 Found 45 hits with Last Name = 'flaugh' and Initial = 'me'
Found 45 hits with Last Name = 'flaugh' and Initial = 'me' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM85195
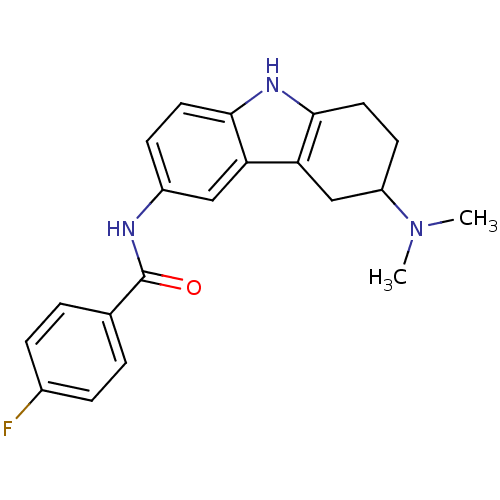
(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(RAT) | BDBM50094670
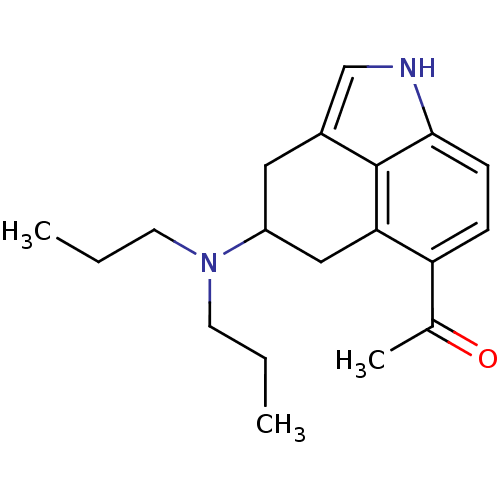
(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 66.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50094670

(1-(4-Dipropylamino-1,3,4,5-tetrahydro-benzo[cd]ind...)Show InChI InChI=1S/C19H26N2O/c1-4-8-21(9-5-2)15-10-14-12-20-18-7-6-16(13(3)22)17(11-15)19(14)18/h6-7,12,15,20H,4-5,8-11H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 270: 1270-81 (1994)
BindingDB Entry DOI: 10.7270/Q2805146 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1E
(Homo sapiens (Human)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(RAT) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(RAT) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM85195

(CAS_5311097 | LY 344864 | NSC_5311097)Show SMILES CN(C)C1CCc2[nH]c3ccc(NC(=O)c4ccc(F)cc4)cc3c2C1 Show InChI InChI=1S/C21H22FN3O/c1-25(2)16-8-10-20-18(12-16)17-11-15(7-9-19(17)24-20)23-21(26)13-3-5-14(22)6-4-13/h3-7,9,11,16,24H,8,10,12H2,1-2H3,(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by PDSP Ki Database
| |
Life Sci 61: 2117-26 (1997)
Article DOI: 10.1016/s0024-3205(97)00885-0
BindingDB Entry DOI: 10.7270/Q2F47MNN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50227629
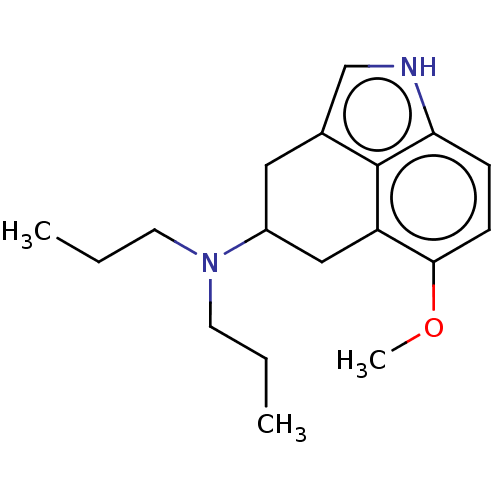
(CHEMBL433084)Show InChI InChI=1S/C18H26N2O/c1-4-8-20(9-5-2)14-10-13-12-19-16-6-7-17(21-3)15(11-14)18(13)16/h6-7,12,14,19H,4-5,8-11H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated serotonin to the 5-hydroxytryptamine 1 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50227633
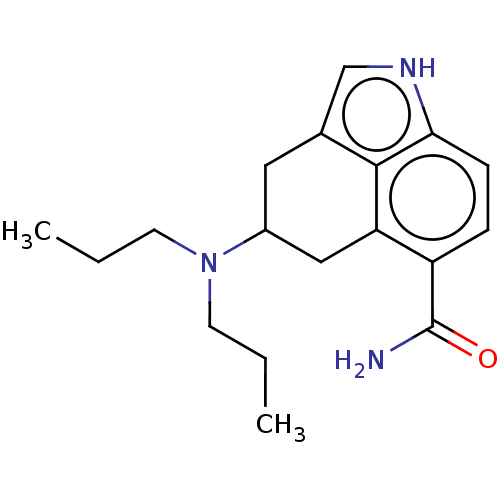
(CHEMBL296753)Show InChI InChI=1S/C18H25N3O/c1-3-7-21(8-4-2)13-9-12-11-20-16-6-5-14(18(19)22)15(10-13)17(12)16/h5-6,11,13,20H,3-4,7-10H2,1-2H3,(H2,19,22) | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated serotonin to the 5-hydroxytryptamine 1 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50227634
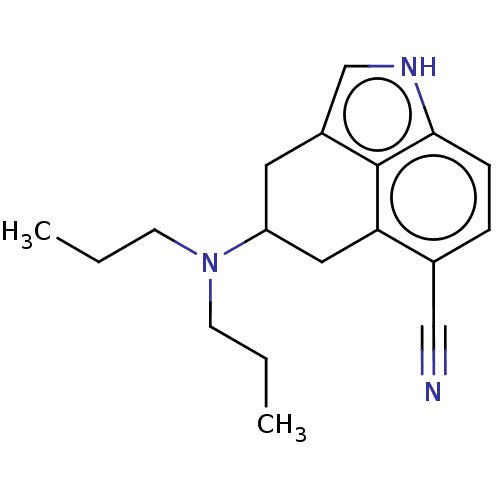
(CHEMBL48617)Show InChI InChI=1S/C18H23N3/c1-3-7-21(8-4-2)15-9-14-12-20-17-6-5-13(11-19)16(10-15)18(14)17/h5-6,12,15,20H,3-4,7-10H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated serotonin to the 5-hydroxytryptamine 1 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50227630
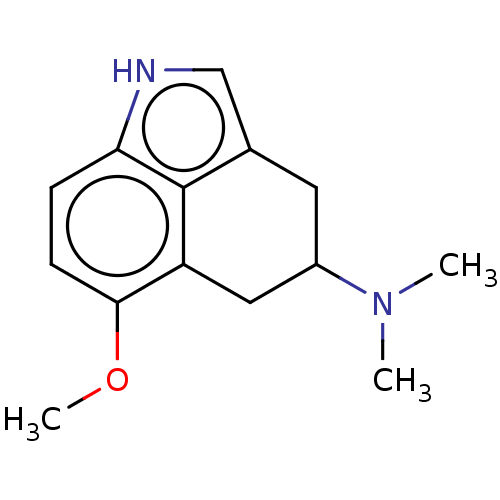
(CHEMBL49309)Show InChI InChI=1S/C14H18N2O/c1-16(2)10-6-9-8-15-12-4-5-13(17-3)11(7-10)14(9)12/h4-5,8,10,15H,6-7H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated serotonin to the 5-hydroxytryptamine 1 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50227635
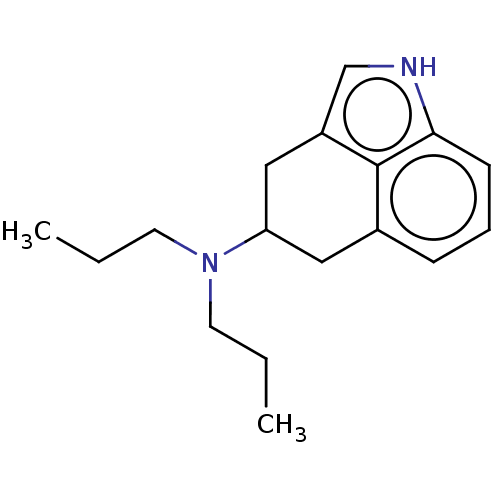
(CHEMBL49027)Show InChI InChI=1S/C17H24N2/c1-3-8-19(9-4-2)15-10-13-6-5-7-16-17(13)14(11-15)12-18-16/h5-7,12,15,18H,3-4,8-11H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated serotonin to the 5-hydroxytryptamine 1 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50227632
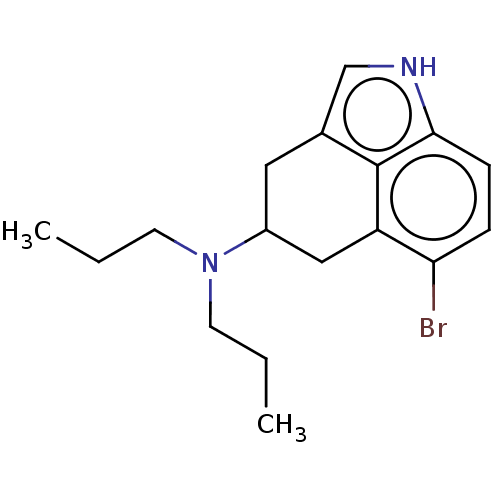
(CHEMBL300246)Show InChI InChI=1S/C17H23BrN2/c1-3-7-20(8-4-2)13-9-12-11-19-16-6-5-15(18)14(10-13)17(12)16/h5-6,11,13,19H,3-4,7-10H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 241 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated serotonin to the 5-hydroxytryptamine 1 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1F
(RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50227636

(CHEMBL300957)Show InChI InChI=1S/C14H17N3O/c1-17(2)9-5-8-7-16-12-4-3-10(14(15)18)11(6-9)13(8)12/h3-4,7,9,16H,5-6H2,1-2H3,(H2,15,18) | PDB
MMDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated serotonin to the 5-hydroxytryptamine 1 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227634

(CHEMBL48617)Show InChI InChI=1S/C18H23N3/c1-3-7-21(8-4-2)15-9-14-12-20-17-6-5-13(11-19)16(10-15)18(14)17/h5-6,12,15,20H,3-4,7-10H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated spiperone to 5-hydroxytryptamine 2 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227635

(CHEMBL49027)Show InChI InChI=1S/C17H24N2/c1-3-8-19(9-4-2)15-10-13-6-5-7-16-17(13)14(11-15)12-18-16/h5-7,12,15,18H,3-4,8-11H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated spiperone to 5-hydroxytryptamine 2 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227629

(CHEMBL433084)Show InChI InChI=1S/C18H26N2O/c1-4-8-20(9-5-2)14-10-13-12-19-16-6-7-17(21-3)15(11-14)18(13)16/h6-7,12,14,19H,4-5,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated spiperone to the 5-hydroxytryptamine 2 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227630

(CHEMBL49309)Show InChI InChI=1S/C14H18N2O/c1-16(2)10-6-9-8-15-12-4-5-13(17-3)11(7-10)14(9)12/h4-5,8,10,15H,6-7H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated spiperone to the 5-hydroxytryptamine 2 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227633

(CHEMBL296753)Show InChI InChI=1S/C18H25N3O/c1-3-7-21(8-4-2)13-9-12-11-20-16-6-5-14(18(19)22)15(10-13)17(12)16/h5-6,11,13,20H,3-4,7-10H2,1-2H3,(H2,19,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated spiperone to the 5-hydroxytryptamine 2 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227636

(CHEMBL300957)Show InChI InChI=1S/C14H17N3O/c1-17(2)9-5-8-7-16-12-4-3-10(14(15)18)11(6-9)13(8)12/h3-4,7,9,16H,5-6H2,1-2H3,(H2,15,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated spiperone to the 5-hydroxytryptamine 2 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50227632

(CHEMBL300246)Show InChI InChI=1S/C17H23BrN2/c1-3-7-20(8-4-2)13-9-12-11-19-16-6-5-15(18)14(10-13)17(12)16/h5-6,11,13,19H,3-4,7-10H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to inhibit binding of titreated spiperone to 5-hydroxytryptamine 2 receptor in rat brain frontal cortex membranes |
J Med Chem 31: 1746-53 (1988)
BindingDB Entry DOI: 10.7270/Q2N58PJQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data