 Found 200 hits with Last Name = 'engelberg' and Initial = 'em'
Found 200 hits with Last Name = 'engelberg' and Initial = 'em' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50283472

(CHEMBL4160748)Show SMILES COc1ccc(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1F |(9.45,-43.77,;10.47,-42.62,;9.99,-41.15,;8.48,-40.84,;7.99,-39.38,;9.02,-38.23,;8.54,-36.77,;9.43,-35.53,;10.97,-35.52,;8.53,-34.29,;7.07,-34.77,;5.74,-34,;4.41,-34.77,;4.41,-36.31,;3.08,-37.09,;5.74,-37.08,;7.08,-36.3,;5.74,-32.46,;7.08,-31.68,;4.41,-31.68,;5.73,-30.92,;10.52,-38.54,;11.55,-37.39,;11.01,-40,;12.52,-40.31,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-6-11(16(19,20)21)24-15(22-7)12(8(2)23-24)9-4-5-10(25-3)14(18)13(9)17/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Displacement of [3H] substance P from recombinant human NK1 receptor expressed in CHO cells after 90 mins by scintillation counting method |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50283479
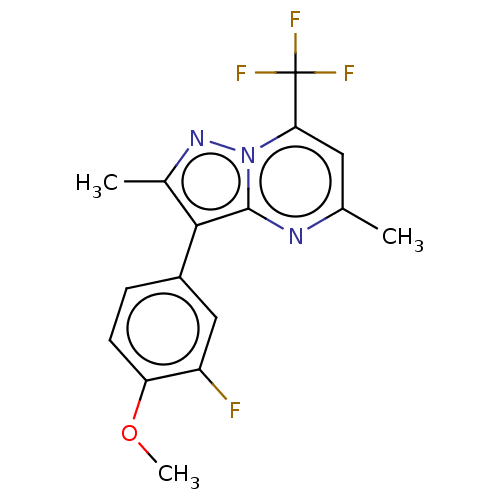
(CHEMBL4165248)Show SMILES COc1ccc(cc1F)-c1c(C)nn2c(cc(C)nc12)C(F)(F)F Show InChI InChI=1S/C16H13F4N3O/c1-8-6-13(16(18,19)20)23-15(21-8)14(9(2)22-23)10-4-5-12(24-3)11(17)7-10/h4-7H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50249435
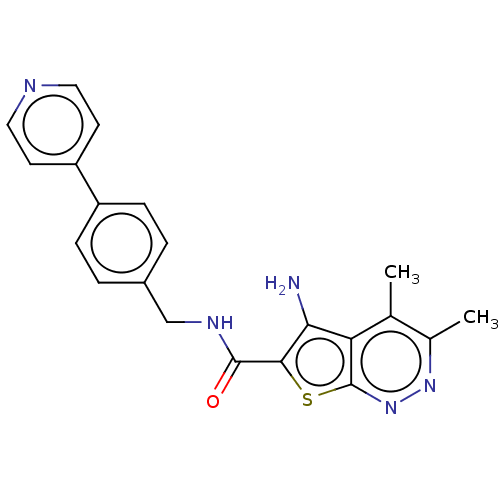
(CHEMBL4070692)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccncc3)c(N)c2c1C Show InChI InChI=1S/C21H19N5OS/c1-12-13(2)25-26-21-17(12)18(22)19(28-21)20(27)24-11-14-3-5-15(6-4-14)16-7-9-23-10-8-16/h3-10H,11,22H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Induction of CYP3A4 in cryopreserved human hepatocytes measured after 48 hrs |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280369
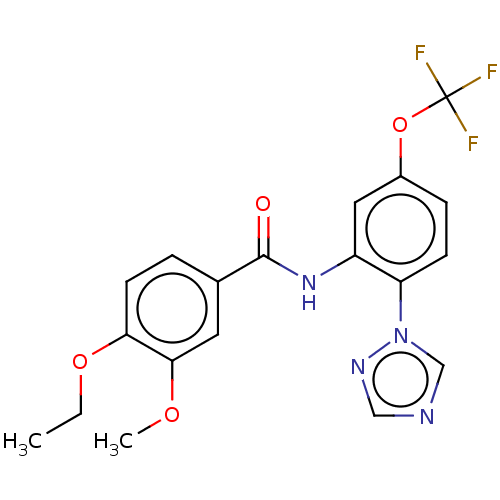
(CHEMBL4169114)Show SMILES CCOc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C19H17F3N4O4/c1-3-29-16-7-4-12(8-17(16)28-2)18(27)25-14-9-13(30-19(20,21)22)5-6-15(14)26-11-23-10-24-26/h4-11H,3H2,1-2H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280369

(CHEMBL4169114)Show SMILES CCOc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C19H17F3N4O4/c1-3-29-16-7-4-12(8-17(16)28-2)18(27)25-14-9-13(30-19(20,21)22)5-6-15(14)26-11-23-10-24-26/h4-11H,3H2,1-2H3,(H,25,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280374
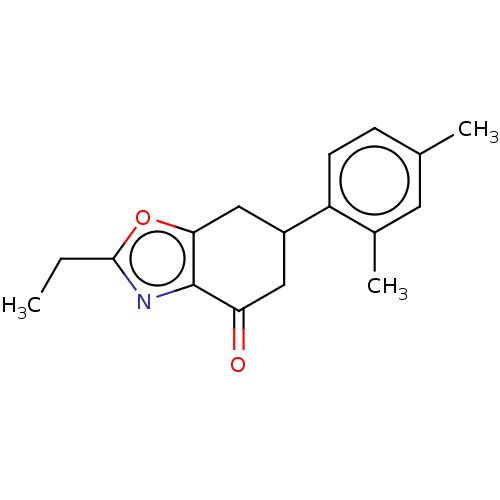
(CHEMBL4174742)Show InChI InChI=1S/C17H19NO2/c1-4-16-18-17-14(19)8-12(9-15(17)20-16)13-6-5-10(2)7-11(13)3/h5-7,12H,4,8-9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of rat mGlu7 receptor expressed in HEK cells co-expressing Galphai5 assessed as decrease in glutamate-induced thallium... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280374

(CHEMBL4174742)Show InChI InChI=1S/C17H19NO2/c1-4-16-18-17-14(19)8-12(9-15(17)20-16)13-6-5-10(2)7-11(13)3/h5-7,12H,4,8-9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280374

(CHEMBL4174742)Show InChI InChI=1S/C17H19NO2/c1-4-16-18-17-14(19)8-12(9-15(17)20-16)13-6-5-10(2)7-11(13)3/h5-7,12H,4,8-9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50283483
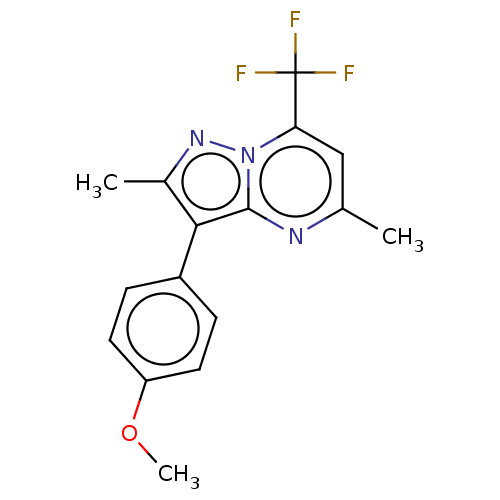
(CHEMBL2361777)Show InChI InChI=1S/C16H14F3N3O/c1-9-8-13(16(17,18)19)22-15(20-9)14(10(2)21-22)11-4-6-12(23-3)7-5-11/h4-8H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280370
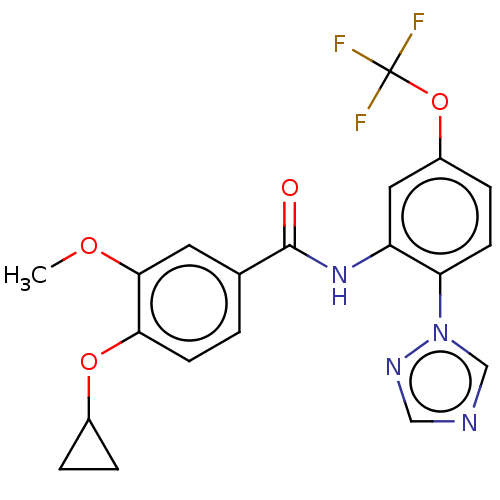
(CHEMBL4160395)Show SMILES COc1cc(ccc1OC1CC1)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C20H17F3N4O4/c1-29-18-8-12(2-7-17(18)30-13-3-4-13)19(28)26-15-9-14(31-20(21,22)23)5-6-16(15)27-11-24-10-25-27/h2,5-11,13H,3-4H2,1H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280370

(CHEMBL4160395)Show SMILES COc1cc(ccc1OC1CC1)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C20H17F3N4O4/c1-29-18-8-12(2-7-17(18)30-13-3-4-13)19(28)26-15-9-14(31-20(21,22)23)5-6-16(15)27-11-24-10-25-27/h2,5-11,13H,3-4H2,1H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280368
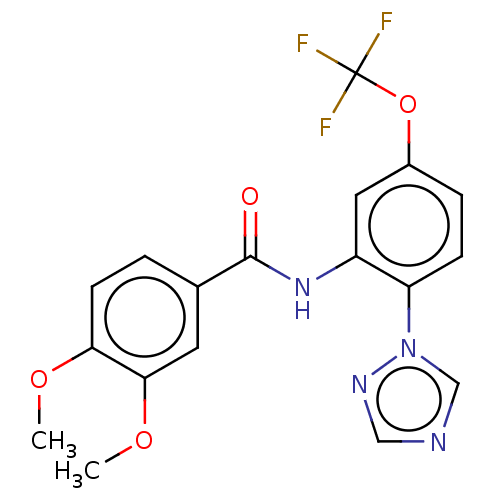
(CHEMBL4176971)Show SMILES COc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C18H15F3N4O4/c1-27-15-6-3-11(7-16(15)28-2)17(26)24-13-8-12(29-18(19,20)21)4-5-14(13)25-10-22-9-23-25/h3-10H,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280368

(CHEMBL4176971)Show SMILES COc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C18H15F3N4O4/c1-27-15-6-3-11(7-16(15)28-2)17(26)24-13-8-12(29-18(19,20)21)4-5-14(13)25-10-22-9-23-25/h3-10H,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280373
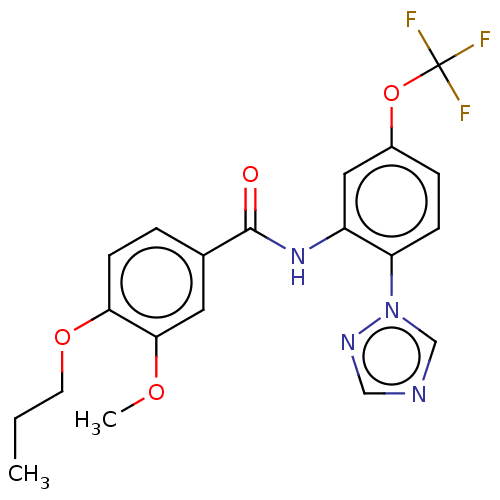
(CHEMBL4161847)Show SMILES CCCOc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C20H19F3N4O4/c1-3-8-30-17-7-4-13(9-18(17)29-2)19(28)26-15-10-14(31-20(21,22)23)5-6-16(15)27-12-24-11-25-27/h4-7,9-12H,3,8H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 776 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280372
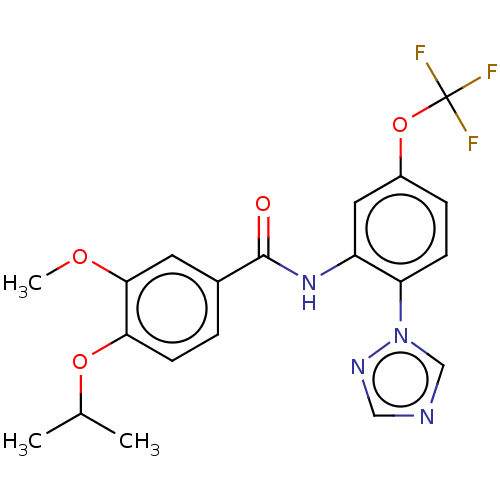
(CHEMBL4165849)Show SMILES COc1cc(ccc1OC(C)C)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C20H19F3N4O4/c1-12(2)30-17-7-4-13(8-18(17)29-3)19(28)26-15-9-14(31-20(21,22)23)5-6-16(15)27-11-24-10-25-27/h4-12H,1-3H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 776 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280372

(CHEMBL4165849)Show SMILES COc1cc(ccc1OC(C)C)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C20H19F3N4O4/c1-12(2)30-17-7-4-13(8-18(17)29-3)19(28)26-15-9-14(31-20(21,22)23)5-6-16(15)27-11-24-10-25-27/h4-12H,1-3H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280373

(CHEMBL4161847)Show SMILES CCCOc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C20H19F3N4O4/c1-3-8-30-17-7-4-13(9-18(17)29-2)19(28)26-15-10-14(31-20(21,22)23)5-6-16(15)27-12-24-11-25-27/h4-7,9-12H,3,8H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50249405

(CHEMBL4102040)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccc(F)nc3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-9-13-3-5-14(6-4-13)15-7-8-16(22)24-10-15/h3-8,10H,9,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50249435

(CHEMBL4070692)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccncc3)c(N)c2c1C Show InChI InChI=1S/C21H19N5OS/c1-12-13(2)25-26-21-17(12)18(22)19(28-21)20(27)24-11-14-3-5-15(6-4-14)16-7-9-23-10-8-16/h3-10H,11,22H2,1-2H3,(H,24,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280367
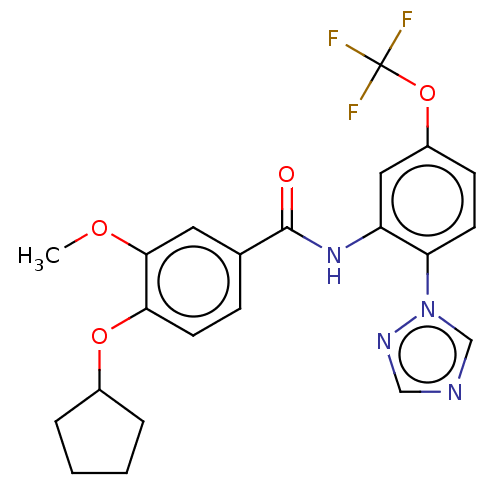
(CHEMBL4170997)Show SMILES COc1cc(ccc1OC1CCCC1)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C22H21F3N4O4/c1-31-20-10-14(6-9-19(20)32-15-4-2-3-5-15)21(30)28-17-11-16(33-22(23,24)25)7-8-18(17)29-13-26-12-27-29/h6-13,15H,2-5H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280367

(CHEMBL4170997)Show SMILES COc1cc(ccc1OC1CCCC1)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C22H21F3N4O4/c1-31-20-10-14(6-9-19(20)32-15-4-2-3-5-15)21(30)28-17-11-16(33-22(23,24)25)7-8-18(17)29-13-26-12-27-29/h6-13,15H,2-5H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of rat mGlu7 expressed in HEK cells co-expressing Galpha15 assessed as reduction in L-AP4-induced intracellular calciu... |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50249435

(CHEMBL4070692)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccncc3)c(N)c2c1C Show InChI InChI=1S/C21H19N5OS/c1-12-13(2)25-26-21-17(12)18(22)19(28-21)20(27)24-11-14-3-5-15(6-4-14)16-7-9-23-10-8-16/h3-10H,11,22H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50249434
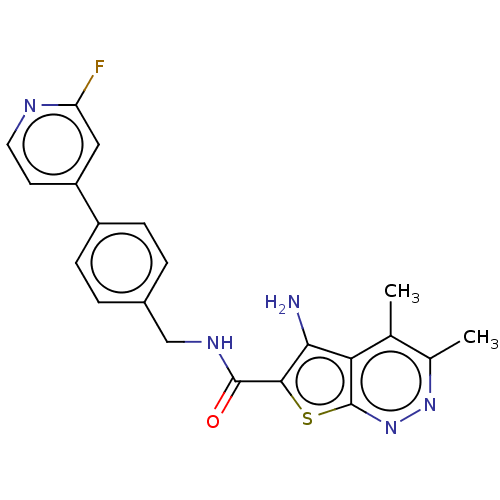
(CHEMBL4094439)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccnc(F)c3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-10-13-3-5-14(6-4-13)15-7-8-24-16(22)9-15/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50283490

(CHEMBL4172016)Show SMILES COc1cc(F)c(cc1F)-c1c(C)nn2c(cc(C)nc12)C(F)(F)F |(24.3,-44,;25.32,-42.84,;24.84,-41.38,;25.87,-40.23,;25.37,-38.77,;26.4,-37.61,;23.87,-38.46,;22.85,-39.61,;23.33,-41.07,;22.3,-42.22,;23.39,-36.99,;24.29,-35.75,;25.83,-35.74,;23.38,-34.52,;21.92,-35,;20.59,-34.22,;19.26,-35,;19.26,-36.54,;17.92,-37.31,;20.59,-37.3,;21.93,-36.53,;20.59,-32.68,;21.92,-31.91,;19.25,-31.91,;20.57,-31.14,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-4-13(16(19,20)21)24-15(22-7)14(8(2)23-24)9-5-11(18)12(25-3)6-10(9)17/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50283472

(CHEMBL4160748)Show SMILES COc1ccc(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1F |(9.45,-43.77,;10.47,-42.62,;9.99,-41.15,;8.48,-40.84,;7.99,-39.38,;9.02,-38.23,;8.54,-36.77,;9.43,-35.53,;10.97,-35.52,;8.53,-34.29,;7.07,-34.77,;5.74,-34,;4.41,-34.77,;4.41,-36.31,;3.08,-37.09,;5.74,-37.08,;7.08,-36.3,;5.74,-32.46,;7.08,-31.68,;4.41,-31.68,;5.73,-30.92,;10.52,-38.54,;11.55,-37.39,;11.01,-40,;12.52,-40.31,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-6-11(16(19,20)21)24-15(22-7)12(8(2)23-24)9-4-5-10(25-3)14(18)13(9)17/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50283472

(CHEMBL4160748)Show SMILES COc1ccc(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1F |(9.45,-43.77,;10.47,-42.62,;9.99,-41.15,;8.48,-40.84,;7.99,-39.38,;9.02,-38.23,;8.54,-36.77,;9.43,-35.53,;10.97,-35.52,;8.53,-34.29,;7.07,-34.77,;5.74,-34,;4.41,-34.77,;4.41,-36.31,;3.08,-37.09,;5.74,-37.08,;7.08,-36.3,;5.74,-32.46,;7.08,-31.68,;4.41,-31.68,;5.73,-30.92,;10.52,-38.54,;11.55,-37.39,;11.01,-40,;12.52,-40.31,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-6-11(16(19,20)21)24-15(22-7)12(8(2)23-24)9-4-5-10(25-3)14(18)13(9)17/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Displacement of [3H] substance P from recombinant human NK1 receptor expressed in CHO cells after 90 mins by scintillation counting method |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280371
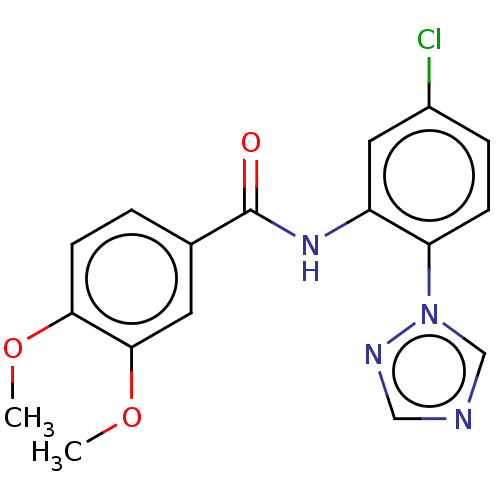
(CHEMBL4160779)Show InChI InChI=1S/C17H15ClN4O3/c1-24-15-6-3-11(7-16(15)25-2)17(23)21-13-8-12(18)4-5-14(13)22-10-19-9-20-22/h3-10H,1-2H3,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 7
(Rattus norvegicus (Rat)) | BDBM50280371

(CHEMBL4160779)Show InChI InChI=1S/C17H15ClN4O3/c1-24-15-6-3-11(7-16(15)25-2)17(23)21-13-8-12(18)4-5-14(13)22-10-19-9-20-22/h3-10H,1-2H3,(H,21,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of rat mGlu7 expressed in HEK cells co-expressing Galpha15 assessed as reduction in L-AP4-induced intracellular calciu... |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50283489

(CHEMBL4168668)Show SMILES COc1cc(F)c(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1 |(75.94,-28.33,;76.96,-27.18,;76.48,-25.72,;74.97,-25.41,;74.49,-23.95,;72.98,-23.63,;75.51,-22.79,;75.03,-21.33,;75.93,-20.09,;77.47,-20.08,;75.01,-18.86,;73.56,-19.33,;72.23,-18.56,;70.9,-19.33,;70.9,-20.87,;69.56,-21.65,;72.23,-21.64,;73.57,-20.87,;72.23,-17.02,;73.56,-16.25,;70.89,-16.25,;72.21,-15.48,;77.01,-23.11,;78.04,-21.95,;77.51,-24.56,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-4-12(16(19,20)21)24-15(22-7)13(8(2)23-24)14-10(17)5-9(25-3)6-11(14)18/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50249435

(CHEMBL4070692)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccncc3)c(N)c2c1C Show InChI InChI=1S/C21H19N5OS/c1-12-13(2)25-26-21-17(12)18(22)19(28-21)20(27)24-11-14-3-5-15(6-4-14)16-7-9-23-10-8-16/h3-10H,11,22H2,1-2H3,(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50283472

(CHEMBL4160748)Show SMILES COc1ccc(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1F |(9.45,-43.77,;10.47,-42.62,;9.99,-41.15,;8.48,-40.84,;7.99,-39.38,;9.02,-38.23,;8.54,-36.77,;9.43,-35.53,;10.97,-35.52,;8.53,-34.29,;7.07,-34.77,;5.74,-34,;4.41,-34.77,;4.41,-36.31,;3.08,-37.09,;5.74,-37.08,;7.08,-36.3,;5.74,-32.46,;7.08,-31.68,;4.41,-31.68,;5.73,-30.92,;10.52,-38.54,;11.55,-37.39,;11.01,-40,;12.52,-40.31,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-6-11(16(19,20)21)24-15(22-7)12(8(2)23-24)9-4-5-10(25-3)14(18)13(9)17/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of rat mGlu5 receptor expressed in HEK cells assessed as decrease in glutamate-induced thallium flux incubated for 142... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 4
(Homo sapiens (Human)) | BDBM50280374

(CHEMBL4174742)Show InChI InChI=1S/C17H19NO2/c1-4-16-18-17-14(19)8-12(9-15(17)20-16)13-6-5-10(2)7-11(13)3/h5-7,12H,4,8-9H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of human mGlu4 receptor expressed in CHO cells co-expressing Gqi5 harboring GIRK channel assessed as decrease in gluta... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 6
(Homo sapiens (Human)) | BDBM50280368

(CHEMBL4176971)Show SMILES COc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C18H15F3N4O4/c1-27-15-6-3-11(7-16(15)28-2)17(26)24-13-8-12(29-18(19,20)21)4-5-14(13)25-10-22-9-23-25/h3-10H,1-2H3,(H,24,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50280368

(CHEMBL4176971)Show SMILES COc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C18H15F3N4O4/c1-27-15-6-3-11(7-16(15)28-2)17(26)24-13-8-12(29-18(19,20)21)4-5-14(13)25-10-22-9-23-25/h3-10H,1-2H3,(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50280368

(CHEMBL4176971)Show SMILES COc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C18H15F3N4O4/c1-27-15-6-3-11(7-16(15)28-2)17(26)24-13-8-12(29-18(19,20)21)4-5-14(13)25-10-22-9-23-25/h3-10H,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 4
(Homo sapiens (Human)) | BDBM50280368

(CHEMBL4176971)Show SMILES COc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C18H15F3N4O4/c1-27-15-6-3-11(7-16(15)28-2)17(26)24-13-8-12(29-18(19,20)21)4-5-14(13)25-10-22-9-23-25/h3-10H,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50280368

(CHEMBL4176971)Show SMILES COc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C18H15F3N4O4/c1-27-15-6-3-11(7-16(15)28-2)17(26)24-13-8-12(29-18(19,20)21)4-5-14(13)25-10-22-9-23-25/h3-10H,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin I converting enzyme of rats. |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 8
(Rattus norvegicus (Rat)) | BDBM50280368

(CHEMBL4176971)Show SMILES COc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C18H15F3N4O4/c1-27-15-6-3-11(7-16(15)28-2)17(26)24-13-8-12(29-18(19,20)21)4-5-14(13)25-10-22-9-23-25/h3-10H,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGlu8 expressed in HEK cells co-expressing Galpha15 assessed as reduction in L-AP4-induced intracellular calcium flux pret... |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50280368

(CHEMBL4176971)Show SMILES COc1ccc(cc1OC)C(=O)Nc1cc(OC(F)(F)F)ccc1-n1cncn1 Show InChI InChI=1S/C18H15F3N4O4/c1-27-15-6-3-11(7-16(15)28-2)17(26)24-13-8-12(29-18(19,20)21)4-5-14(13)25-10-22-9-23-25/h3-10H,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGlu2 receptor (unknown origin) |
ACS Med Chem Lett 8: 1326-1330 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00429
BindingDB Entry DOI: 10.7270/Q25X2CG2 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Rattus norvegicus (Rat)) | BDBM50283472

(CHEMBL4160748)Show SMILES COc1ccc(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1F |(9.45,-43.77,;10.47,-42.62,;9.99,-41.15,;8.48,-40.84,;7.99,-39.38,;9.02,-38.23,;8.54,-36.77,;9.43,-35.53,;10.97,-35.52,;8.53,-34.29,;7.07,-34.77,;5.74,-34,;4.41,-34.77,;4.41,-36.31,;3.08,-37.09,;5.74,-37.08,;7.08,-36.3,;5.74,-32.46,;7.08,-31.68,;4.41,-31.68,;5.73,-30.92,;10.52,-38.54,;11.55,-37.39,;11.01,-40,;12.52,-40.31,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-6-11(16(19,20)21)24-15(22-7)12(8(2)23-24)9-4-5-10(25-3)14(18)13(9)17/h4-6H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of rat mGlu3 receptor expressed in HEK cells harboring GIRK channel assessed as decrease in glutamate-induced thallium... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Rattus norvegicus) | BDBM50283472

(CHEMBL4160748)Show SMILES COc1ccc(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1F |(9.45,-43.77,;10.47,-42.62,;9.99,-41.15,;8.48,-40.84,;7.99,-39.38,;9.02,-38.23,;8.54,-36.77,;9.43,-35.53,;10.97,-35.52,;8.53,-34.29,;7.07,-34.77,;5.74,-34,;4.41,-34.77,;4.41,-36.31,;3.08,-37.09,;5.74,-37.08,;7.08,-36.3,;5.74,-32.46,;7.08,-31.68,;4.41,-31.68,;5.73,-30.92,;10.52,-38.54,;11.55,-37.39,;11.01,-40,;12.52,-40.31,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-6-11(16(19,20)21)24-15(22-7)12(8(2)23-24)9-4-5-10(25-3)14(18)13(9)17/h4-6H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of rat mGlu2 receptor expressed in HEK cells assessed as decrease in glutamate-induced thallium flux incubated for 142... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 8
(Rattus norvegicus (Rat)) | BDBM50280374

(CHEMBL4174742)Show InChI InChI=1S/C17H19NO2/c1-4-16-18-17-14(19)8-12(9-15(17)20-16)13-6-5-10(2)7-11(13)3/h5-7,12H,4,8-9H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of rat mGlu8 receptor expressed in HEK cells co-expressing Galphai5 assessed as decrease in glutamate-induced thallium... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 6
(Homo sapiens (Human)) | BDBM50283472

(CHEMBL4160748)Show SMILES COc1ccc(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1F |(9.45,-43.77,;10.47,-42.62,;9.99,-41.15,;8.48,-40.84,;7.99,-39.38,;9.02,-38.23,;8.54,-36.77,;9.43,-35.53,;10.97,-35.52,;8.53,-34.29,;7.07,-34.77,;5.74,-34,;4.41,-34.77,;4.41,-36.31,;3.08,-37.09,;5.74,-37.08,;7.08,-36.3,;5.74,-32.46,;7.08,-31.68,;4.41,-31.68,;5.73,-30.92,;10.52,-38.54,;11.55,-37.39,;11.01,-40,;12.52,-40.31,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-6-11(16(19,20)21)24-15(22-7)12(8(2)23-24)9-4-5-10(25-3)14(18)13(9)17/h4-6H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of human mGlu6 receptor expressed in HEK cells harboring GIRK channel assessed as decrease in glutamate-induced thalli... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50283472

(CHEMBL4160748)Show SMILES COc1ccc(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1F |(9.45,-43.77,;10.47,-42.62,;9.99,-41.15,;8.48,-40.84,;7.99,-39.38,;9.02,-38.23,;8.54,-36.77,;9.43,-35.53,;10.97,-35.52,;8.53,-34.29,;7.07,-34.77,;5.74,-34,;4.41,-34.77,;4.41,-36.31,;3.08,-37.09,;5.74,-37.08,;7.08,-36.3,;5.74,-32.46,;7.08,-31.68,;4.41,-31.68,;5.73,-30.92,;10.52,-38.54,;11.55,-37.39,;11.01,-40,;12.52,-40.31,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-6-11(16(19,20)21)24-15(22-7)12(8(2)23-24)9-4-5-10(25-3)14(18)13(9)17/h4-6H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of rat mGlu1 receptor expressed in HEK cells assessed as decrease in glutamate-induced thallium flux incubated for 142... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50249403
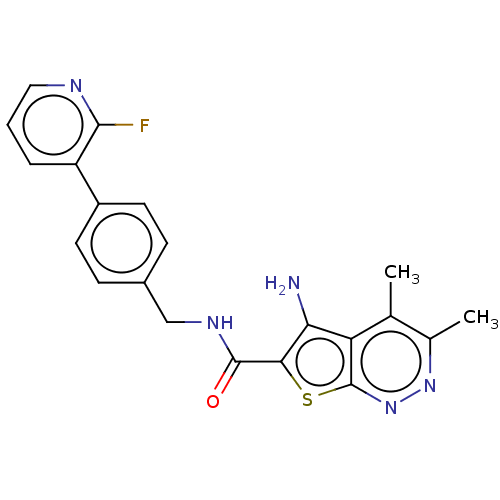
(CHEMBL4091821)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3cccnc3F)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-16(11)17(23)18(29-21)20(28)25-10-13-5-7-14(8-6-13)15-4-3-9-24-19(15)22/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50249403

(CHEMBL4091821)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3cccnc3F)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-16(11)17(23)18(29-21)20(28)25-10-13-5-7-14(8-6-13)15-4-3-9-24-19(15)22/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50249434

(CHEMBL4094439)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccnc(F)c3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-10-13-3-5-14(6-4-13)15-7-8-24-16(22)9-15/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Induction of CYP3A4 in cryopreserved human hepatocytes measured after 48 hrs |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50283472

(CHEMBL4160748)Show SMILES COc1ccc(-c2c(C)nn3c(cc(C)nc23)C(F)(F)F)c(F)c1F |(9.45,-43.77,;10.47,-42.62,;9.99,-41.15,;8.48,-40.84,;7.99,-39.38,;9.02,-38.23,;8.54,-36.77,;9.43,-35.53,;10.97,-35.52,;8.53,-34.29,;7.07,-34.77,;5.74,-34,;4.41,-34.77,;4.41,-36.31,;3.08,-37.09,;5.74,-37.08,;7.08,-36.3,;5.74,-32.46,;7.08,-31.68,;4.41,-31.68,;5.73,-30.92,;10.52,-38.54,;11.55,-37.39,;11.01,-40,;12.52,-40.31,)| Show InChI InChI=1S/C16H12F5N3O/c1-7-6-11(16(19,20)21)24-15(22-7)12(8(2)23-24)9-4-5-10(25-3)14(18)13(9)17/h4-6H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... |
ACS Med Chem Lett 8: 1110-1115 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00317
BindingDB Entry DOI: 10.7270/Q2B85BN1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50249434

(CHEMBL4094439)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccnc(F)c3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-10-13-3-5-14(6-4-13)15-7-8-24-16(22)9-15/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50249405

(CHEMBL4102040)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3ccc(F)nc3)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-17(11)18(23)19(29-21)20(28)25-9-13-3-5-14(6-4-13)15-7-8-16(22)24-10-15/h3-8,10H,9,23H2,1-2H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data