

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50248476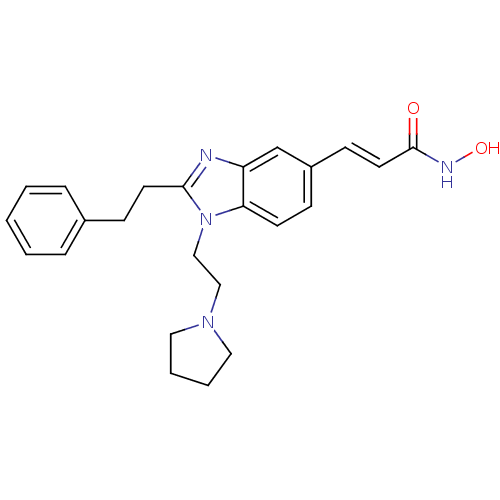 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC4 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50248476 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC3 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50248476 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC11 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50248476 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC5 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyamine deacetylase HDAC10 (Homo sapiens (Human)) | BDBM50248476 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC10 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50248476 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC9 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50248476 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC6 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50248476 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC2 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50248476 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC8 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50173468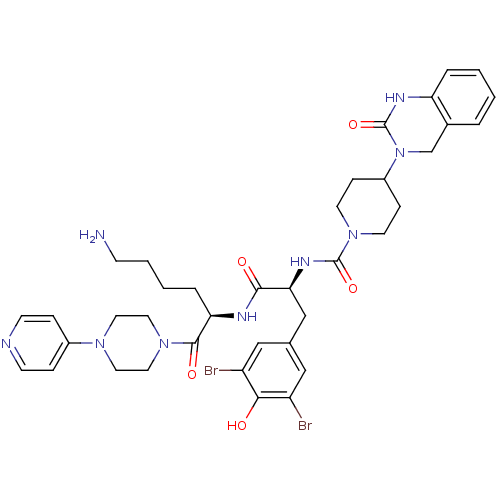 (4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Affinity against human calcitonin gene related peptide receptor (1 uM) expressed in SK-N-MC cells using [125I]-CGRP as radioligand after 180 minutes ... | J Med Chem 48: 5921-31 (2005) Article DOI: 10.1021/jm0490641 BindingDB Entry DOI: 10.7270/Q2KS6R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50173482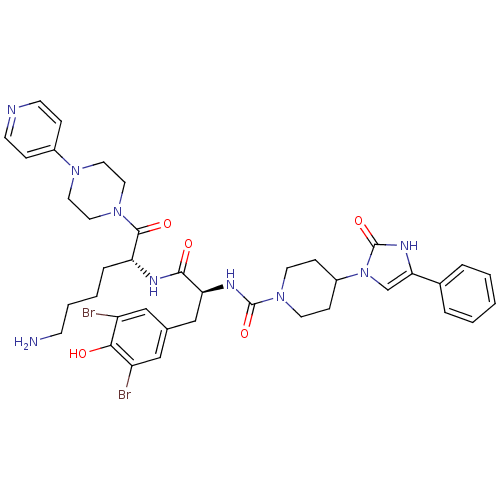 (4-(2-Oxo-4-phenyl-2,3-dihydro-imidazol-1-yl)-piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Affinity against human calcitonin gene related peptide receptor (1 uM) expressed in SK-N-MC cells using [125I]-CGRP as radioligand after 180 minutes ... | J Med Chem 48: 5921-31 (2005) Article DOI: 10.1021/jm0490641 BindingDB Entry DOI: 10.7270/Q2KS6R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50173468 (4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Affinity against calcitonin gene related peptide receptor (1 uM) in marmoset tissue homogenates using [125I]-CGRP as radioligand after 180 minutes of... | J Med Chem 48: 5921-31 (2005) Article DOI: 10.1021/jm0490641 BindingDB Entry DOI: 10.7270/Q2KS6R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50173475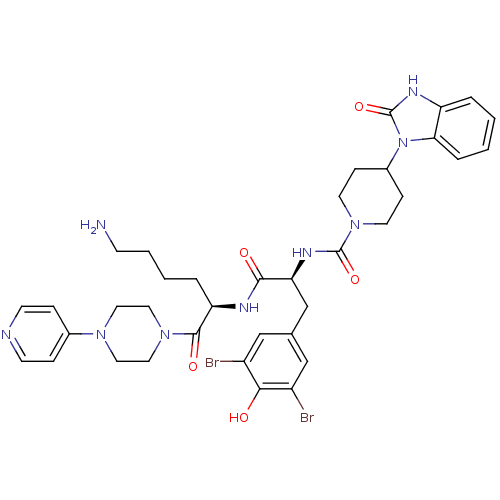 (4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Affinity against human calcitonin gene related peptide receptor (1 uM) expressed in SK-N-MC cells using [125I]-CGRP as radioligand after 180 minutes ... | J Med Chem 48: 5921-31 (2005) Article DOI: 10.1021/jm0490641 BindingDB Entry DOI: 10.7270/Q2KS6R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50173475 (4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Affinity against calcitonin gene related peptide receptor (1 uM) in marmoset tissue homogenates using [125I]-CGRP as radioligand after 180 minutes of... | J Med Chem 48: 5921-31 (2005) Article DOI: 10.1021/jm0490641 BindingDB Entry DOI: 10.7270/Q2KS6R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043257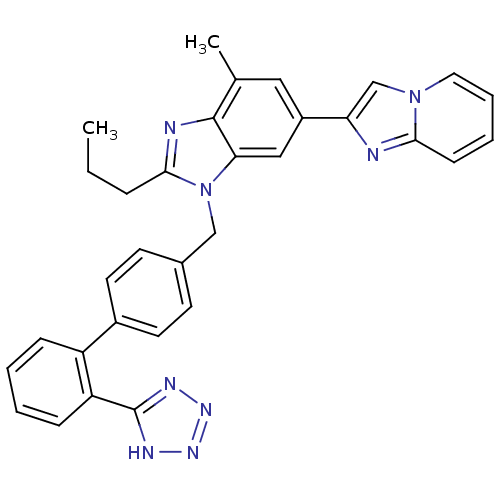 (6-Imidazo[1,2-a]pyridin-2-yl-4-methyl-2-propyl-1-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043260 (2-Butyl-6-(1,1-dioxo-1lambda*6*-[1,2]thiazinan-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043280 (4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043244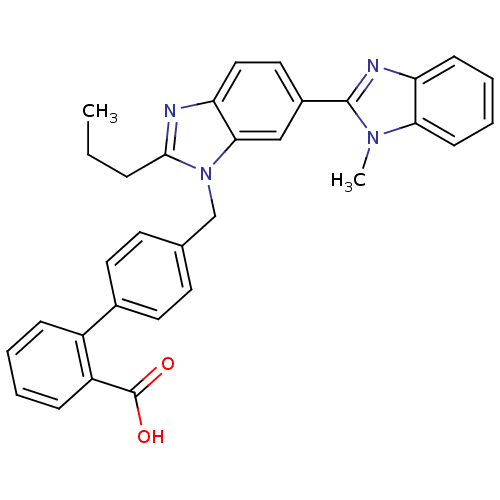 (4'-(1-Methyl-2'-propyl-1H-[2,5']bibenzoimidazolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043277 (1-{2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043246 (4'-(6-Imidazo[1,2-a]pyridin-2-yl-4-methyl-2-propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50173472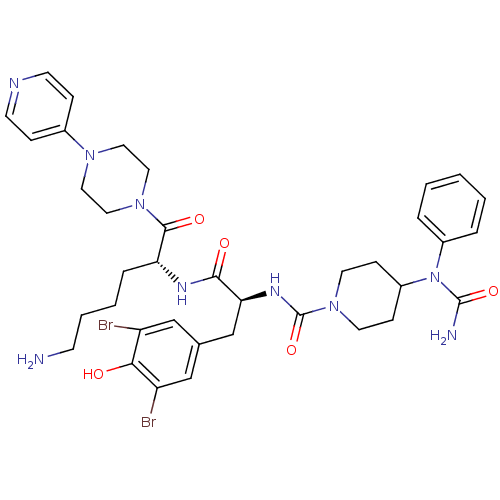 (4-(1-Phenyl-ureido)-piperidine-1-carboxylic acid [...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Affinity against human calcitonin gene related peptide receptor (1 uM) expressed in SK-N-MC cells using [125I]-CGRP as radioligand after 180 minutes ... | J Med Chem 48: 5921-31 (2005) Article DOI: 10.1021/jm0490641 BindingDB Entry DOI: 10.7270/Q2KS6R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043247 (4-Methyl-2-propyl-6-pyridin-2-yl-1-[2'-(1H-tetrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043279 (1-Methyl-2'-propyl-3'-[2'-(1H-tetrazol-5-yl)-biphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Rattus norvegicus) | BDBM50173468 (4-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)-piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Affinity against calcitonin gene related peptide receptor (1 uM) in rat spleen using [125I]-CGRP as radioligand after 180 minutes of incubation at pH... | J Med Chem 48: 5921-31 (2005) Article DOI: 10.1021/jm0490641 BindingDB Entry DOI: 10.7270/Q2KS6R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043248 (3-{2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043263 (1-{2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043255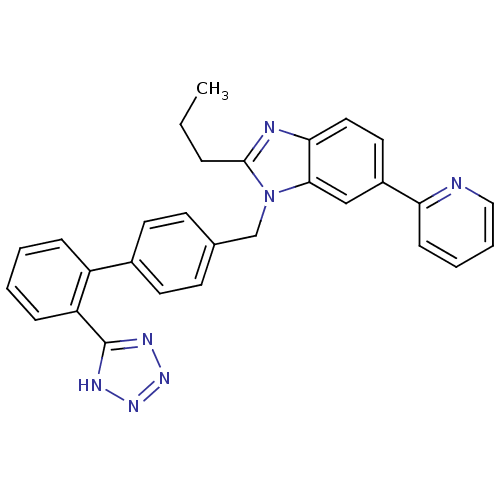 (2-Propyl-6-pyridin-2-yl-1-[2'-(1H-tetrazol-5-yl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043271 (1,7'-Dimethyl-2'-propyl-3'-[2'-(1H-tetrazol-5-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043252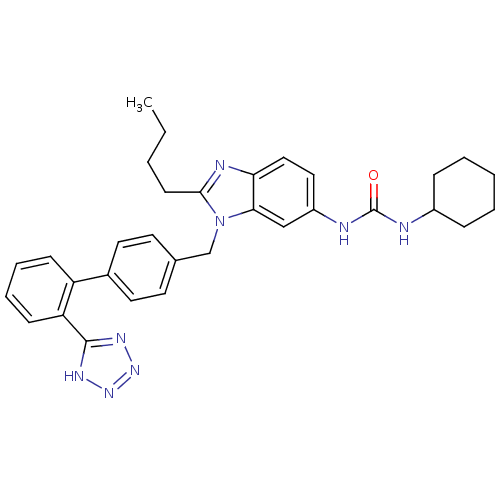 (1-{2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043269 (4'-[2-Butyl-6-(3,3-dimethyl-ureido)-benzoimidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043268 (4'-[2-Butyl-6-(3-cyclohexyl-1-methyl-ureido)-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50248522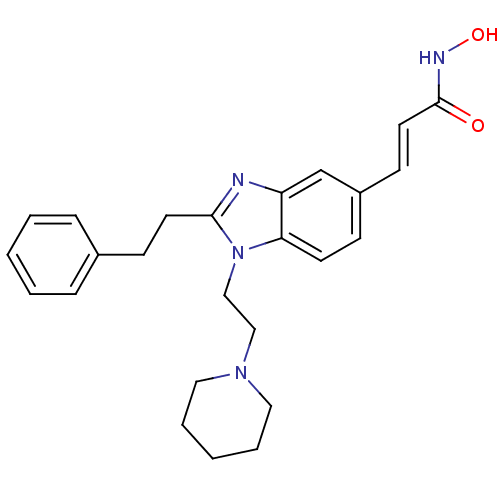 (CHEMBL489332 | N-hydroxy-3-(2-phenethyl-1-(2-(pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50035429 (4'-[2-Butyl-6-(3-cyclohexyl-ureido)-benzoimidazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50062162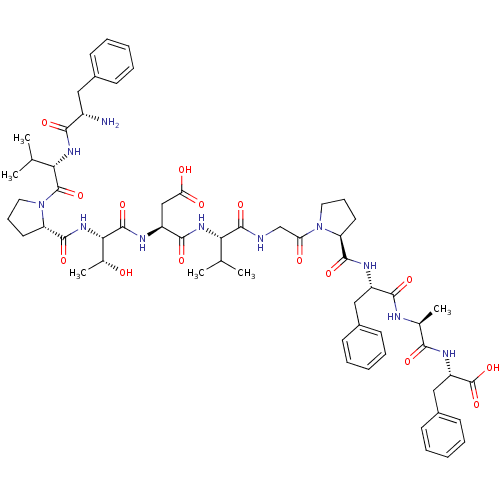 (CHEMBL264010 | FVPTDVGPFAF) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zürich Curated by ChEMBL | Assay Description CGRP1 receptor affinity on human neuroblastoma cells SK-N-MC, which selectively express the human CGRP1 receptor. | J Med Chem 41: 117-23 (1998) Article DOI: 10.1021/jm970533r BindingDB Entry DOI: 10.7270/Q2736Q0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043281 (4'-{2-Butyl-6-[methyl-(propane-1-sulfonyl)-amino]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043249 (4'-[2-Butyl-6-(1,1-dioxo-1lambda*6*-[1,2]thiazinan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50248476 (CHEMBL491316 | N-hydroxy-3-(2-phenethyl-1-(2-(pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50009714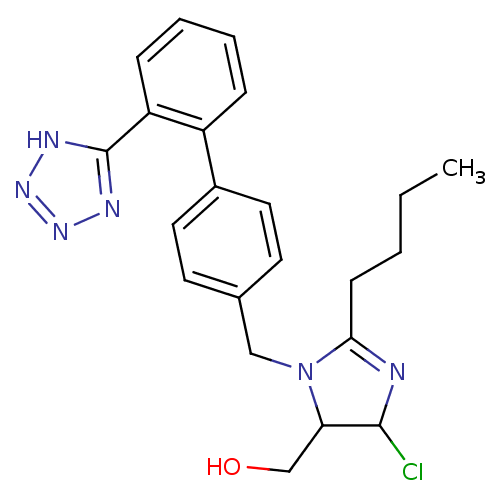 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50248570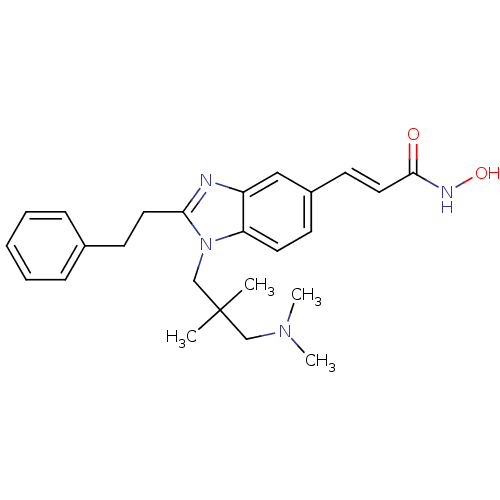 (3-(1-(3-(dimethylamino)-2,2-dimethylpropyl)-2-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50173486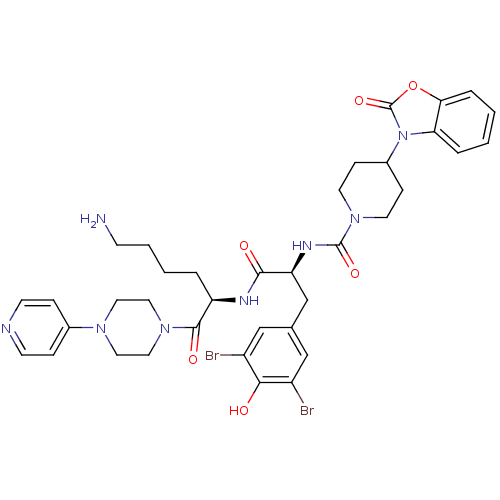 (4-(2-Oxo-benzooxazol-3-yl)-piperidine-1-carboxylic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Affinity against human calcitonin gene related peptide receptor (1 uM) expressed in SK-N-MC cells using [125I]-CGRP as radioligand after 180 minutes ... | J Med Chem 48: 5921-31 (2005) Article DOI: 10.1021/jm0490641 BindingDB Entry DOI: 10.7270/Q2KS6R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50248375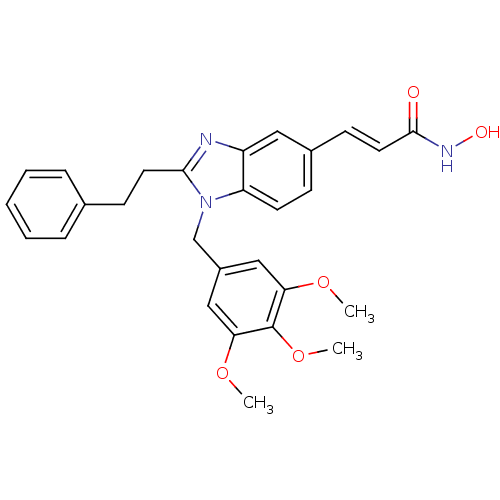 (CHEMBL491688 | N-hydroxy-3-(2-phenethyl-1-(3,4,5-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50248475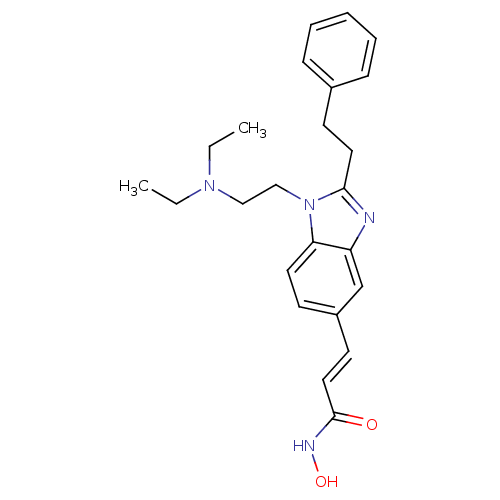 (3-(1-(2-(diethylamino)ethyl)-2-phenethyl-1H-benzo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Rattus norvegicus) | BDBM50173475 (4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56.7 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Affinity against calcitonin gene related peptide receptor (1 uM) in rat spleen using [125I]-CGRP as radioligand after 180 minutes of incubation at pH... | J Med Chem 48: 5921-31 (2005) Article DOI: 10.1021/jm0490641 BindingDB Entry DOI: 10.7270/Q2KS6R3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50248314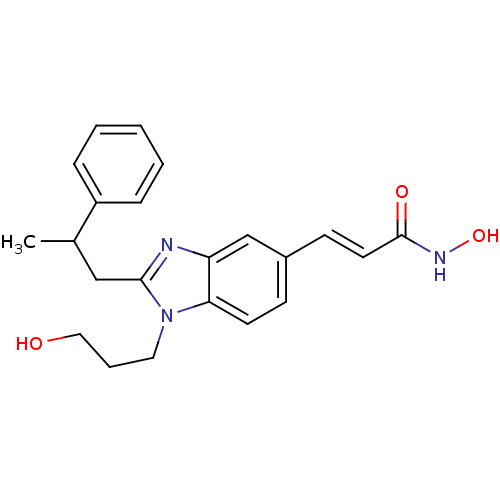 (CHEMBL474746 | N-hydroxy-3-(1-(3-hydroxypropyl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043270 (4'-[2-Butyl-6-(2-oxo-piperidin-1-yl)-benzoimidazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043265 (4'-(2-Butyl-6-pentanoylamino-benzoimidazol-1-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50248261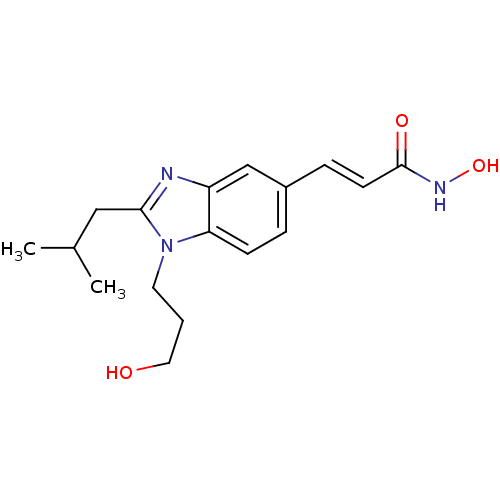 (CHEMBL475145 | N-hydroxy-3-(1-(3-hydroxypropyl)-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
S *BIO Pte Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant HDAC1 by fluorimetric assay | Bioorg Med Chem Lett 19: 1403-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.041 BindingDB Entry DOI: 10.7270/Q2FT8KX3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50030559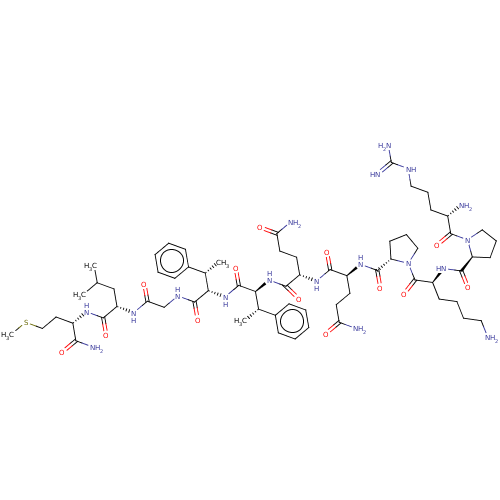 (7S,8S-(betaMeF)2SP | CHEMBL2371143 | FVPTDVG-Tic-F...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zürich Curated by ChEMBL | Assay Description CGRP1 receptor affinity on human neuroblastoma cells SK-N-MC, which selectively express the human CGRP1 receptor. | J Med Chem 41: 117-23 (1998) Article DOI: 10.1021/jm970533r BindingDB Entry DOI: 10.7270/Q2736Q0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50043275 (4'-(6-Dimethylaminomethyl-4-methyl-2-propyl-benzoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Dr. Karl Thomae GmbH Curated by ChEMBL | Assay Description Inhibition of specific binding of [125I]-angiotensin-II to angiotensin 1 receptor in rat lung membrane preparation | J Med Chem 36: 4040-51 (1994) BindingDB Entry DOI: 10.7270/Q2V98746 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 176 total ) | Next | Last >> |