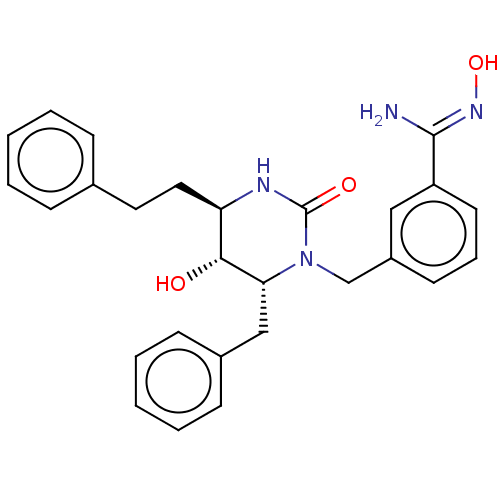 (CHEMBL286159) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1090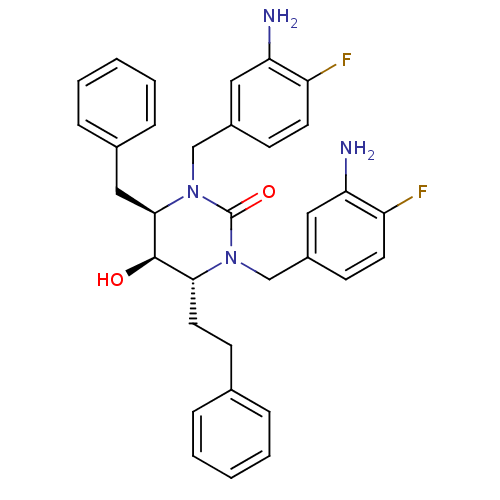 ((4R,5R,6R)-1,3-bis[(3-amino-4-fluorophenyl)methyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135635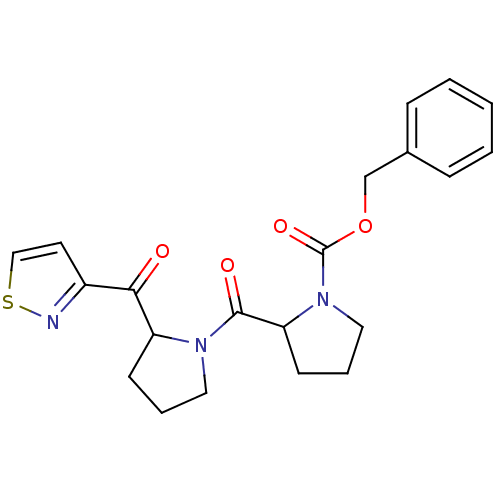 (2-[2-(Isothiazole-3-carbonyl)-pyrrolidine-1-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50135633 ((S)-2-[(S)-2-(5-Benzyloxymethyl-isoxazole-3-carbon...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description In vitro inhibitory activity of the compound determined against prolyl oligopeptidase (Tc80) in Trypanosoma cruzi | Bioorg Med Chem Lett 13: 2875-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50473997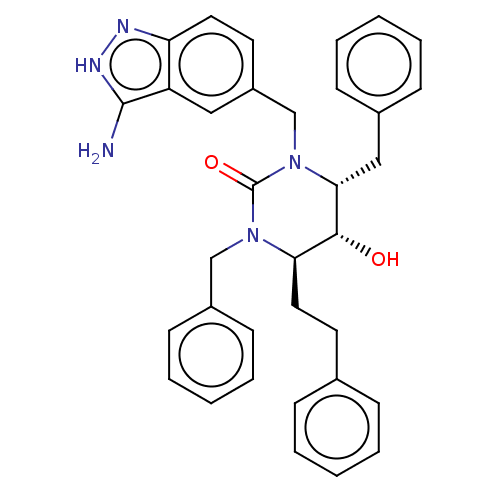 (CHEMBL36581) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 45: 973-83 (2002) Article DOI: 10.1021/jm010417v BindingDB Entry DOI: 10.7270/Q2JH3PX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9215 total ) | Next | Last >> |