 Found 160 hits with Last Name = 'fiorucci' and Initial = 's'
Found 160 hits with Last Name = 'fiorucci' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50596184
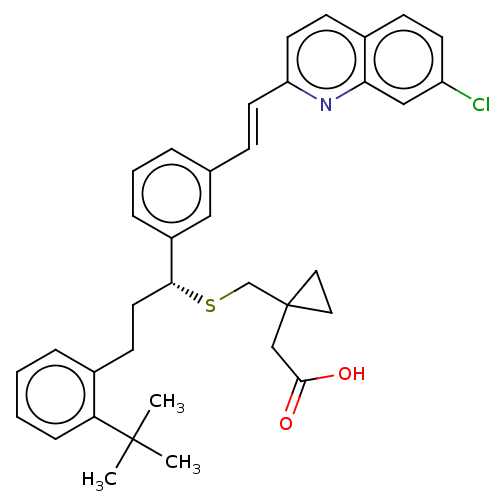
(CHEMBL5205127)Show SMILES [H][C@](CCc1ccccc1C(C)(C)C)(SCC1(CC(O)=O)CC1)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50023198

(8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O4/c33-23-17-24(26-29-31-32-30-26)36-25-21(23)10-6-11-22(25)28-27(34)19-12-14-20(15-13-19)35-16-5-4-9-18-7-2-1-3-8-18/h1-3,6-8,10-15,17H,4-5,9,16H2,(H,28,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535422
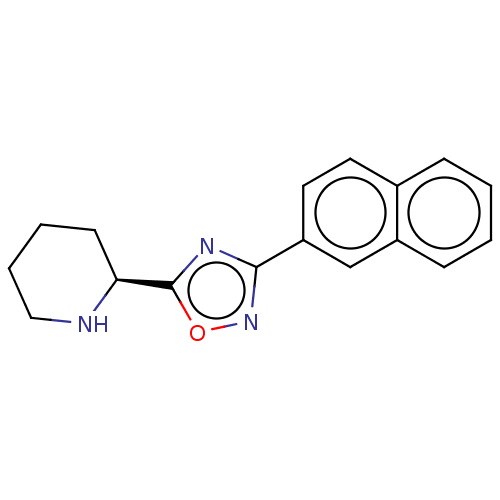
(CHEMBL4527193)Show InChI InChI=1S/C17H17N3O/c1-2-6-13-11-14(9-8-12(13)5-1)16-19-17(21-20-16)15-7-3-4-10-18-15/h1-2,5-6,8-9,11,15,18H,3-4,7,10H2/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535427
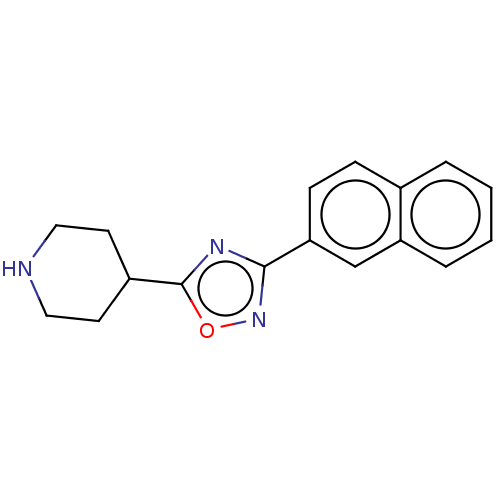
(CHEMBL4464270)Show InChI InChI=1S/C17H17N3O/c1-2-4-14-11-15(6-5-12(14)3-1)16-19-17(21-20-16)13-7-9-18-10-8-13/h1-6,11,13,18H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50018938
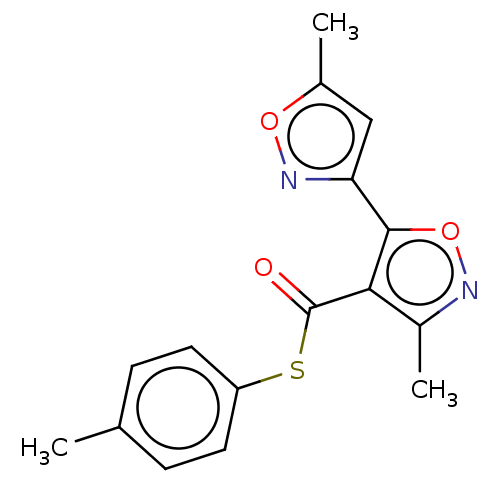
(CHEMBL1445606)Show InChI InChI=1S/C16H14N2O3S/c1-9-4-6-12(7-5-9)22-16(19)14-11(3)17-21-15(14)13-8-10(2)20-18-13/h4-8H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Antagonist activity at PXR (unknown origin) transfected in human HepG2 cells assessed as inhibition of rifampicin-induced reporter gene transcription |
J Med Chem 57: 4819-33 (2014)
Article DOI: 10.1021/jm500351m
BindingDB Entry DOI: 10.7270/Q2XD137W |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50130734
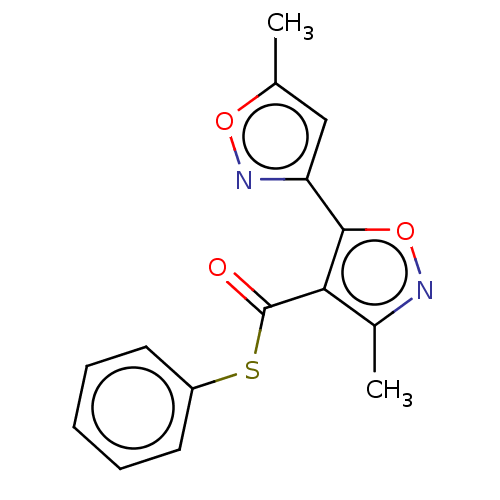
(CHEMBL3632931)Show InChI InChI=1S/C15H12N2O3S/c1-9-8-12(17-19-9)14-13(10(2)16-20-14)15(18)21-11-6-4-3-5-7-11/h3-8H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Antagonist activity at PXR (unknown origin) |
Eur J Med Chem 103: 551-62 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.005
BindingDB Entry DOI: 10.7270/Q2N87CMJ |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 2
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50012434
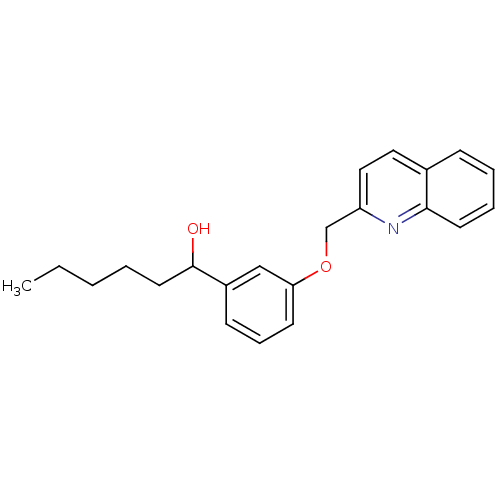
((REV-5,901)1-[3-(Quinolin-2-ylmethoxy)-phenyl]-hex...)Show InChI InChI=1S/C22H25NO2/c1-2-3-4-12-22(24)18-9-7-10-20(15-18)25-16-19-14-13-17-8-5-6-11-21(17)23-19/h5-11,13-15,22,24H,2-4,12,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50012434

((REV-5,901)1-[3-(Quinolin-2-ylmethoxy)-phenyl]-hex...)Show InChI InChI=1S/C22H25NO2/c1-2-3-4-12-22(24)18-9-7-10-20(15-18)25-16-19-14-13-17-8-5-6-11-21(17)23-19/h5-11,13-15,22,24H,2-4,12,16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535416
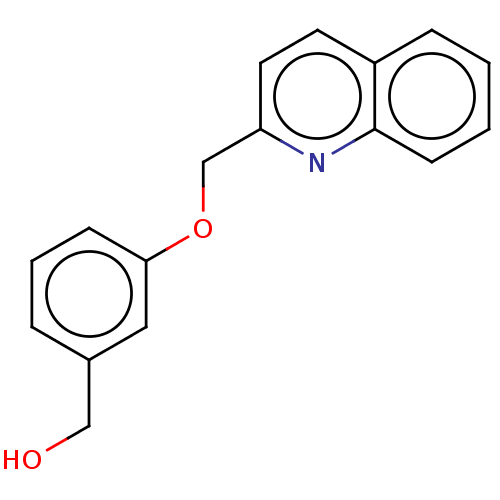
(CHEMBL4451559)Show InChI InChI=1S/C21H25N3O2/c25-14-4-3-11-24-12-9-17(10-13-24)21-22-20(23-26-21)19-8-7-16-5-1-2-6-18(16)15-19/h1-2,5-8,15,17,25H,3-4,9-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50596187
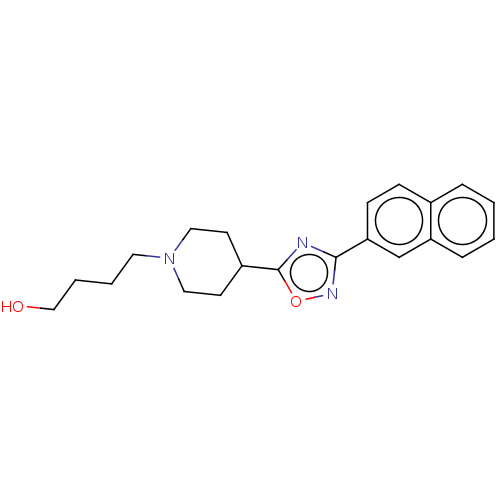
(CHEMBL5174405) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50596185
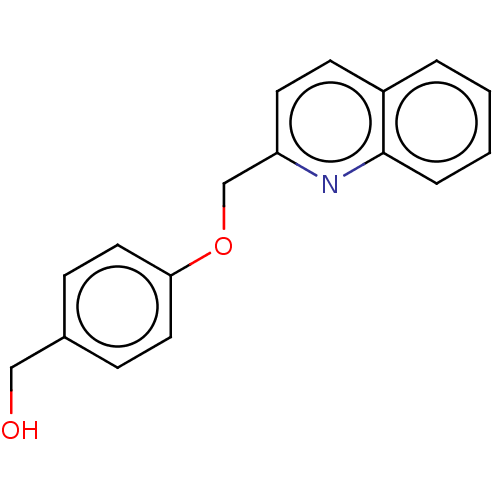
(CHEMBL5181326) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50596188
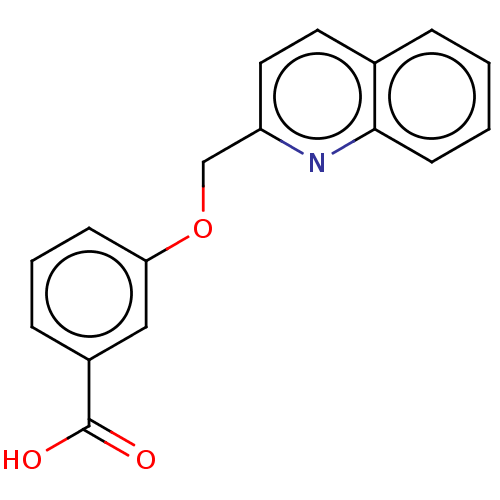
(CHEMBL5169385) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 2
(Homo sapiens (Human)) | BDBM50023198

(8-[4-(4-phenylbutyloxy)benzoyl]amino-2-(tetrazol-5...)Show SMILES O=C(Nc1cccc2c1oc(cc2=O)-c1nnn[nH]1)c1ccc(OCCCCc2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O4/c33-23-17-24(26-29-31-32-30-26)36-25-21(23)10-6-11-22(25)28-27(34)19-12-14-20(15-13-19)35-16-5-4-9-18-7-2-1-3-8-18/h1-3,6-8,10-15,17H,4-5,9,16H2,(H,28,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50596186
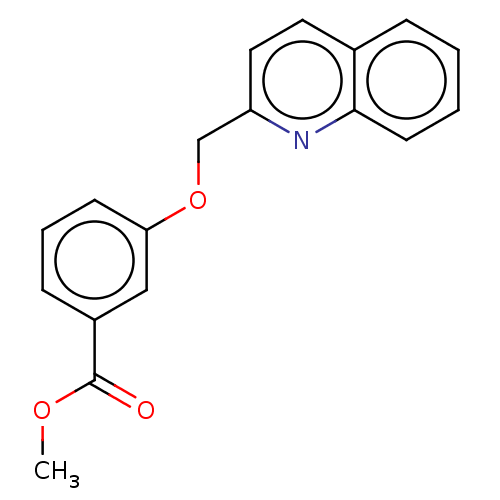
(CHEMBL5173307) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 2
(Homo sapiens (Human)) | BDBM50596184

(CHEMBL5205127)Show SMILES [H][C@](CCc1ccccc1C(C)(C)C)(SCC1(CC(O)=O)CC1)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50596189
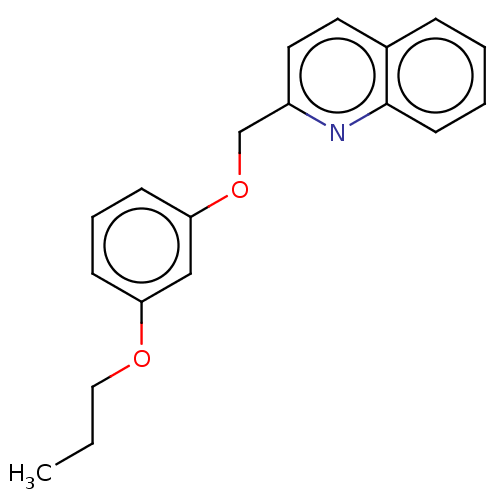
(CHEMBL5189181) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535421
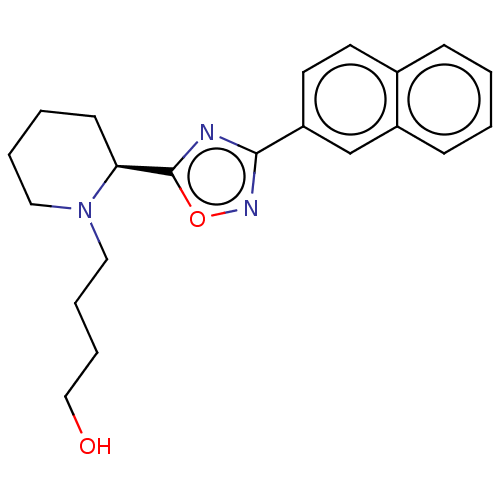
(CHEMBL4519624)Show SMILES OCCCCN1CCCC[C@H]1c1nc(no1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C21H25N3O2/c25-14-6-5-13-24-12-4-3-9-19(24)21-22-20(23-26-21)18-11-10-16-7-1-2-8-17(16)15-18/h1-2,7-8,10-11,15,19,25H,3-6,9,12-14H2/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50596190
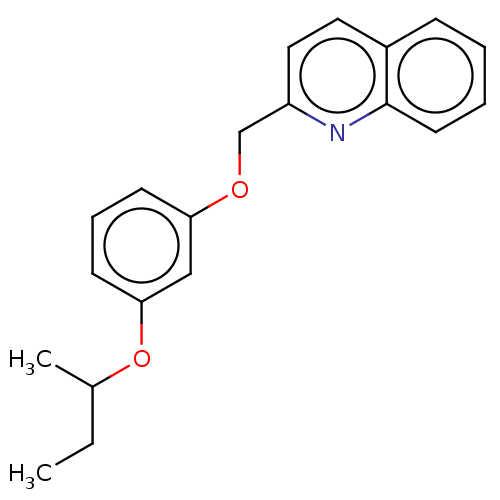
(CHEMBL5192002) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01078
BindingDB Entry DOI: 10.7270/Q2T72NGC |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535420
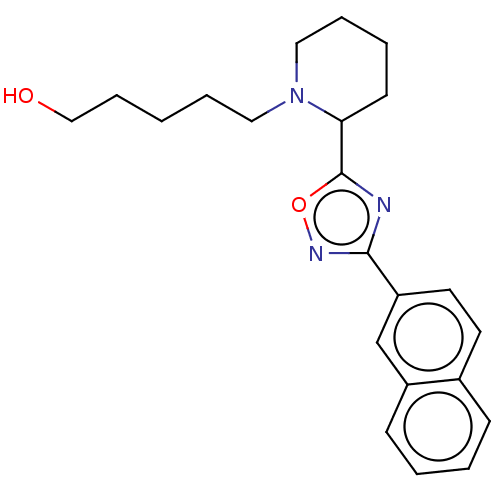
(CHEMBL4541043)Show InChI InChI=1S/C22H27N3O2/c26-15-7-1-5-13-25-14-6-4-10-20(25)22-23-21(24-27-22)19-12-11-17-8-2-3-9-18(17)16-19/h2-3,8-9,11-12,16,20,26H,1,4-7,10,13-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50018939
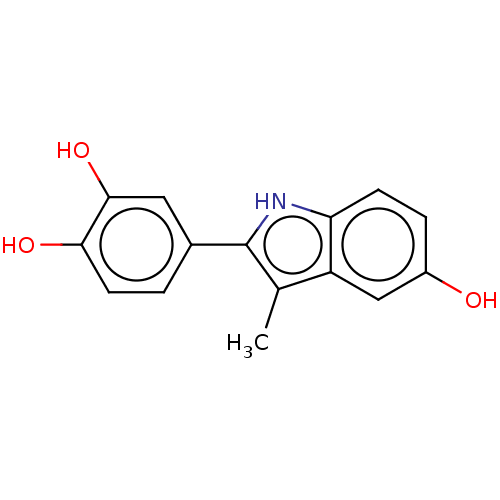
(CHEMBL3287132)Show InChI InChI=1S/C15H13NO3/c1-8-11-7-10(17)3-4-12(11)16-15(8)9-2-5-13(18)14(19)6-9/h2-7,16-19H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Antagonist activity at human PXR transfected in human HepG2 cells co-transfected with pSG5-RXR/pCMV-beta-galactosidase/p(CYP3A4)-TK-Luc assessed as i... |
J Med Chem 57: 4819-33 (2014)
Article DOI: 10.1021/jm500351m
BindingDB Entry DOI: 10.7270/Q2XD137W |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM23451
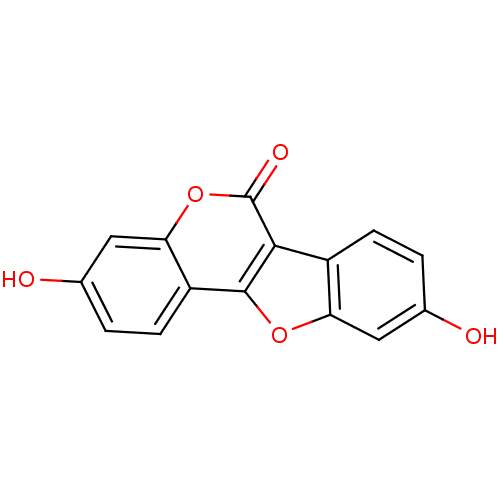
(5,14-dihydroxy-8,17-dioxatetracyclo[8.7.0.0^{2,7}....)Show InChI InChI=1S/C15H8O5/c16-7-1-3-9-11(5-7)19-14-10-4-2-8(17)6-12(10)20-15(18)13(9)14/h1-6,16-17H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Antagonist activity at human PXR transfected in African green monkey CV1 cells assessed as inhibition of SR12813-induced transactivation after 24 hrs... |
J Med Chem 57: 4819-33 (2014)
Article DOI: 10.1021/jm500351m
BindingDB Entry DOI: 10.7270/Q2XD137W |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50018940

(CHEMBL3287136)Show InChI InChI=1S/C18H19NO4/c1-11-14-10-13(23-8-2-7-20)4-5-15(14)19-18(11)12-3-6-16(21)17(22)9-12/h3-6,9-10,19-22H,2,7-8H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Antagonist activity at human PXR transfected in human HepG2 cells co-transfected with pSG5-RXR/pCMV-beta-galactosidase/p(CYP3A4)-TK-Luc assessed as i... |
J Med Chem 57: 4819-33 (2014)
Article DOI: 10.1021/jm500351m
BindingDB Entry DOI: 10.7270/Q2XD137W |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM20625
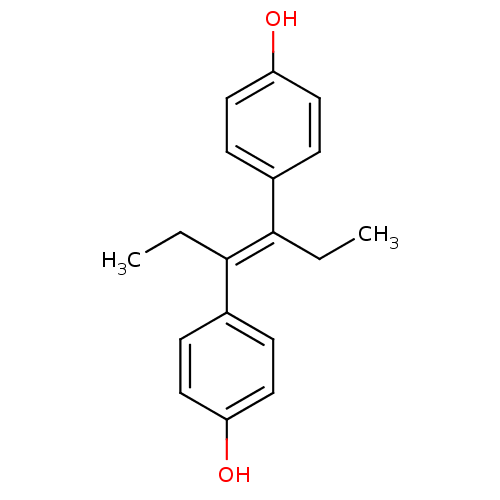
(4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...)Show InChI InChI=1S/C18H20O2/c1-3-17(13-5-9-15(19)10-6-13)18(4-2)14-7-11-16(20)12-8-14/h5-12,19-20H,3-4H2,1-2H3/b18-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human PXR transfected in human HepG2 cells assessed as reduction in rifaximin-induced receptor transactivation aft... |
Eur J Med Chem 103: 551-62 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.005
BindingDB Entry DOI: 10.7270/Q2N87CMJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535425
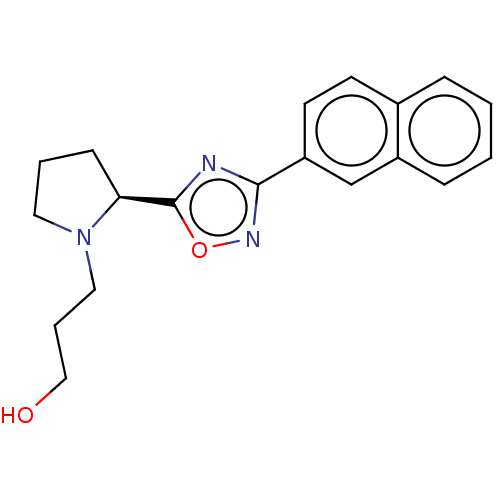
(CHEMBL4562404)Show SMILES OCCCN1CCC[C@H]1c1nc(no1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H21N3O2/c23-12-4-11-22-10-3-7-17(22)19-20-18(21-24-19)16-9-8-14-5-1-2-6-15(14)13-16/h1-2,5-6,8-9,13,17,23H,3-4,7,10-12H2/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535417
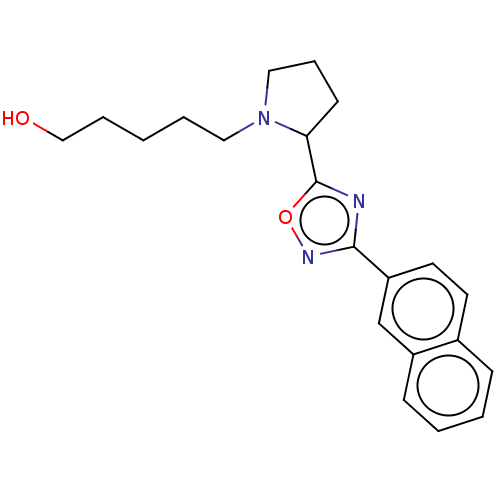
(CHEMBL4575954)Show InChI InChI=1S/C21H25N3O2/c25-14-5-1-4-12-24-13-6-9-19(24)21-22-20(23-26-21)18-11-10-16-7-2-3-8-17(16)15-18/h2-3,7-8,10-11,15,19,25H,1,4-6,9,12-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535423
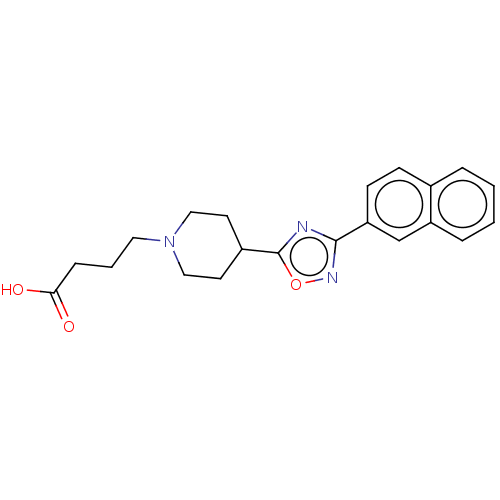
(CHEMBL4573467)Show SMILES OC(=O)CCCN1CCC(CC1)c1nc(no1)-c1ccc2ccccc2c1 Show InChI InChI=1S/C21H23N3O3/c25-19(26)6-3-11-24-12-9-16(10-13-24)21-22-20(23-27-21)18-8-7-15-4-1-2-5-17(15)14-18/h1-2,4-5,7-8,14,16H,3,6,9-13H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535426
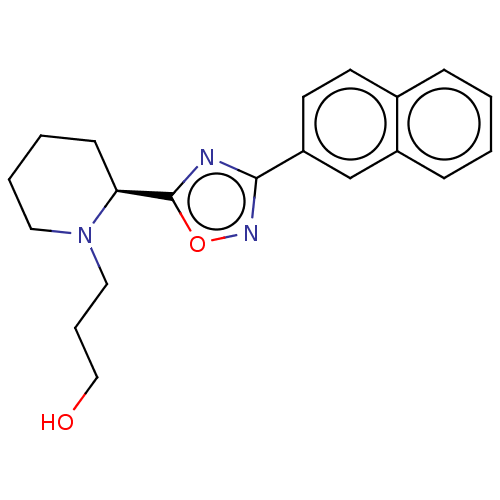
(CHEMBL4539531)Show SMILES OCCCN1CCCC[C@H]1c1nc(no1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H23N3O2/c24-13-5-12-23-11-4-3-8-18(23)20-21-19(22-25-20)17-10-9-15-6-1-2-7-16(15)14-17/h1-2,6-7,9-10,14,18,24H,3-5,8,11-13H2/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50130718
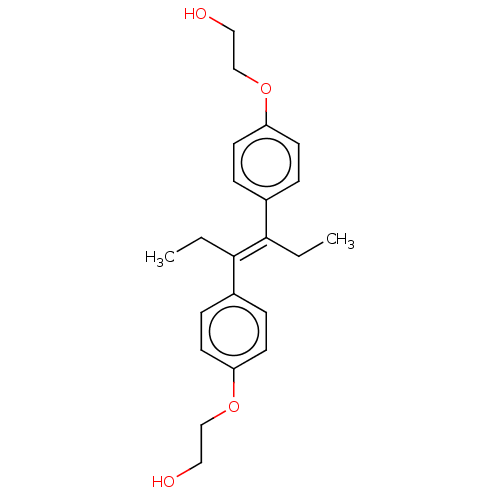
(CHEMBL3632924)Show InChI InChI=1S/C22H28O4/c1-3-21(17-5-9-19(10-6-17)25-15-13-23)22(4-2)18-7-11-20(12-8-18)26-16-14-24/h5-12,23-24H,3-4,13-16H2,1-2H3/b22-21+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human PXR transfected in human HepG2 cells assessed as reduction in rifaximin-induced receptor transactivation aft... |
Eur J Med Chem 103: 551-62 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.005
BindingDB Entry DOI: 10.7270/Q2N87CMJ |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535419
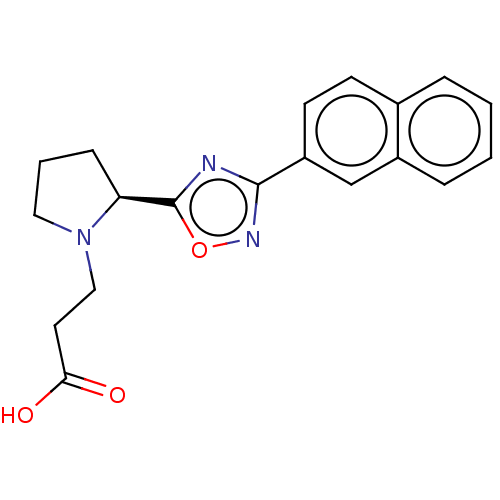
(CHEMBL4464005)Show SMILES OC(=O)CCN1CCC[C@H]1c1nc(no1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C19H19N3O3/c23-17(24)9-11-22-10-3-6-16(22)19-20-18(21-25-19)15-8-7-13-4-1-2-5-14(13)12-15/h1-2,4-5,7-8,12,16H,3,6,9-11H2,(H,23,24)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535418
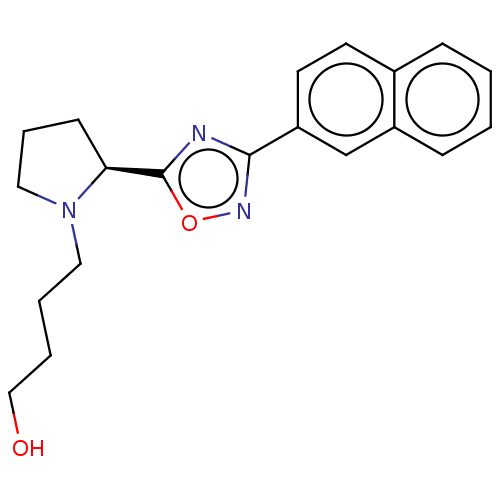
(CHEMBL4457628)Show SMILES OCCCCN1CCC[C@H]1c1nc(no1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H23N3O2/c24-13-4-3-11-23-12-5-8-18(23)20-21-19(22-25-20)17-10-9-15-6-1-2-7-16(15)14-17/h1-2,6-7,9-10,14,18,24H,3-5,8,11-13H2/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535428
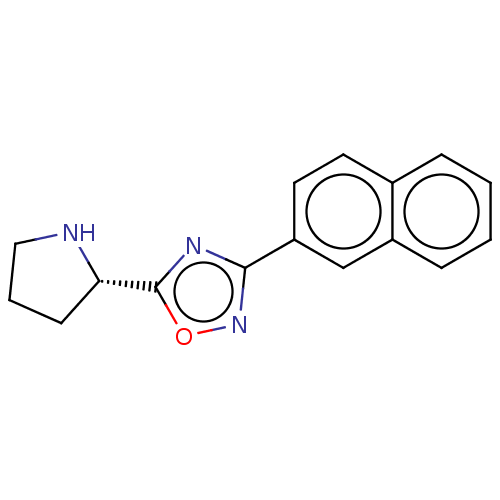
(CHEMBL4452655)Show InChI InChI=1S/C16H15N3O/c1-2-5-12-10-13(8-7-11(12)4-1)15-18-16(20-19-15)14-6-3-9-17-14/h1-2,4-5,7-8,10,14,17H,3,6,9H2/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50535424

(CHEMBL4564891)Show InChI InChI=1S/C20H23N3O2/c24-13-3-10-23-11-8-16(9-12-23)20-21-19(22-25-20)18-7-6-15-4-1-2-5-17(15)14-18/h1-2,4-7,14,16,24H,3,8-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Antagonist activity at human FXR transfected in HepG2 cells assessed as inhibition of CDCA-induced receptor transactivation by luciferase reporter as... |
ACS Med Chem Lett 10: 504-510 (2019)
Article DOI: 10.1021/acsmedchemlett.8b00534
BindingDB Entry DOI: 10.7270/Q2445R07 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50002612
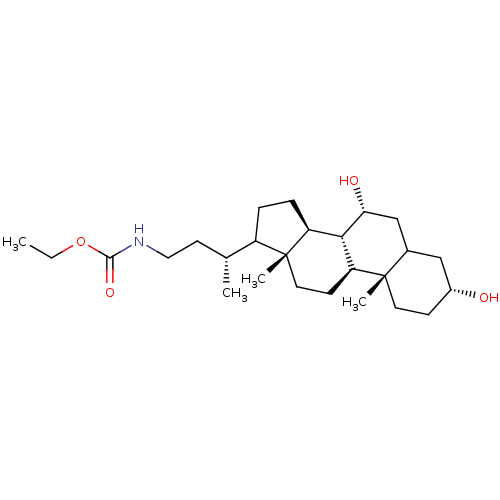
(CHEMBL3138403)Show SMILES [H][C@@]12CCC([C@H](C)CCNC(=O)OCC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](O)CC2C[C@H](O)CC[C@]12C Show InChI InChI=1S/C26H45NO4/c1-5-31-24(30)27-13-10-16(2)19-6-7-20-23-21(9-12-26(19,20)4)25(3)11-8-18(28)14-17(25)15-22(23)29/h16-23,28-29H,5-15H2,1-4H3,(H,27,30)/t16-,17?,18-,19?,20+,21+,22-,23+,25+,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Binding affinity to FXR assessed as ligand-dependent SRC1 recruitment by FRET based co-activator assay |
J Med Chem 49: 4208-15 (2006)
Article DOI: 10.1021/jm060294k
BindingDB Entry DOI: 10.7270/Q23779H2 |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21675
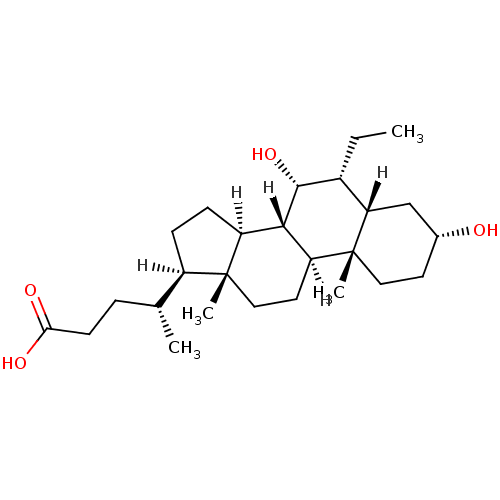
((4R)-4-[(1S,2S,5R,7S,8R,9R,10S,11S,14R,15R)-8-ethy...)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O Show InChI InChI=1S/C26H44O4/c1-5-17-21-14-16(27)10-12-26(21,4)20-11-13-25(3)18(15(2)6-9-22(28)29)7-8-19(25)23(20)24(17)30/h15-21,23-24,27,30H,5-14H2,1-4H3,(H,28,29)/t15-,16-,17-,18-,19+,20+,21+,23+,24-,25-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 98 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Binding affinity to FXR assessed as ligand-dependent SRC1 recruitment by FRET based co-activator assay |
J Med Chem 49: 4208-15 (2006)
Article DOI: 10.1021/jm060294k
BindingDB Entry DOI: 10.7270/Q23779H2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50002614
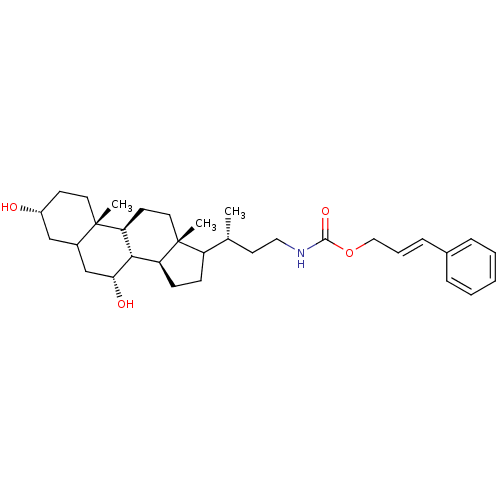
(CHEMBL3138092)Show SMILES [H][C@@]12CCC([C@H](C)CCNC(=O)OC\C=C\c3ccccc3)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](O)CC2C[C@H](O)CC[C@]12C Show InChI InChI=1S/C33H49NO4/c1-22(15-18-34-31(37)38-19-7-10-23-8-5-4-6-9-23)26-11-12-27-30-28(14-17-33(26,27)3)32(2)16-13-25(35)20-24(32)21-29(30)36/h4-10,22,24-30,35-36H,11-21H2,1-3H3,(H,34,37)/b10-7+/t22-,24?,25-,26?,27+,28+,29-,30+,32+,33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 590 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Binding affinity to FXR assessed as ligand-dependent SRC1 recruitment by FRET based co-activator assay |
J Med Chem 49: 4208-15 (2006)
Article DOI: 10.1021/jm060294k
BindingDB Entry DOI: 10.7270/Q23779H2 |
More data for this
Ligand-Target Pair | |
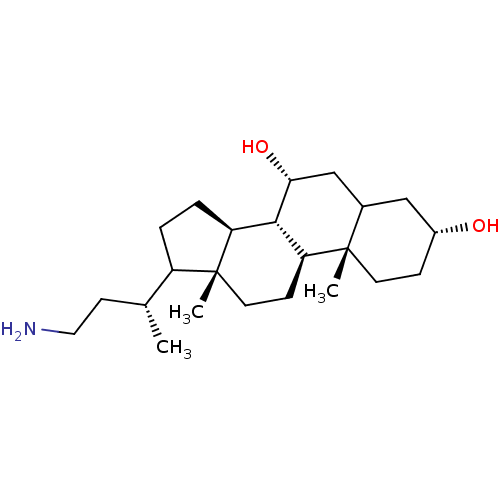
Bile acid receptor
(Homo sapiens (Human)) | BDBM50002611
(CHEMBL3138044)Show SMILES [H][C@@]12CCC([C@H](C)CCN)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])[C@H](O)CC2C[C@H](O)CC[C@]12C Show InChI InChI=1S/C23H41NO2/c1-14(8-11-24)17-4-5-18-21-19(7-10-23(17,18)3)22(2)9-6-16(25)12-15(22)13-20(21)26/h14-21,25-26H,4-13,24H2,1-3H3/t14-,15?,16-,17?,18+,19+,20-,21+,22+,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.68E+3 | n/a | n/a | n/a | n/a |
Universit£ di Perugia
Curated by ChEMBL
| Assay Description
Binding affinity to FXR assessed as ligand-dependent SRC1 recruitment by FRET based co-activator assay |
J Med Chem 49: 4208-15 (2006)
Article DOI: 10.1021/jm060294k
BindingDB Entry DOI: 10.7270/Q23779H2 |
More data for this
Ligand-Target Pair | |
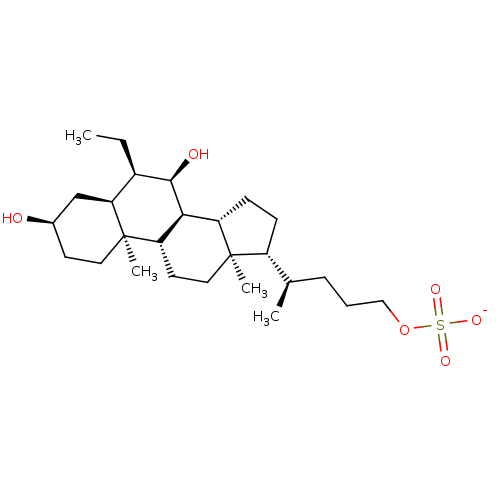
Bile acid receptor
(Homo sapiens (Human)) | BDBM50011369
(CHEMBL3260994)Show SMILES [Na+].[H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCOS([O-])(=O)=O |r| Show InChI InChI=1S/C26H46O6S.Na/c1-5-18-22-15-17(27)10-12-26(22,4)21-11-13-25(3)19(8-9-20(25)23(21)24(18)28)16(2)7-6-14-32-33(29,30)31;/h16-24,27-28H,5-15H2,1-4H3,(H,29,30,31);/q;+1/p-1/t16-,17-,18-,19-,20+,21+,22+,23+,24-,25-,26-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia
Curated by ChEMBL
| Assay Description
Transactivation of human FXR transfected in human HepG2 cells by beta-galactosidase reporter gene assay |
J Med Chem 57: 937-54 (2014)
Article DOI: 10.1021/jm401873d
BindingDB Entry DOI: 10.7270/Q2RR20S3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50011369

(CHEMBL3260994)Show SMILES [Na+].[H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCOS([O-])(=O)=O |r| Show InChI InChI=1S/C26H46O6S.Na/c1-5-18-22-15-17(27)10-12-26(22,4)21-11-13-25(3)19(8-9-20(25)23(21)24(18)28)16(2)7-6-14-32-33(29,30)31;/h16-24,27-28H,5-15H2,1-4H3,(H,29,30,31);/q;+1/p-1/t16-,17-,18-,19-,20+,21+,22+,23+,24-,25-,26-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia
Curated by ChEMBL
| Assay Description
Transactivation of human GP-BAR1 transfected in HEK293T cells assessed as induction of intracellular cAMP production after 18 hrs by cAMP responsive ... |
J Med Chem 57: 937-54 (2014)
Article DOI: 10.1021/jm401873d
BindingDB Entry DOI: 10.7270/Q2RR20S3 |
More data for this
Ligand-Target Pair | |
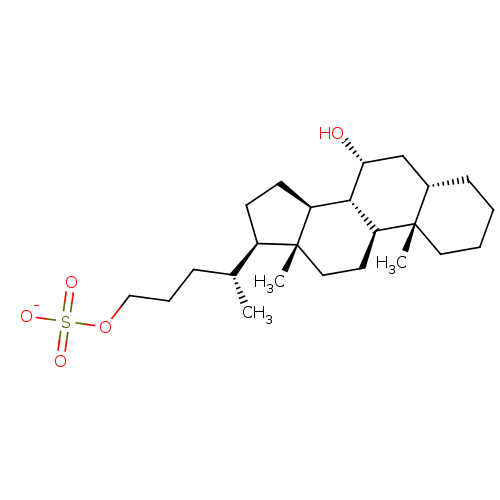
Bile acid receptor
(Homo sapiens (Human)) | BDBM50028026
(CHEMBL3342065)Show SMILES [Na+].[H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])CCCC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCOS([O-])(=O)=O |r| Show InChI InChI=1S/C24H42O5S/c1-16(7-6-14-29-30(26,27)28)18-9-10-19-22-20(11-13-24(18,19)3)23(2)12-5-4-8-17(23)15-21(22)25/h16-22,25H,4-15H2,1-3H3,(H,26,27,28)/p-1/t16-,17+,18-,19+,20+,21-,22+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a |
University of Naples"Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) expressed in human HepG2 cells assessed as receptor transactivation at 100 nM to 50 uM incubated for 16 hrs ... |
J Med Chem 57: 8477-95 (2014)
Article DOI: 10.1021/jm501273r
BindingDB Entry DOI: 10.7270/Q2RF5WMN |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50028027
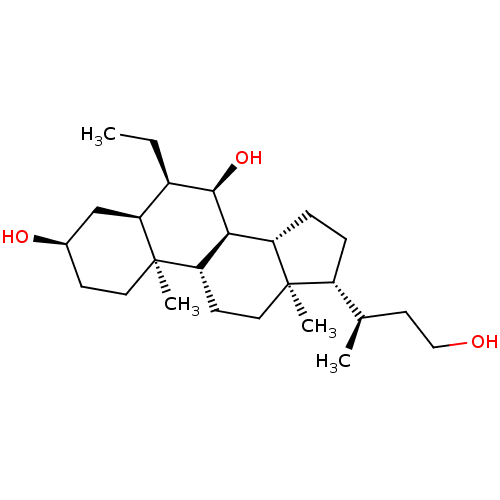
(CHEMBL3342086)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCO |r| Show InChI InChI=1S/C25H44O3/c1-5-17-21-14-16(27)8-11-25(21,4)20-9-12-24(3)18(15(2)10-13-26)6-7-19(24)22(20)23(17)28/h15-23,26-28H,5-14H2,1-4H3/t15-,16-,17-,18-,19+,20+,21+,22+,23-,24-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
University of Naples"Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at FXR (unknown origin) expressed in human HepG2 cells assessed as receptor transactivation at 100 nM to 50 uM incubated for 16 hrs ... |
J Med Chem 57: 8477-95 (2014)
Article DOI: 10.1021/jm501273r
BindingDB Entry DOI: 10.7270/Q2RF5WMN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50028028
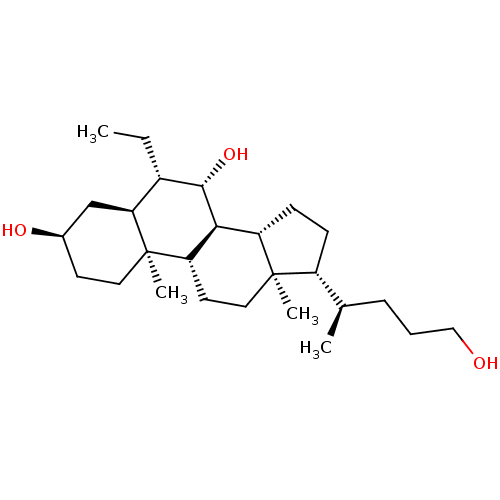
(CHEMBL3342082)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)[C@@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCO |r| Show InChI InChI=1S/C26H46O3/c1-5-18-22-15-17(28)10-12-26(22,4)21-11-13-25(3)19(16(2)7-6-14-27)8-9-20(25)23(21)24(18)29/h16-24,27-29H,5-15H2,1-4H3/t16-,17-,18+,19-,20+,21+,22+,23+,24+,25-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a |
University of Naples"Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at GP-BAR1 (unknown origin) in human HEK293 cells assessed as receptor transactivation at 100 nM to 50 uM incubated for 16 hrs by CR... |
J Med Chem 57: 8477-95 (2014)
Article DOI: 10.1021/jm501273r
BindingDB Entry DOI: 10.7270/Q2RF5WMN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50028027

(CHEMBL3342086)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)[C@H](CC)[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCO |r| Show InChI InChI=1S/C25H44O3/c1-5-17-21-14-16(27)8-11-25(21,4)20-9-12-24(3)18(15(2)10-13-26)6-7-19(24)22(20)23(17)28/h15-23,26-28H,5-14H2,1-4H3/t15-,16-,17-,18-,19+,20+,21+,22+,23-,24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
University of Naples"Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at GP-BAR1 (unknown origin) in human HEK293 cells assessed as receptor transactivation at 100 nM to 50 uM incubated for 16 hrs by CR... |
J Med Chem 57: 8477-95 (2014)
Article DOI: 10.1021/jm501273r
BindingDB Entry DOI: 10.7270/Q2RF5WMN |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50098804
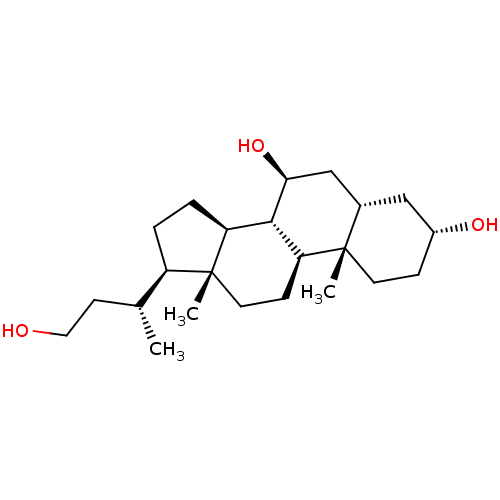
(CHEMBL3329940)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCO |r| Show InChI InChI=1S/C23H40O3/c1-14(8-11-24)17-4-5-18-21-19(7-10-23(17,18)3)22(2)9-6-16(25)12-15(22)13-20(21)26/h14-21,24-26H,4-13H2,1-3H3/t14-,15+,16-,17-,18+,19+,20+,21+,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a |
University of Naples"Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at human GPBAR1 expressed in HEK293T cells assessed as stimulation of cAMP response element-mediated receptor transactivation by luc... |
J Med Chem 57: 7687-701 (2014)
Article DOI: 10.1021/jm500889f
BindingDB Entry DOI: 10.7270/Q2WQ05J5 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50098805
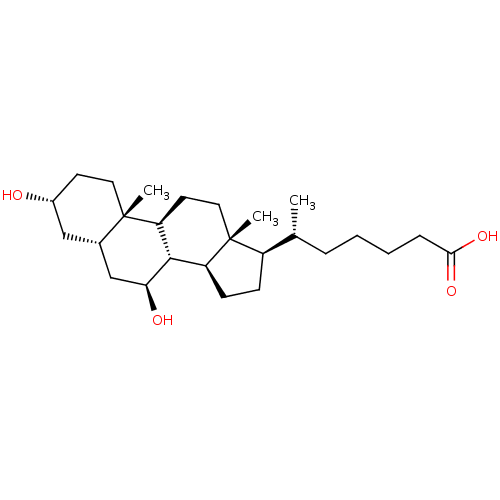
(CHEMBL3330019)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCCC(O)=O |r| Show InChI InChI=1S/C26H44O4/c1-16(6-4-5-7-23(29)30)19-8-9-20-24-21(11-13-26(19,20)3)25(2)12-10-18(27)14-17(25)15-22(24)28/h16-22,24,27-28H,4-15H2,1-3H3,(H,29,30)/t16-,17+,18-,19-,20+,21+,22+,24+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a |
University of Naples"Federico II"
Curated by ChEMBL
| Assay Description
Agonist activity at human GPBAR1 expressed in HEK293T cells assessed as stimulation of cAMP response element-mediated receptor transactivation by luc... |
J Med Chem 57: 7687-701 (2014)
Article DOI: 10.1021/jm500889f
BindingDB Entry DOI: 10.7270/Q2WQ05J5 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50130717
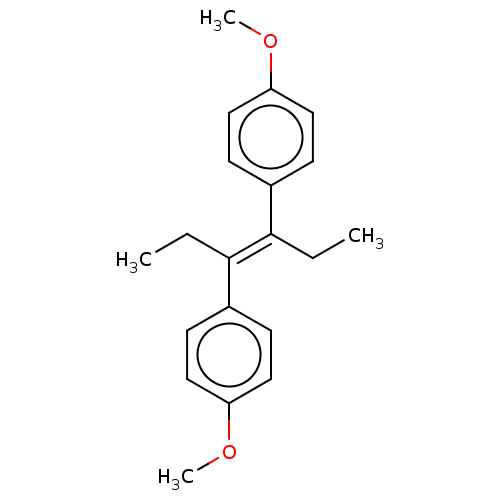
(CHEBI:34701 | CHEMBL113650)Show InChI InChI=1S/C20H24O2/c1-5-19(15-7-11-17(21-3)12-8-15)20(6-2)16-9-13-18(22-4)14-10-16/h7-14H,5-6H2,1-4H3/b20-19+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Transactivation of full length human PXR transfected in human HepG2 cells after 18 hrs by luciferase reporter assay relative to rifaximin |
Eur J Med Chem 103: 551-62 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.005
BindingDB Entry DOI: 10.7270/Q2N87CMJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50347620
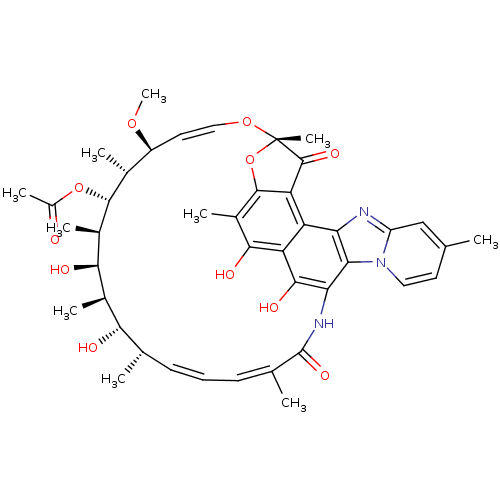
(RIFAXIMIN | Rifacol | Rifaxidin)Show SMILES CO[C@H]1\C=C\O[C@@]2(C)Oc3c(C2=O)c2c4nc5cc(C)ccn5c4c(NC(=O)\C(C)=C/C=C/[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@@H]1C)c(O)c2c(O)c3C |r,c:32,t:3,34| Show InChI InChI=1S/C43H51N3O11/c1-19-14-16-46-28(18-19)44-32-29-30-37(50)25(7)40-31(29)41(52)43(9,57-40)55-17-15-27(54-10)22(4)39(56-26(8)47)24(6)36(49)23(5)35(48)20(2)12-11-13-21(3)42(53)45-33(34(32)46)38(30)51/h11-18,20,22-24,27,35-36,39,48-51H,1-10H3,(H,45,53)/b12-11+,17-15+,21-13-/t20-,22+,23+,24+,27-,35-,36+,39+,43-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a |
University of Ljubljana
Curated by ChEMBL
| Assay Description
Transactivation of full length human PXR transfected in human HepG2 cells after 18 hrs by luciferase reporter assay relative to rifaximin |
Eur J Med Chem 103: 551-62 (2015)
Article DOI: 10.1016/j.ejmech.2015.09.005
BindingDB Entry DOI: 10.7270/Q2N87CMJ |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50347619
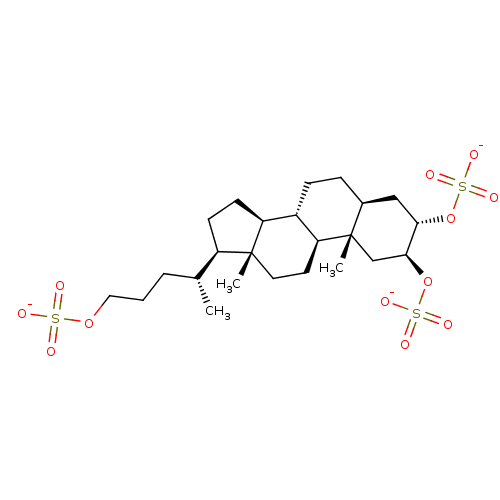
(CHEMBL1649712 | Solomonsterol A)Show SMILES C[C@H](CCCOS([O-])(=O)=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](OS([O-])(=O)=O)[C@H](C[C@]4(C)[C@H]3CC[C@]12C)OS([O-])(=O)=O |r| Show InChI InChI=1S/C24H42O12S3/c1-15(5-4-12-34-37(25,26)27)18-8-9-19-17-7-6-16-13-21(35-38(28,29)30)22(36-39(31,32)33)14-24(16,3)20(17)10-11-23(18,19)2/h15-22H,4-14H2,1-3H3,(H,25,26,27)(H,28,29,30)(H,31,32,33)/p-3/t15-,16+,17+,18-,19+,20+,21+,22+,23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a |
Universita di Napoli Federico II
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in HepG2 cells after 18 hrs by luciferase reporter gene assay |
J Med Chem 54: 4590-9 (2011)
Article DOI: 10.1021/jm200241s
BindingDB Entry DOI: 10.7270/Q2W37WP3 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50347620

(RIFAXIMIN | Rifacol | Rifaxidin)Show SMILES CO[C@H]1\C=C\O[C@@]2(C)Oc3c(C2=O)c2c4nc5cc(C)ccn5c4c(NC(=O)\C(C)=C/C=C/[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@@H]1C)c(O)c2c(O)c3C |r,c:32,t:3,34| Show InChI InChI=1S/C43H51N3O11/c1-19-14-16-46-28(18-19)44-32-29-30-37(50)25(7)40-31(29)41(52)43(9,57-40)55-17-15-27(54-10)22(4)39(56-26(8)47)24(6)36(49)23(5)35(48)20(2)12-11-13-21(3)42(53)45-33(34(32)46)38(30)51/h11-18,20,22-24,27,35-36,39,48-51H,1-10H3,(H,45,53)/b12-11+,17-15+,21-13-/t20-,22+,23+,24+,27-,35-,36+,39+,43-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
Universita di Napoli Federico II
Curated by ChEMBL
| Assay Description
Transactivation of human PXR expressed in HepG2 cells after 18 hrs by luciferase reporter gene assay |
J Med Chem 54: 4590-9 (2011)
Article DOI: 10.1021/jm200241s
BindingDB Entry DOI: 10.7270/Q2W37WP3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data