

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lethal factor (Bacillus anthracis) | BDBM8443 (2-[(5Z)-5-({5-[2-chloro-5-(trifluoromethyl)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8435 (3-[(5Z)-5-{[5-(4-chlorophenyl)furan-2-yl]methylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 800 | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM10215 (2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4-[3-(trifluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute | Assay Description Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a... | J Med Chem 48: 1649-56 (2005) Article DOI: 10.1021/jm0493212 BindingDB Entry DOI: 10.7270/Q2GT5KDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10215 (2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4-[3-(trifluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30E+3 | -30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Burnham Institute | Assay Description Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a... | J Med Chem 48: 1649-56 (2005) Article DOI: 10.1021/jm0493212 BindingDB Entry DOI: 10.7270/Q2GT5KDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10215 (2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4-[3-(trifluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute | Assay Description Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a... | J Med Chem 48: 1649-56 (2005) Article DOI: 10.1021/jm0493212 BindingDB Entry DOI: 10.7270/Q2GT5KDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM10216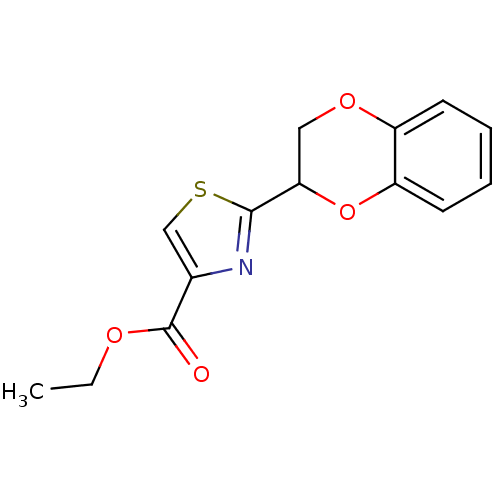 (BI-7E7 | Burnham Institute Compound 2 | ethyl 2-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute | Assay Description Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a... | J Med Chem 48: 1649-56 (2005) Article DOI: 10.1021/jm0493212 BindingDB Entry DOI: 10.7270/Q2GT5KDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8442 (2-[(5Z)-5-{[5-(3,4-dichlorophenyl)furan-2-yl]methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8441 (2-[(5Z)-5-{[5-(3-chloro-4-methoxyphenyl)furan-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8439 (2-[(5Z)-5-{[5-(4-chloro-2-nitrophenyl)furan-2-yl]m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8444 (2-[(5Z)-4-oxo-5-[(5-phenylthiophen-2-yl)methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8438 (2-[(5Z)-5-{[5-(4-bromophenyl)furan-2-yl]methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8437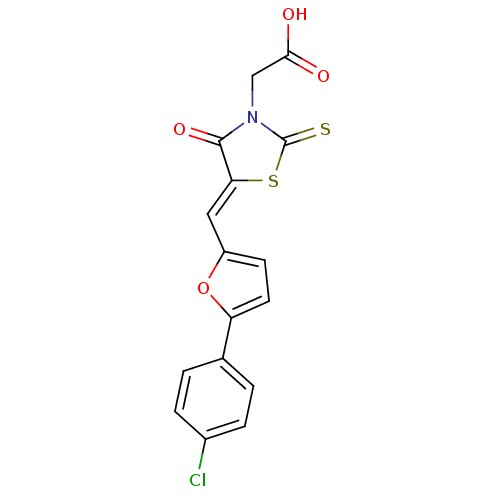 (2-[(5Z)-5-{[5-(4-chlorophenyl)furan-2-yl]methylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8447 (2-[(5Z)-5-[(5-{[(5Z)-3-(carboxymethyl)-4-oxo-2-sul...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8434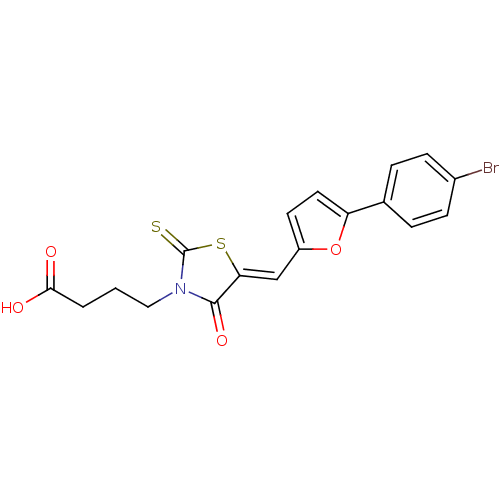 (4-[(5Z)-5-{[5-(4-bromophenyl)furan-2-yl]methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8432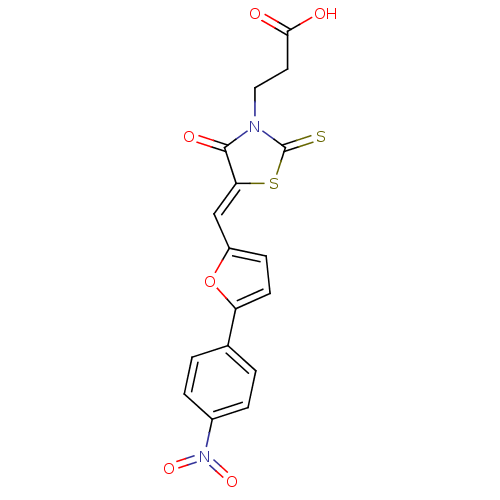 (3-[(5Z)-5-{[5-(4-nitrophenyl)furan-2-yl]methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8433 (2-chloro-4-(5-{[(5Z)-4-oxo-3-(prop-2-en-1-yl)-2-su...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8431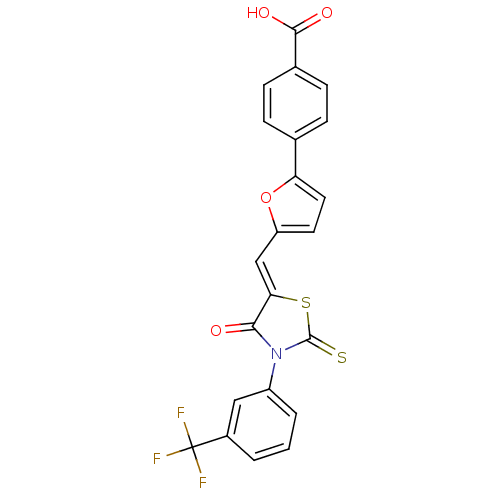 (4-(5-{[(5Z)-4-oxo-2-sulfanylidene-3-[3-(trifluorom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8430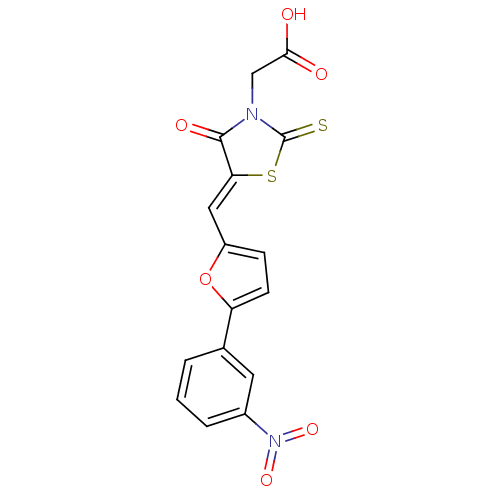 (2-[(5Z)-5-{[5-(3-nitrophenyl)furan-2-yl]methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8440 (2-[(5Z)-5-{[5-(2-nitrophenyl)furan-2-yl]methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244994 (CHEMBL4092239) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of PE-conjugated 12G5 antibody binding | J Med Chem 61: 2910-2923 (2018) Article DOI: 10.1021/acs.jmedchem.7b01830 BindingDB Entry DOI: 10.7270/Q2G44SPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8446 (2-[(5Z)-5-{[5-(4-chlorophenyl)thiophen-2-yl]methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8429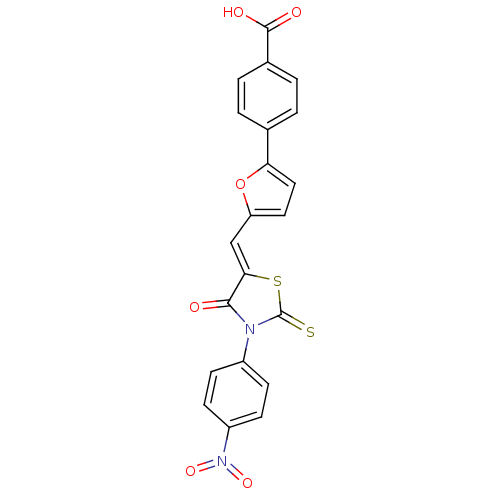 (4-(5-{[(5Z)-3-(4-nitrophenyl)-4-oxo-2-sulfanyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8428 (2-[(5Z)-5-{[5-(4-iodophenyl)furan-2-yl]methylidene...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8427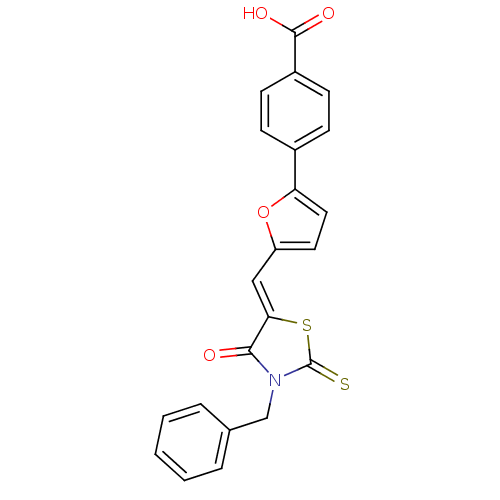 (4-(5-{[(5Z)-3-benzyl-4-oxo-2-sulfanylidene-1,3-thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8426 ((5Z)-5-{[5-(4-bromo-3-chlorophenyl)furan-2-yl]meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8425 ((5Z)-5-{[5-(3,4-dichlorophenyl)furan-2-yl]methylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8424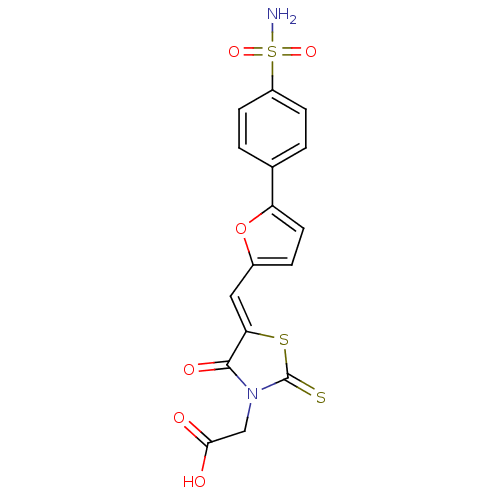 (2-[(5Z)-4-oxo-5-{[5-(4-sulfamoylphenyl)furan-2-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8423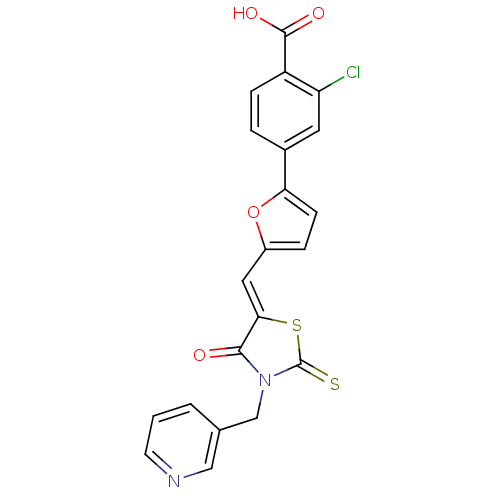 (2-chloro-4-(5-{[(5Z)-4-oxo-3-(pyridin-3-ylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244995 (CHEMBL4083765) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of PE-conjugated 12G5 antibody binding | J Med Chem 61: 2910-2923 (2018) Article DOI: 10.1021/acs.jmedchem.7b01830 BindingDB Entry DOI: 10.7270/Q2G44SPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244996 (CHEMBL4101762) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of PE-conjugated 12G5 antibody binding | J Med Chem 61: 2910-2923 (2018) Article DOI: 10.1021/acs.jmedchem.7b01830 BindingDB Entry DOI: 10.7270/Q2G44SPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244997 (CHEMBL4070596) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II Curated by ChEMBL | Assay Description Binding affinity at [3H]CD radiolabeled muscarinic M2 receptor in rat cortex. | J Med Chem 61: 2910-2923 (2018) Article DOI: 10.1021/acs.jmedchem.7b01830 BindingDB Entry DOI: 10.7270/Q2G44SPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244998 (CHEMBL4064541) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of PE-conjugated 12G5 antibody binding | J Med Chem 61: 2910-2923 (2018) Article DOI: 10.1021/acs.jmedchem.7b01830 BindingDB Entry DOI: 10.7270/Q2G44SPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244999 (CHEMBL4094209) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of PE-conjugated 12G5 antibody binding | J Med Chem 61: 2910-2923 (2018) Article DOI: 10.1021/acs.jmedchem.7b01830 BindingDB Entry DOI: 10.7270/Q2G44SPB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8422 ((5Z)-3-(furan-2-ylmethyl)-5-{[5-(4-nitrophenyl)fur...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8421 (3-[(5Z)-5-{[5-(2-nitrophenyl)furan-2-yl]methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8420 (4-[(5Z)-5-{[5-(3-nitrophenyl)furan-2-yl]methyliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50223446 (CHEMBL389147 | c[Tyr-Tyr-Asp-Glu-Gly-Leu-Glu-Glu]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of biotinylated VEGF165 binding to human recombinant VEGFR1 domain 1 to 3 by competitive binding assay | J Med Chem 53: 4428-40 (2010) Article DOI: 10.1021/jm1002167 BindingDB Entry DOI: 10.7270/Q2JM29SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50319610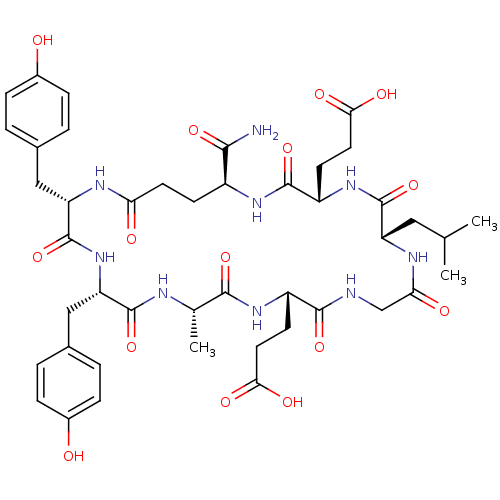 (CHEMBL1082940 | c[YYAEGLEE]-NH2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of biotinylated VEGF165 binding to human recombinant VEGFR1 domain 1 to 3 by competitive binding assay | J Med Chem 53: 4428-40 (2010) Article DOI: 10.1021/jm1002167 BindingDB Entry DOI: 10.7270/Q2JM29SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8419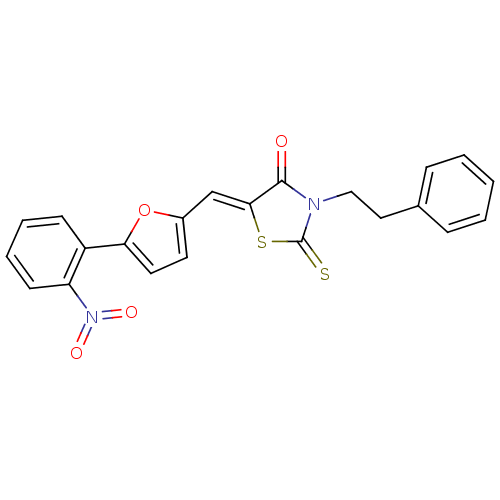 ((5Z)-5-{[5-(2-nitrophenyl)furan-2-yl]methylidene}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.19E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50223446 (CHEMBL389147 | c[Tyr-Tyr-Asp-Glu-Gly-Leu-Glu-Glu]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of biotinylated VEGF165 binding to human recombinant VEGFR1 extracellular domain by competitive binding assay | J Med Chem 53: 4428-40 (2010) Article DOI: 10.1021/jm1002167 BindingDB Entry DOI: 10.7270/Q2JM29SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM50319610 (CHEMBL1082940 | c[YYAEGLEE]-NH2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of biotinylated VEGF165 binding to human recombinant VEGFR1 extracellular domain by competitive binding assay | J Med Chem 53: 4428-40 (2010) Article DOI: 10.1021/jm1002167 BindingDB Entry DOI: 10.7270/Q2JM29SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8418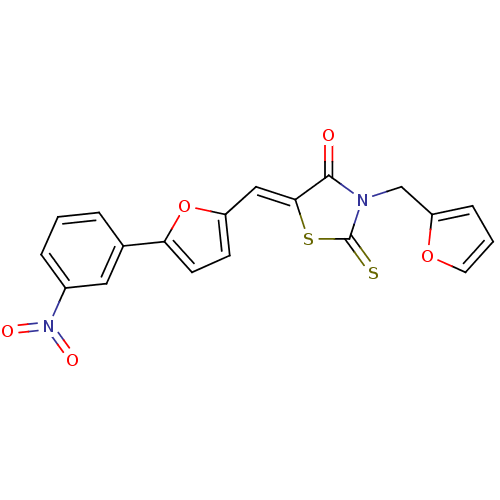 ((5Z)-3-(furan-2-ylmethyl)-5-{[5-(3-nitrophenyl)fur...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8417 ((5Z)-3-(4-hydroxyphenyl)-5-{[5-(4-nitrophenyl)fura...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10216 (BI-7E7 | Burnham Institute Compound 2 | ethyl 2-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Burnham Institute | Assay Description Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a... | J Med Chem 48: 1649-56 (2005) Article DOI: 10.1021/jm0493212 BindingDB Entry DOI: 10.7270/Q2GT5KDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10218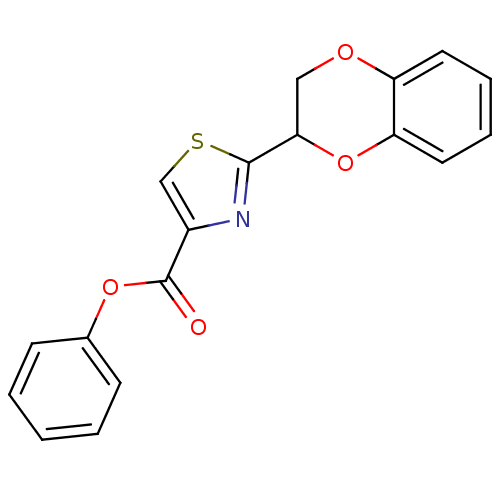 (BI-9C3 | Burnham Institute Compound 4 | phenyl 2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Burnham Institute | Assay Description Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a... | J Med Chem 48: 1649-56 (2005) Article DOI: 10.1021/jm0493212 BindingDB Entry DOI: 10.7270/Q2GT5KDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-7 (Homo sapiens (Human)) | BDBM10216 (BI-7E7 | Burnham Institute Compound 2 | ethyl 2-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute | Assay Description Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a... | J Med Chem 48: 1649-56 (2005) Article DOI: 10.1021/jm0493212 BindingDB Entry DOI: 10.7270/Q2GT5KDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal factor (Bacillus anthracis) | BDBM8416 ((5Z)-5-{[5-(4-fluorophenyl)furan-2-yl]methylidene}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Burnham Institute for Medical Research | Assay Description Fluorescence peptide cleavage assay was performed in a 96-well plate. Each reaction contained MAPKKide, LF, and the small-molecule inhibitor. The C-t... | Proc Natl Acad Sci U S A 102: 9499-504 (2005) Article DOI: 10.1073/pnas.0502733102 BindingDB Entry DOI: 10.7270/Q2TM78BQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM10219 (BI-9B11 | Burnham Institute Compound 5 | [2-(2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Burnham Institute | Assay Description Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a... | J Med Chem 48: 1649-56 (2005) Article DOI: 10.1021/jm0493212 BindingDB Entry DOI: 10.7270/Q2GT5KDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM10217 (2-(2,3-dihydro-1,4-benzodioxin-2-yl)-N-(furan-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute | Assay Description Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a... | J Med Chem 48: 1649-56 (2005) Article DOI: 10.1021/jm0493212 BindingDB Entry DOI: 10.7270/Q2GT5KDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-8 (Homo sapiens (Human)) | BDBM10218 (BI-9C3 | Burnham Institute Compound 4 | phenyl 2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute | Assay Description Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a... | J Med Chem 48: 1649-56 (2005) Article DOI: 10.1021/jm0493212 BindingDB Entry DOI: 10.7270/Q2GT5KDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 83 total ) | Next | Last >> |