 Found 871 hits with Last Name = 'fang' and Initial = 'q'
Found 871 hits with Last Name = 'fang' and Initial = 'q' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor
(Homo sapiens (Human)) | BDBM50462974
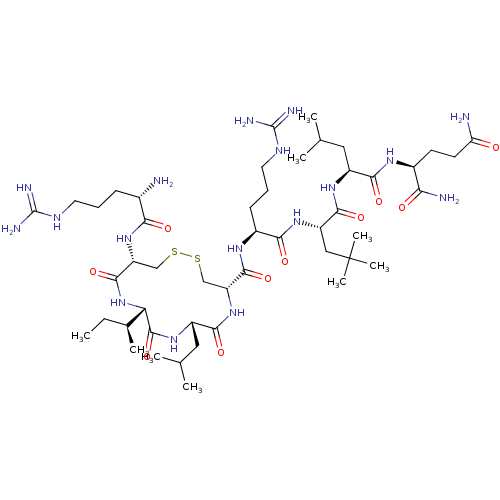
(CHEMBL4240100)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N |r| Show InChI InChI=1S/C48H89N17O10S2/c1-10-26(6)36-45(75)61-31(20-25(4)5)41(71)64-33(22-76-77-23-34(44(74)65-36)63-38(68)27(49)13-11-17-56-46(52)53)43(73)59-29(14-12-18-57-47(54)55)39(69)62-32(21-48(7,8)9)42(72)60-30(19-24(2)3)40(70)58-28(37(51)67)15-16-35(50)66/h24-34,36H,10-23,49H2,1-9H3,(H2,50,66)(H2,51,67)(H,58,70)(H,59,73)(H,60,72)(H,61,75)(H,62,69)(H,63,68)(H,64,71)(H,65,74)(H4,52,53,56)(H4,54,55,57)/t26-,27-,28-,29-,30-,31-,32-,33+,34+,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Antagonist activity at full length recombinant human ERalpha expressed in baculovirus expression system assessed as inhibition of estradiol-induced E... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50131114
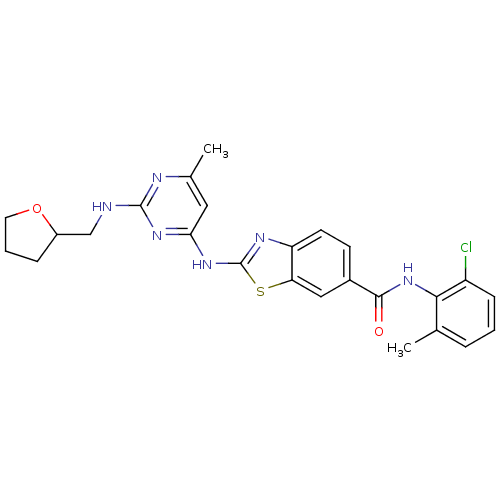
(2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...)Show SMILES Cc1cc(Nc2nc3ccc(cc3s2)C(=O)Nc2c(C)cccc2Cl)nc(NCC2CCCO2)n1 Show InChI InChI=1S/C25H25ClN6O2S/c1-14-5-3-7-18(26)22(14)32-23(33)16-8-9-19-20(12-16)35-25(29-19)31-21-11-15(2)28-24(30-21)27-13-17-6-4-10-34-17/h3,5,7-9,11-12,17H,4,6,10,13H2,1-2H3,(H,32,33)(H2,27,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM13357
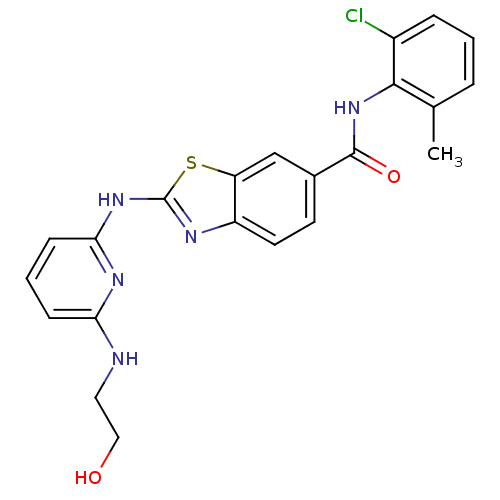
(CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...)Show SMILES Cc1cccc(Cl)c1NC(=O)c1ccc2nc(Nc3cccc(NCCO)n3)sc2c1 Show InChI InChI=1S/C22H20ClN5O2S/c1-13-4-2-5-15(23)20(13)28-21(30)14-8-9-16-17(12-14)31-22(25-16)27-19-7-3-6-18(26-19)24-10-11-29/h2-9,12,29H,10-11H2,1H3,(H,28,30)(H2,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50131114

(2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...)Show SMILES Cc1cc(Nc2nc3ccc(cc3s2)C(=O)Nc2c(C)cccc2Cl)nc(NCC2CCCO2)n1 Show InChI InChI=1S/C25H25ClN6O2S/c1-14-5-3-7-18(26)22(14)32-23(33)16-8-9-19-20(12-16)35-25(29-19)31-21-11-15(2)28-24(30-21)27-13-17-6-4-10-34-17/h3,5,7-9,11-12,17H,4,6,10,13H2,1-2H3,(H,32,33)(H2,27,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Fyn protein kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50462974

(CHEMBL4240100)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N |r| Show InChI InChI=1S/C48H89N17O10S2/c1-10-26(6)36-45(75)61-31(20-25(4)5)41(71)64-33(22-76-77-23-34(44(74)65-36)63-38(68)27(49)13-11-17-56-46(52)53)43(73)59-29(14-12-18-57-47(54)55)39(69)62-32(21-48(7,8)9)42(72)60-30(19-24(2)3)40(70)58-28(37(51)67)15-16-35(50)66/h24-34,36H,10-23,49H2,1-9H3,(H2,50,66)(H2,51,67)(H,58,70)(H,59,73)(H,60,72)(H,61,75)(H,62,69)(H,63,68)(H,64,71)(H,65,74)(H4,52,53,56)(H4,54,55,57)/t26-,27-,28-,29-,30-,31-,32-,33+,34+,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Antagonist activity at full length recombinant human ERbeta expressed in baculovirus expression system assessed as inhibition of estradiol-induced Eu... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433372
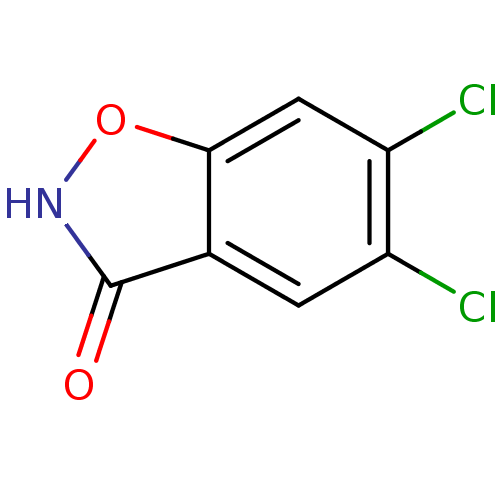
(CHEMBL2375520)Show InChI InChI=1S/C7H3Cl2NO2/c8-4-1-3-6(2-5(4)9)12-10-7(3)11/h1-2H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM13357

(CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...)Show SMILES Cc1cccc(Cl)c1NC(=O)c1ccc2nc(Nc3cccc(NCCO)n3)sc2c1 Show InChI InChI=1S/C22H20ClN5O2S/c1-13-4-2-5-15(23)20(13)28-21(30)14-8-9-16-17(12-14)31-22(25-16)27-19-7-3-6-18(26-19)24-10-11-29/h2-9,12,29H,10-11H2,1H3,(H,28,30)(H2,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Fyn protein kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50131114

(2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...)Show SMILES Cc1cc(Nc2nc3ccc(cc3s2)C(=O)Nc2c(C)cccc2Cl)nc(NCC2CCCO2)n1 Show InChI InChI=1S/C25H25ClN6O2S/c1-14-5-3-7-18(26)22(14)32-23(33)16-8-9-19-20(12-16)35-25(29-19)31-21-11-15(2)28-24(30-21)27-13-17-6-4-10-34-17/h3,5,7-9,11-12,17H,4,6,10,13H2,1-2H3,(H,32,33)(H2,27,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725
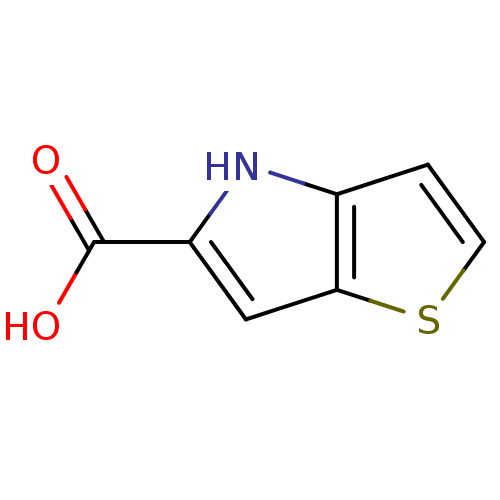
(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600600
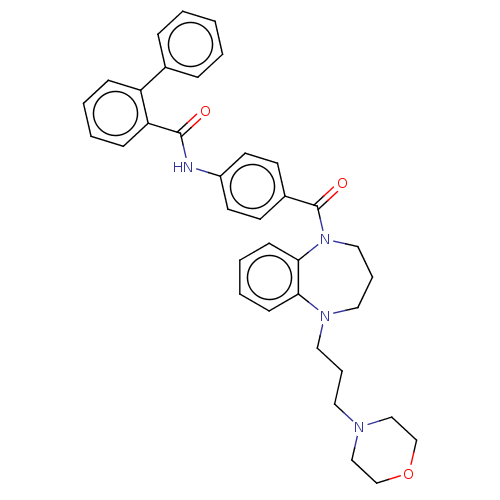
(CHEMBL5207885)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50129307
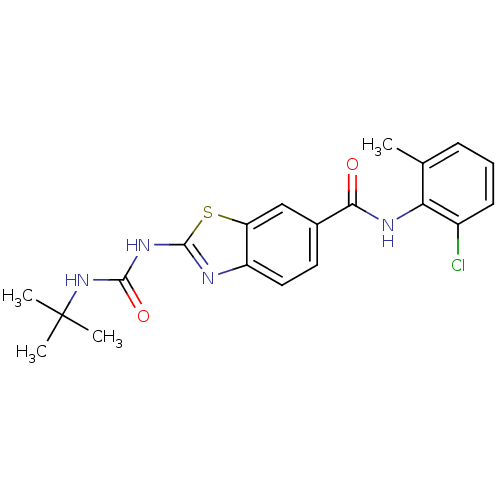
(2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...)Show SMILES Cc1cccc(Cl)c1NC(=O)c1ccc2nc(NC(=O)NC(C)(C)C)sc2c1 Show InChI InChI=1S/C20H21ClN4O2S/c1-11-6-5-7-13(21)16(11)23-17(26)12-8-9-14-15(10-12)28-19(22-14)24-18(27)25-20(2,3)4/h5-10H,1-4H3,(H,23,26)(H2,22,24,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50131131

(2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...)Show SMILES CNc1cc(Nc2nc3ccc(cc3s2)C(=O)Nc2c(C)cccc2Cl)ncn1 Show InChI InChI=1S/C20H17ClN6OS/c1-11-4-3-5-13(21)18(11)27-19(28)12-6-7-14-15(8-12)29-20(25-14)26-17-9-16(22-2)23-10-24-17/h3-10H,1-2H3,(H,27,28)(H2,22,23,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50462976
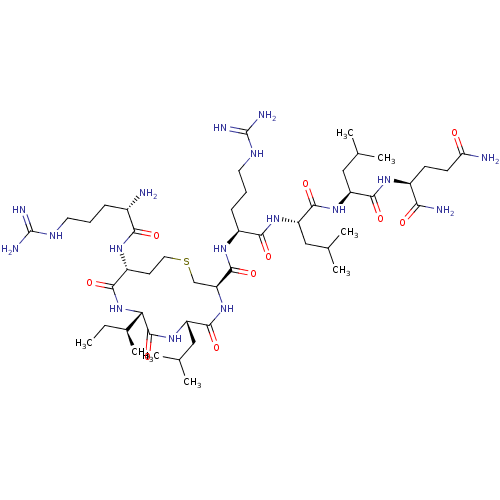
(CHEMBL4250447)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CCSC[C@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N |r| Show InChI InChI=1S/C48H89N17O10S/c1-9-27(8)37-46(75)63-34(22-26(6)7)44(73)64-35(23-76-19-16-31(41(70)65-37)59-39(68)28(49)12-10-17-56-47(52)53)45(74)60-30(13-11-18-57-48(54)55)40(69)61-33(21-25(4)5)43(72)62-32(20-24(2)3)42(71)58-29(38(51)67)14-15-36(50)66/h24-35,37H,9-23,49H2,1-8H3,(H2,50,66)(H2,51,67)(H,58,71)(H,59,68)(H,60,74)(H,61,69)(H,62,72)(H,63,75)(H,64,73)(H,65,70)(H4,52,53,56)(H4,54,55,57)/t27-,28-,29-,30-,31+,32-,33-,34-,35-,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Antagonist activity at full length recombinant human ERalpha expressed in baculovirus expression system assessed as inhibition of estradiol-induced E... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260722
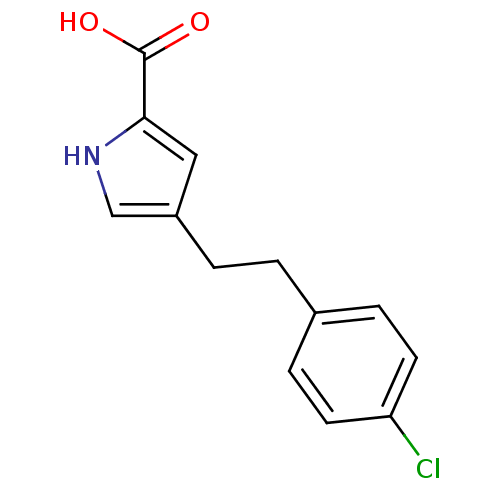
(4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...)Show InChI InChI=1S/C13H12ClNO2/c14-11-5-3-9(4-6-11)1-2-10-7-12(13(16)17)15-8-10/h3-8,15H,1-2H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50131131

(2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...)Show SMILES CNc1cc(Nc2nc3ccc(cc3s2)C(=O)Nc2c(C)cccc2Cl)ncn1 Show InChI InChI=1S/C20H17ClN6OS/c1-11-4-3-5-13(21)18(11)27-19(28)12-6-7-14-15(8-12)29-20(25-14)26-17-9-16(22-2)23-10-24-17/h3-10H,1-2H3,(H,27,28)(H2,22,23,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Fyn protein kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600605
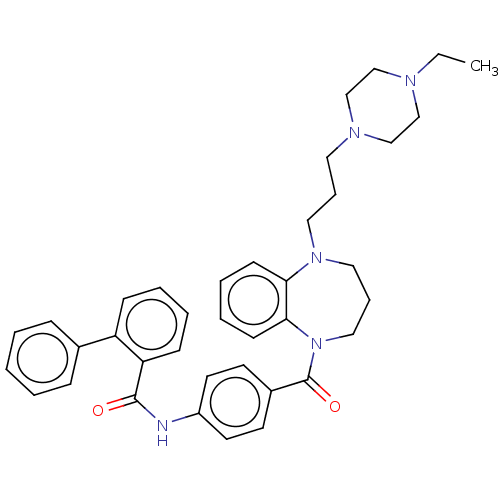
(CHEMBL5208751)Show SMILES CCN1CCN(CCCN2CCCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc23)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600607

(CHEMBL5207405)Show SMILES CCN(CC)CCCN1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3-c3ccccc3)cc2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50462987

(CHEMBL4250610)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N |r| Show InChI InChI=1S/C47H87N17O10S2/c1-9-26(8)36-45(74)61-32(20-25(6)7)42(71)63-33(21-75-76-22-34(44(73)64-36)62-38(67)27(48)12-10-16-55-46(51)52)43(72)58-29(13-11-17-56-47(53)54)39(68)59-31(19-24(4)5)41(70)60-30(18-23(2)3)40(69)57-28(37(50)66)14-15-35(49)65/h23-34,36H,9-22,48H2,1-8H3,(H2,49,65)(H2,50,66)(H,57,69)(H,58,72)(H,59,68)(H,60,70)(H,61,74)(H,62,67)(H,63,71)(H,64,73)(H4,51,52,55)(H4,53,54,56)/t26-,27-,28-,29-,30-,31-,32-,33+,34+,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Antagonist activity at full length recombinant human ERalpha expressed in baculovirus expression system assessed as inhibition of estradiol-induced E... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600603

(CHEMBL5192961)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCCCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600611
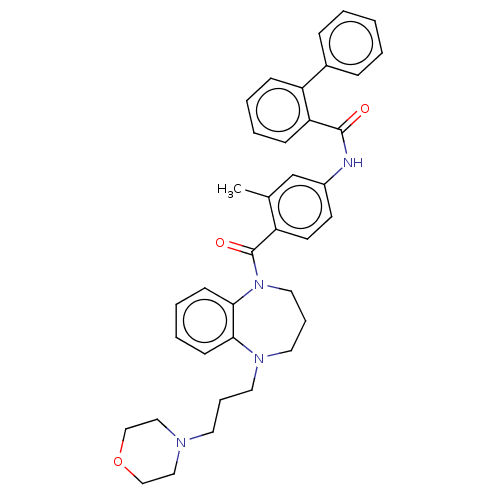
(CHEMBL5199628)Show SMILES Cc1cc(NC(=O)c2ccccc2-c2ccccc2)ccc1C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50206007
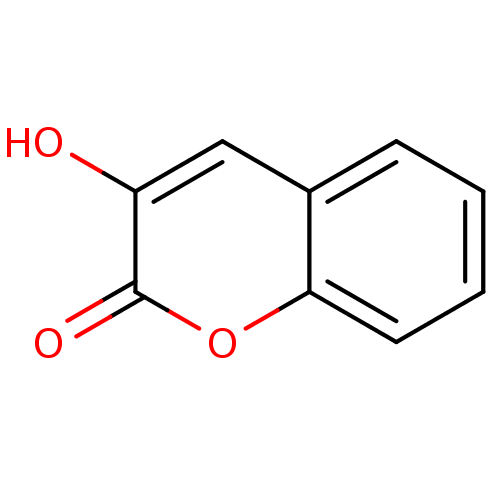
(3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...)Show InChI InChI=1S/C9H6O3/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13357

(CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...)Show SMILES Cc1cccc(Cl)c1NC(=O)c1ccc2nc(Nc3cccc(NCCO)n3)sc2c1 Show InChI InChI=1S/C22H20ClN5O2S/c1-13-4-2-5-15(23)20(13)28-21(30)14-8-9-16-17(12-14)31-22(25-16)27-19-7-3-6-18(26-19)24-10-11-29/h2-9,12,29H,10-11H2,1H3,(H,28,30)(H2,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600604
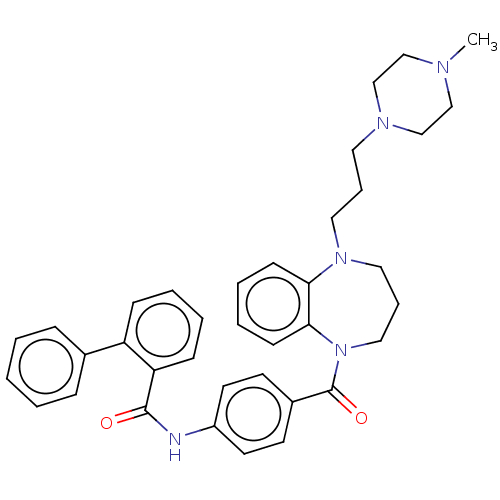
(CHEMBL5193621)Show SMILES CN1CCN(CCCN2CCCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc23)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50131131

(2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...)Show SMILES CNc1cc(Nc2nc3ccc(cc3s2)C(=O)Nc2c(C)cccc2Cl)ncn1 Show InChI InChI=1S/C20H17ClN6OS/c1-11-4-3-5-13(21)18(11)27-19(28)12-6-7-14-15(8-12)29-20(25-14)26-17-9-16(22-2)23-10-24-17/h3-10H,1-2H3,(H,27,28)(H2,22,23,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600601
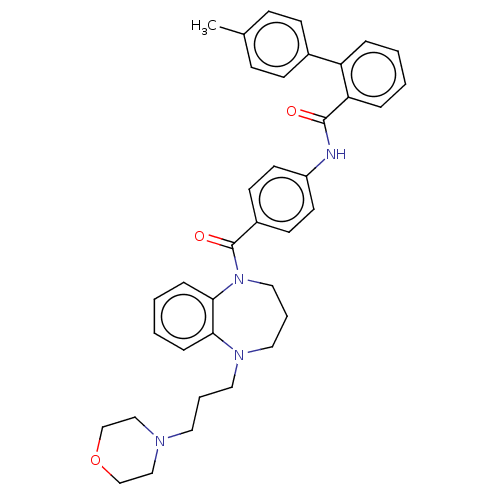
(CHEMBL5181318)Show SMILES Cc1ccc(cc1)-c1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600610
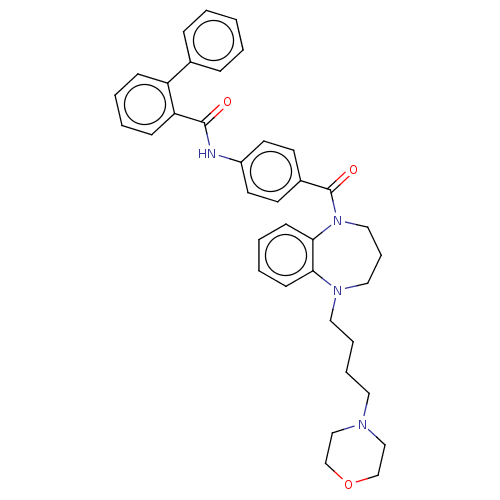
(CHEMBL5179440)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCCN2CCOCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50462993

(CHEMBL4244604)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C60H105N17O12S/c1-32(2)23-41(71-55(85)45(27-36(9)10)74-57(87)48-17-14-21-77(48)58(88)38(61)28-37-29-65-31-67-37)52(82)69-40(18-22-90-11)51(81)68-39(15-12-19-66-60(63)64)50(80)70-42(24-33(3)4)53(83)72-43(25-34(5)6)54(84)73-44(26-35(7)8)56(86)75-46(30-78)59(89)76-20-13-16-47(76)49(62)79/h29,31-36,38-48,78H,12-28,30,61H2,1-11H3,(H2,62,79)(H,65,67)(H,68,81)(H,69,82)(H,70,80)(H,71,85)(H,72,83)(H,73,84)(H,74,87)(H,75,86)(H4,63,64,66)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Displacement of FAM-LTERHKILHRLLQEGSPSD from ERbeta (unknown origin) (260 to 502 residues) expressed in Escherichia coli rosetta (DE3) after 1 hr by ... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50462973
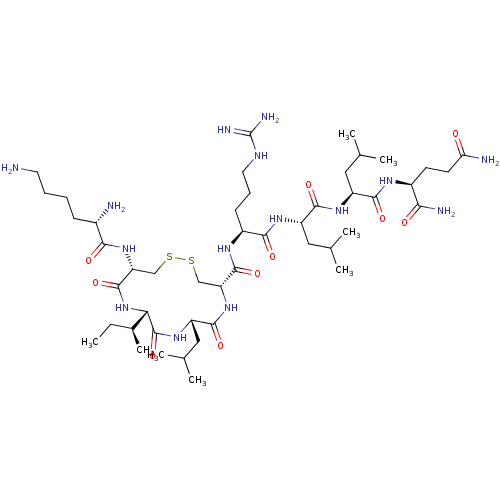
(CHEMBL4246022)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCCN |r| Show InChI InChI=1S/C47H87N15O10S2/c1-9-27(8)37-46(72)59-33(21-26(6)7)43(69)61-34(22-73-74-23-35(45(71)62-37)60-39(65)28(49)13-10-11-17-48)44(70)56-30(14-12-18-54-47(52)53)40(66)57-32(20-25(4)5)42(68)58-31(19-24(2)3)41(67)55-29(38(51)64)15-16-36(50)63/h24-35,37H,9-23,48-49H2,1-8H3,(H2,50,63)(H2,51,64)(H,55,67)(H,56,70)(H,57,66)(H,58,68)(H,59,72)(H,60,65)(H,61,69)(H,62,71)(H4,52,53,54)/t27-,28-,29-,30-,31-,32-,33-,34+,35+,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Antagonist activity at full length recombinant human ERalpha expressed in baculovirus expression system assessed as inhibition of estradiol-induced E... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600609

(CHEMBL5172250)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCN2CCOCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600612

(CHEMBL5187046)Show SMILES Clc1ccc2N(CCCN3CCOCC3)CCCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c2c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... |
Eur J Med Chem 157: 161-176 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.005
BindingDB Entry DOI: 10.7270/Q29P34C1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600608
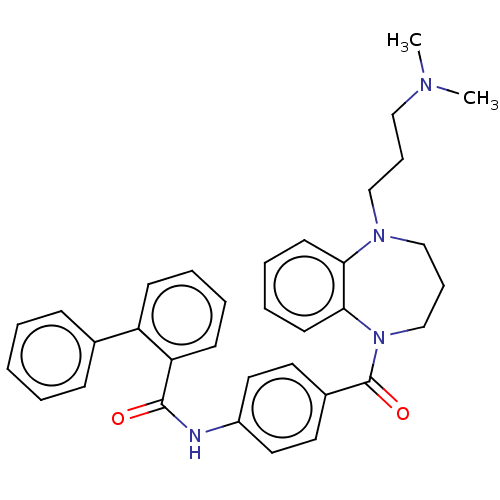
(CHEMBL5192496)Show SMILES CN(C)CCCN1CCCN(C(=O)c2ccc(NC(=O)c3ccccc3-c3ccccc3)cc2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600593

(CHEMBL5188374)Show SMILES Cc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50462976

(CHEMBL4250447)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CCSC[C@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N |r| Show InChI InChI=1S/C48H89N17O10S/c1-9-27(8)37-46(75)63-34(22-26(6)7)44(73)64-35(23-76-19-16-31(41(70)65-37)59-39(68)28(49)12-10-17-56-47(52)53)45(74)60-30(13-11-18-57-48(54)55)40(69)61-33(21-25(4)5)43(72)62-32(20-24(2)3)42(71)58-29(38(51)67)14-15-36(50)66/h24-35,37H,9-23,49H2,1-8H3,(H2,50,66)(H2,51,67)(H,58,71)(H,59,68)(H,60,74)(H,61,69)(H,62,72)(H,63,75)(H,64,73)(H,65,70)(H4,52,53,56)(H4,54,55,57)/t27-,28-,29-,30-,31+,32-,33-,34-,35-,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Antagonist activity at full length recombinant human ERbeta expressed in baculovirus expression system assessed as inhibition of estradiol-induced Eu... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600594
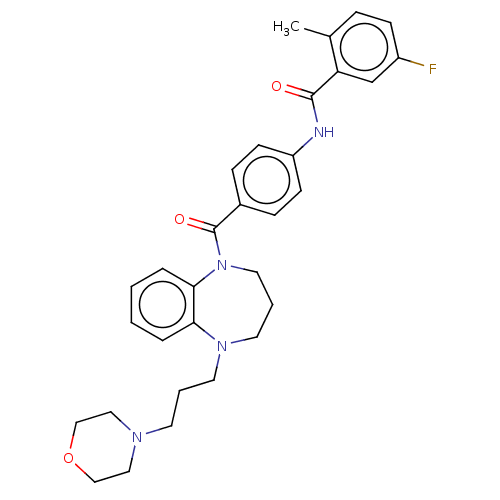
(CHEMBL5172734)Show SMILES Cc1ccc(F)cc1C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
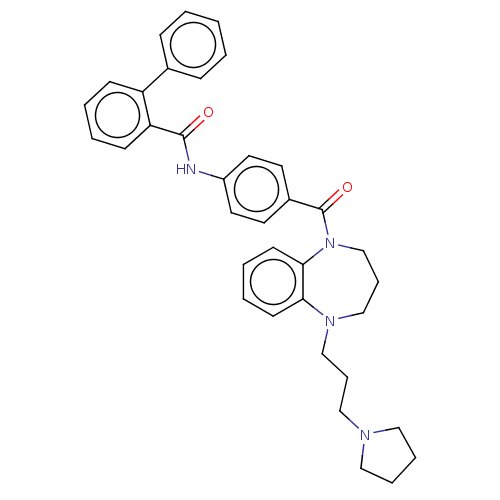
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600606
(CHEMBL5181341)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCCC2)c2ccccc12)c1ccccc1-c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50462987

(CHEMBL4250610)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N |r| Show InChI InChI=1S/C47H87N17O10S2/c1-9-26(8)36-45(74)61-32(20-25(6)7)42(71)63-33(21-75-76-22-34(44(73)64-36)62-38(67)27(48)12-10-16-55-46(51)52)43(72)58-29(13-11-17-56-47(53)54)39(68)59-31(19-24(4)5)41(70)60-30(18-23(2)3)40(69)57-28(37(50)66)14-15-35(49)65/h23-34,36H,9-22,48H2,1-8H3,(H2,49,65)(H2,50,66)(H,57,69)(H,58,72)(H,59,68)(H,60,70)(H,61,74)(H,62,67)(H,63,71)(H,64,73)(H4,51,52,55)(H4,53,54,56)/t26-,27-,28-,29-,30-,31-,32-,33+,34+,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Antagonist activity at full length recombinant human ERbeta expressed in baculovirus expression system assessed as inhibition of estradiol-induced Eu... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600596
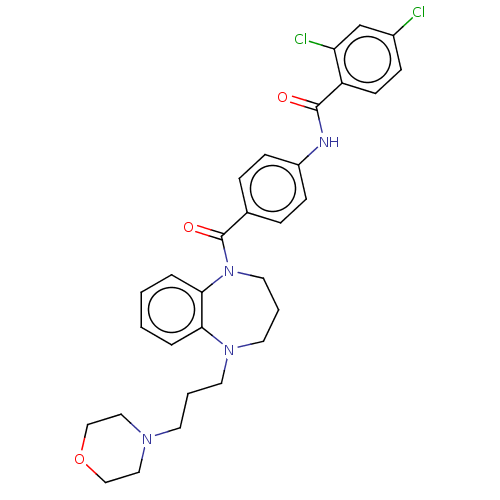
(CHEMBL5207214)Show SMILES Clc1ccc(C(=O)Nc2ccc(cc2)C(=O)N2CCCN(CCCN3CCOCC3)c3ccccc23)c(Cl)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50129307

(2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...)Show SMILES Cc1cccc(Cl)c1NC(=O)c1ccc2nc(NC(=O)NC(C)(C)C)sc2c1 Show InChI InChI=1S/C20H21ClN4O2S/c1-11-6-5-7-13(21)16(11)23-17(26)12-8-9-14-15(10-12)28-19(22-14)24-18(27)25-20(2,3)4/h5-10H,1-4H3,(H,23,26)(H2,22,24,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Fyn protein kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600595
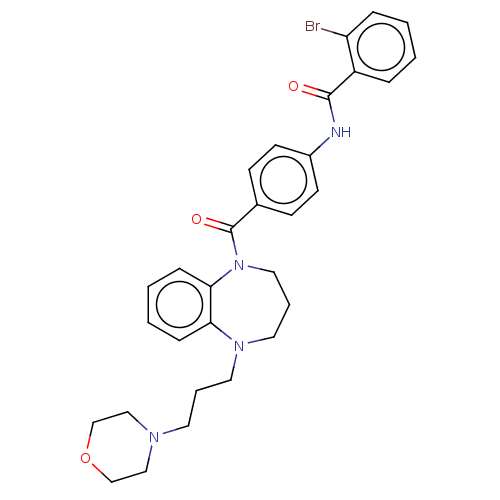
(CHEMBL5207597)Show SMILES Brc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50600605

(CHEMBL5208751)Show SMILES CCN1CCN(CCCN2CCCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc23)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50462993

(CHEMBL4244604)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C60H105N17O12S/c1-32(2)23-41(71-55(85)45(27-36(9)10)74-57(87)48-17-14-21-77(48)58(88)38(61)28-37-29-65-31-67-37)52(82)69-40(18-22-90-11)51(81)68-39(15-12-19-66-60(63)64)50(80)70-42(24-33(3)4)53(83)72-43(25-34(5)6)54(84)73-44(26-35(7)8)56(86)75-46(30-78)59(89)76-20-13-16-47(76)49(62)79/h29,31-36,38-48,78H,12-28,30,61H2,1-11H3,(H2,62,79)(H,65,67)(H,68,81)(H,69,82)(H,70,80)(H,71,85)(H,72,83)(H,73,84)(H,74,87)(H,75,86)(H4,63,64,66)/t38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Displacement of FAM-LTERHKILHRLLQEGSPSD from ERalpha (unknown origin) (302 to 552 residues) expressed in Escherichia coli rosetta (DE3) after 1 hr by... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433371
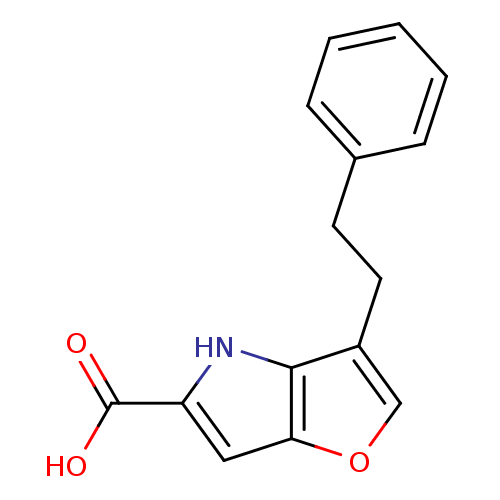
(CHEMBL2375519)Show InChI InChI=1S/C15H13NO3/c17-15(18)12-8-13-14(16-12)11(9-19-13)7-6-10-4-2-1-3-5-10/h1-5,8-9,16H,6-7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50129307

(2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...)Show SMILES Cc1cccc(Cl)c1NC(=O)c1ccc2nc(NC(=O)NC(C)(C)C)sc2c1 Show InChI InChI=1S/C20H21ClN4O2S/c1-11-6-5-7-13(21)16(11)23-17(26)12-8-9-14-15(10-12)28-19(22-14)24-18(27)25-20(2,3)4/h5-10H,1-4H3,(H,23,26)(H2,22,24,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Src protein tryrosine kinase |
Bioorg Med Chem Lett 13: 2587-90 (2003)
BindingDB Entry DOI: 10.7270/Q2FX78V4 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50462973

(CHEMBL4246022)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCCN |r| Show InChI InChI=1S/C47H87N15O10S2/c1-9-27(8)37-46(72)59-33(21-26(6)7)43(69)61-34(22-73-74-23-35(45(71)62-37)60-39(65)28(49)13-10-11-17-48)44(70)56-30(14-12-18-54-47(52)53)40(66)57-32(20-25(4)5)42(68)58-31(19-24(2)3)41(67)55-29(38(51)64)15-16-36(50)63/h24-35,37H,9-23,48-49H2,1-8H3,(H2,50,63)(H2,51,64)(H,55,67)(H,56,70)(H,57,66)(H,58,68)(H,59,72)(H,60,65)(H,61,69)(H,62,71)(H4,52,53,54)/t27-,28-,29-,30-,31-,32-,33-,34+,35+,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Antagonist activity at full length recombinant human ERbeta expressed in baculovirus expression system assessed as inhibition of estradiol-induced Eu... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600599
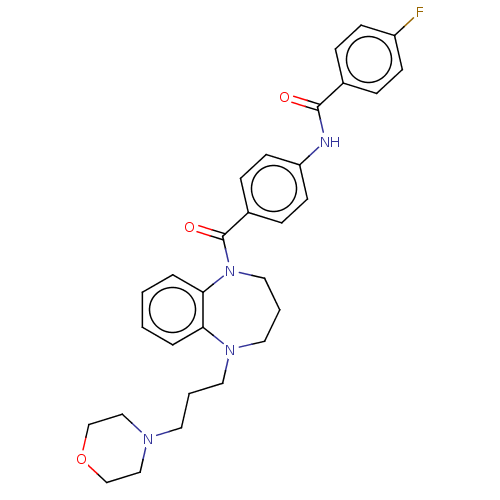
(CHEMBL5183482)Show SMILES Fc1ccc(cc1)C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600598
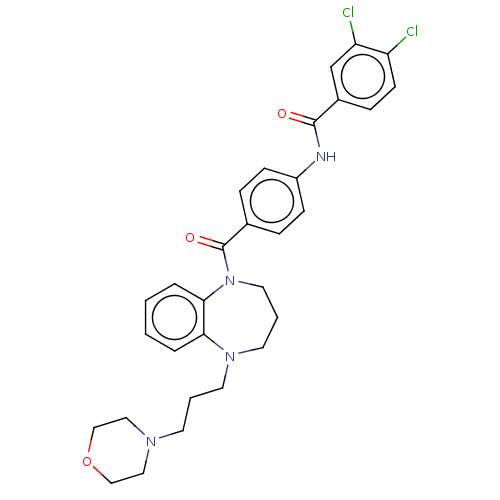
(CHEMBL5179419)Show SMILES Clc1ccc(cc1Cl)C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 638 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50600597
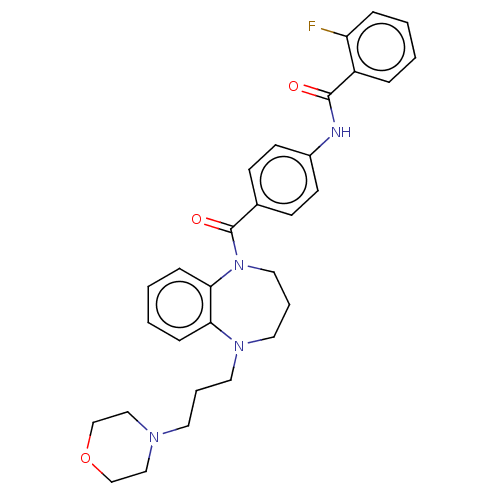
(CHEMBL5187312)Show SMILES Fc1ccccc1C(=O)Nc1ccc(cc1)C(=O)N1CCCN(CCCN2CCOCC2)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00567
BindingDB Entry DOI: 10.7270/Q2RR2392 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data