

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-galactosidase (Escherichia coli) | BDBM50150470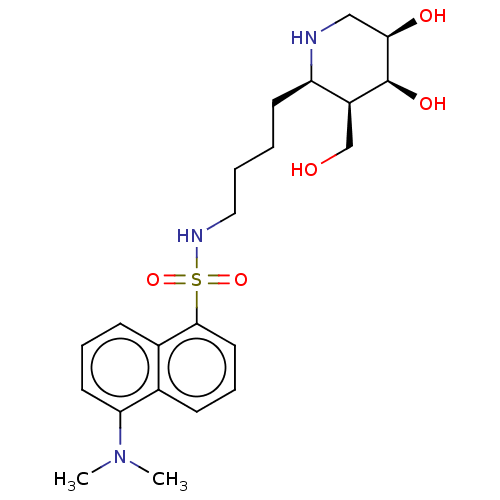 (CHEMBL3771185) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lacZ beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed b... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50150470 (CHEMBL3771185) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed by substra... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50350758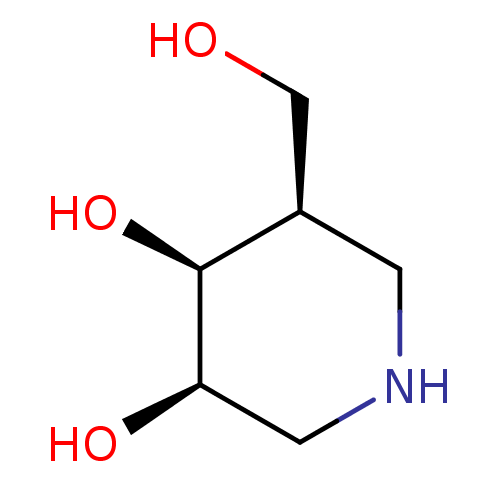 (CHEMBL1818433) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lacZ beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed b... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50150471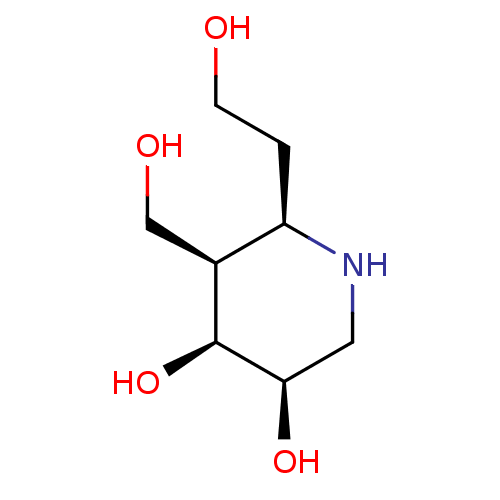 (CHEMBL3770764) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lacZ beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed b... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Escherichia coli) | BDBM50150472 (CHEMBL3770736) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lacZ beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed b... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50358321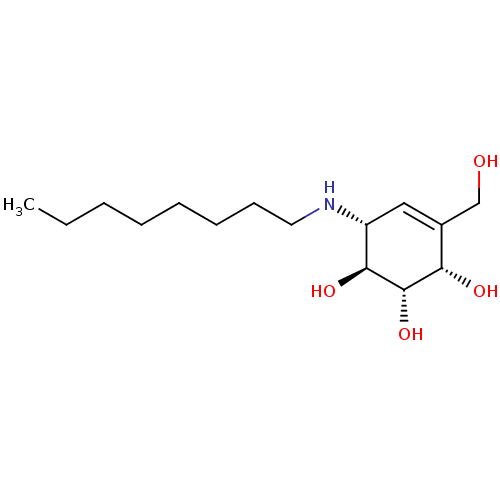 (CHEMBL1922579 | CHEMBL1922581) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50150471 (CHEMBL3770764) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed by substra... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50150472 (CHEMBL3770736) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed by substra... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Bos taurus (Bovine)) | BDBM50350758 (CHEMBL1818433) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of bovine liver beta-galactosidase using 4-nitrophenyl-beta-D-galactopyranoside as substrate preincubated up to 5 mins followed by substra... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase A (Homo sapiens (Human)) | BDBM50150471 (CHEMBL3770764) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant lysosomal alpha-galactosidase using 2,4-dinitrophenyl-alpha-D-galactopyranoside as substrate preincubated up to 5 min... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase A (Homo sapiens (Human)) | BDBM50150472 (CHEMBL3770736) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant lysosomal alpha-galactosidase using 2,4-dinitrophenyl-alpha-D-galactopyranoside as substrate preincubated up to 5 min... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase A (Homo sapiens (Human)) | BDBM50150470 (CHEMBL3771185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant lysosomal alpha-galactosidase using 2,4-dinitrophenyl-alpha-D-galactopyranoside as substrate preincubated up to 5 min... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-galactosidase A (Homo sapiens (Human)) | BDBM50350758 (CHEMBL1818433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant lysosomal alpha-galactosidase using 2,4-dinitrophenyl-alpha-D-galactopyranoside as substrate preincubated up to 5 min... | Bioorg Med Chem Lett 26: 1438-42 (2016) Article DOI: 10.1016/j.bmcl.2016.01.059 BindingDB Entry DOI: 10.7270/Q2HQ41SR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by fluorescence method | J Med Chem 62: 10124-10143 (2019) Article DOI: 10.1021/acs.jmedchem.9b00952 BindingDB Entry DOI: 10.7270/Q2QF8XB9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by fluorescence method | J Med Chem 62: 10124-10143 (2019) Article DOI: 10.1021/acs.jmedchem.9b00952 BindingDB Entry DOI: 10.7270/Q2QF8XB9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A4 expressed in supersomes using 7-benzyloxyquinoline as substrate by fluorescence method | J Med Chem 62: 10124-10143 (2019) Article DOI: 10.1021/acs.jmedchem.9b00952 BindingDB Entry DOI: 10.7270/Q2QF8XB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Southwestern Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A4 expressed in supersomes using 7-benzyloxyquinoline as substrate by fluorescence method | J Med Chem 62: 10124-10143 (2019) Article DOI: 10.1021/acs.jmedchem.9b00952 BindingDB Entry DOI: 10.7270/Q2QF8XB9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220140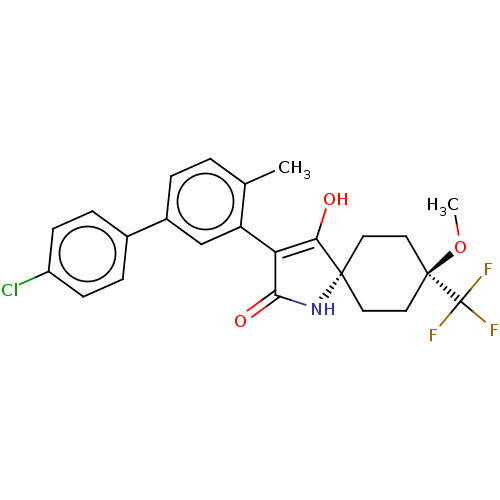 (US9278925, 1-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220130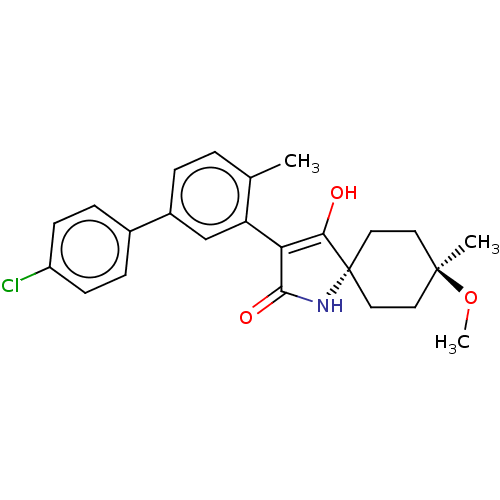 (US9278925, V.1-a | US9278925, V.3-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220130 (US9278925, V.1-a | US9278925, V.3-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220134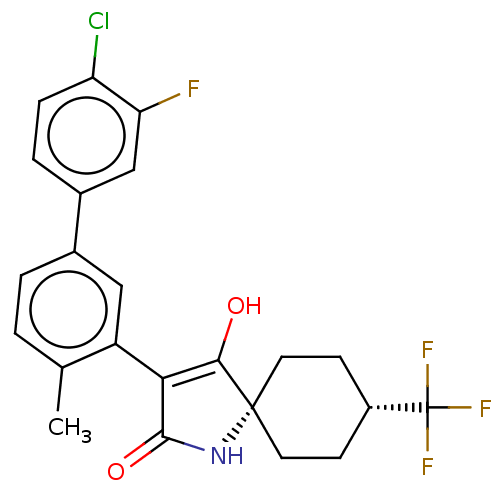 (US9278925, V.2-b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133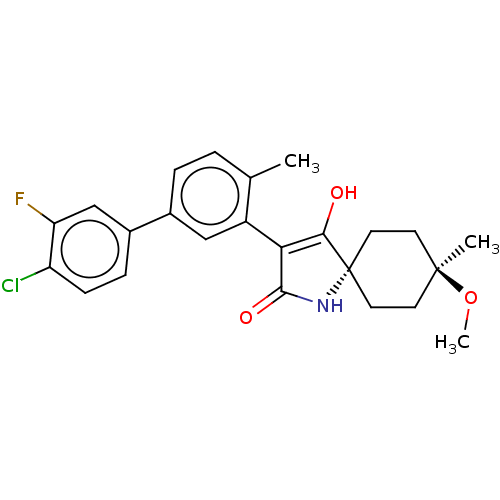 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220140 (US9278925, 1-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220134 (US9278925, V.2-b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220140 (US9278925, 1-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 69 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220139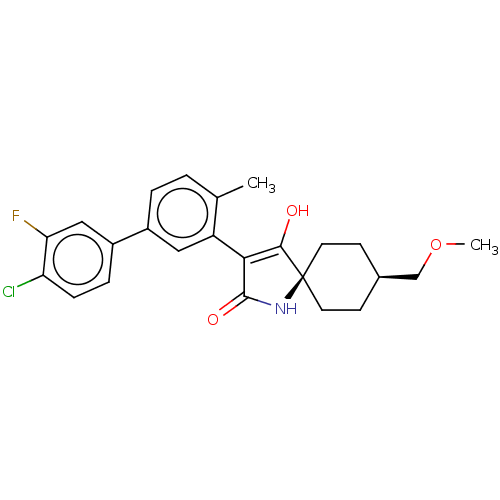 (US9278925, V.4-b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 73 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220140 (US9278925, 1-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 78 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220130 (US9278925, V.1-a | US9278925, V.3-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220130 (US9278925, V.1-a | US9278925, V.3-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-967] (Homo sapiens (Human)) | BDBM196504 (US9212140, C.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bayer Intellectual Property GmbH US Patent | Assay Description Assay B1 (=(B1)): The hACC1-inhibitory action of the substances of the present invention was measured in the hACC 1 assay described in the paragraphs... | US Patent US9212140 (2015) BindingDB Entry DOI: 10.7270/Q22Z14BD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220143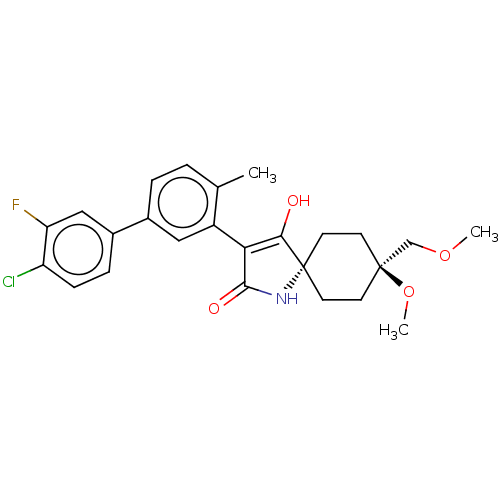 (US9278925, 1-4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220140 (US9278925, 1-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220133 (US9278925, V.2-a | US9278925, V.4-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220130 (US9278925, V.1-a | US9278925, V.3-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220130 (US9278925, V.1-a | US9278925, V.3-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220137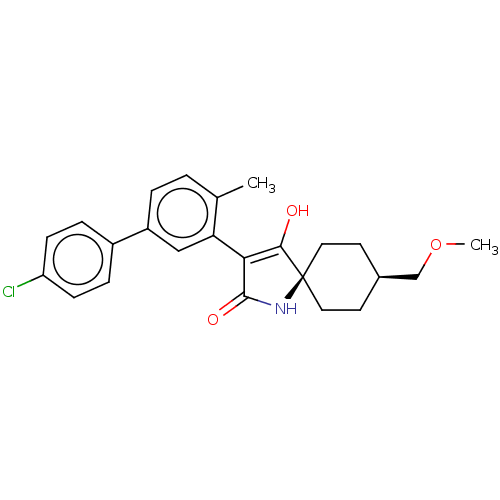 (US9278925, V.3-b) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 92 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220141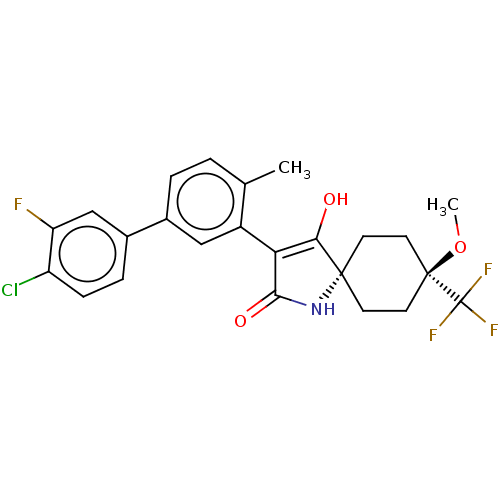 (US9278925, 1-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 92 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220141 (US9278925, 1-2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 94 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220130 (US9278925, V.1-a | US9278925, V.3-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 97 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 [39-2346] (Homo sapiens (Human)) | BDBM220130 (US9278925, V.1-a | US9278925, V.3-a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 97 | n/a | n/a | n/a | n/a | 7.5 | 22 |
BAYER INTELLECTUAL PROPERTY GMBH US Patent | Assay Description For the assay, 50 nl of a 100-times concentrated solution of the test substance in DMSO were pipetted into a white low-volume 384-well microtitre pla... | US Patent US9278925 (2016) BindingDB Entry DOI: 10.7270/Q2VM4B3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 307 total ) | Next | Last >> |