

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50002662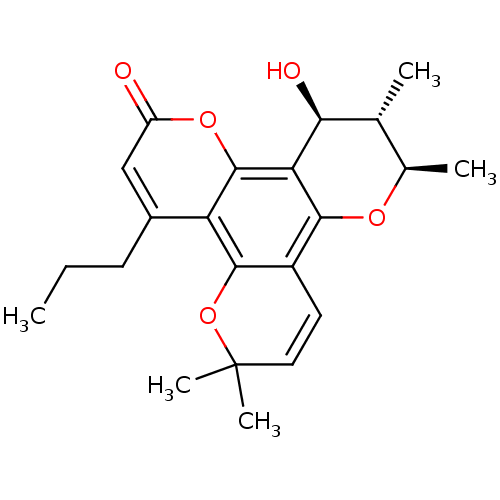 ((+)-calanolide A | (+)-calnolide A | (10R,11S,12S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against DNA -dependent DNA polymerase(DDDP) of HIV-1 RT Nucleic Acid polymerase | J Med Chem 39: 1303-13 (1996) Article DOI: 10.1021/jm950797i BindingDB Entry DOI: 10.7270/Q2WD3ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50002662 ((+)-calanolide A | (+)-calnolide A | (10R,11S,12S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against DNA -dependent DNA polymerase(DDDP) of HIV-1 RT Nucleic Acid polymerase | J Med Chem 39: 1303-13 (1996) Article DOI: 10.1021/jm950797i BindingDB Entry DOI: 10.7270/Q2WD3ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50050430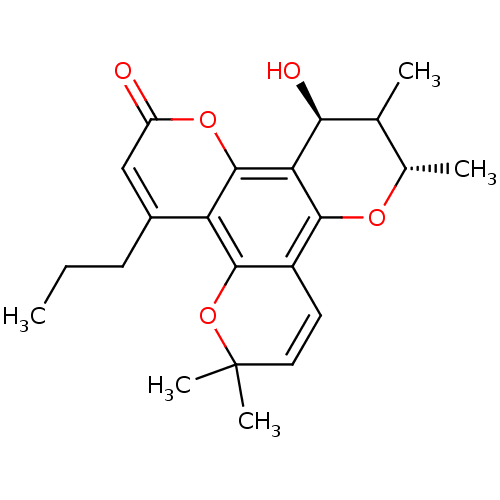 ((10S,12S)-12-Hydroxy-6,6,10,11-tetramethyl-4-propy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against DNA -dependent DNA polymerase(DDDP) of HIV-1 RT Nucleic Acid polymerase | J Med Chem 39: 1303-13 (1996) Article DOI: 10.1021/jm950797i BindingDB Entry DOI: 10.7270/Q2WD3ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50050430 ((10S,12S)-12-Hydroxy-6,6,10,11-tetramethyl-4-propy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against RNA-dependent DNA polymerase(RDDP) of HIV-1 RT Nucleic Acid polymerase | J Med Chem 39: 1303-13 (1996) Article DOI: 10.1021/jm950797i BindingDB Entry DOI: 10.7270/Q2WD3ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50226536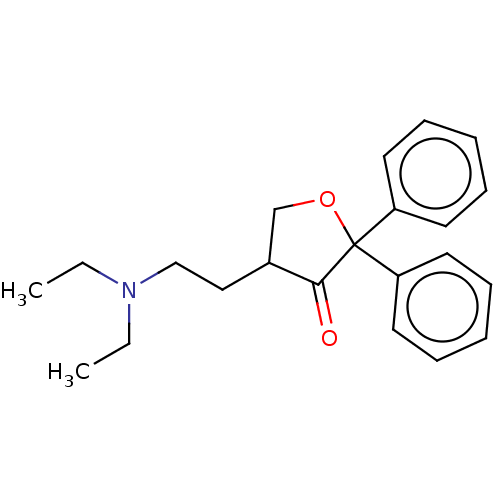 (CHEMBL98190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against sigma receptor | J Med Chem 30: 278-85 (1987) BindingDB Entry DOI: 10.7270/Q2GT5QCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50002662 ((+)-calanolide A | (+)-calnolide A | (10R,11S,12S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against TIBO-resistant HIV-1 RT Nucleic Acid polymerase | J Med Chem 39: 1303-13 (1996) Article DOI: 10.1021/jm950797i BindingDB Entry DOI: 10.7270/Q2WD3ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50336799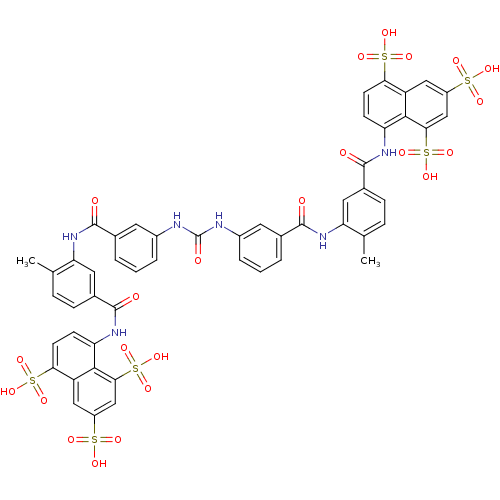 (5,5',5''-[1,3,6-naphthalenetriyltris(sulfonylimino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50050430 ((10S,12S)-12-Hydroxy-6,6,10,11-tetramethyl-4-propy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against TIBO-resistant HIV-1 RT Nucleic Acid polymerase | J Med Chem 39: 1303-13 (1996) Article DOI: 10.1021/jm950797i BindingDB Entry DOI: 10.7270/Q2WD3ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50226537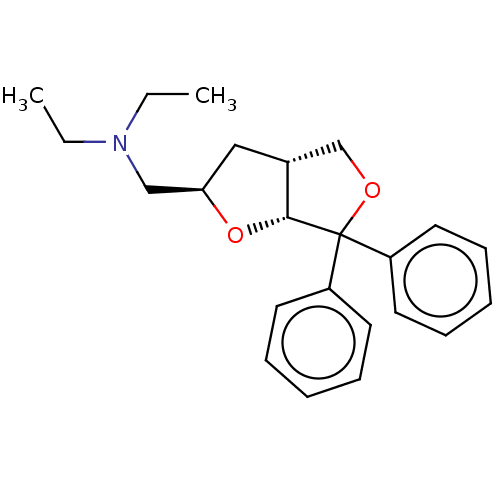 (CHEMBL319799) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 278-85 (1987) BindingDB Entry DOI: 10.7270/Q2GT5QCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50050431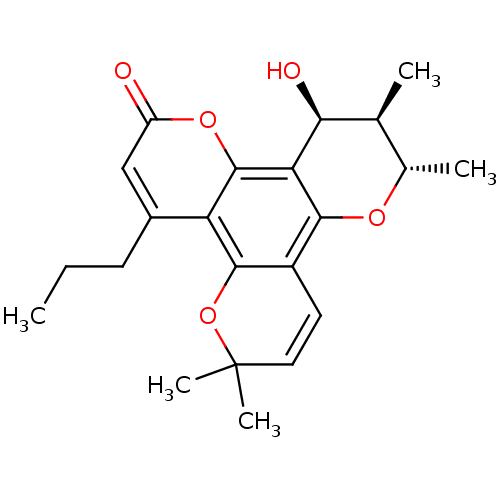 ((-)-calanolide B | (10S,11R,12S)-12-Hydroxy-6,6,10...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against RNA-dependent DNA polymerase(RDDP) of HIV-1 RT Nucleic Acid polymerase | J Med Chem 39: 1303-13 (1996) Article DOI: 10.1021/jm950797i BindingDB Entry DOI: 10.7270/Q2WD3ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50323214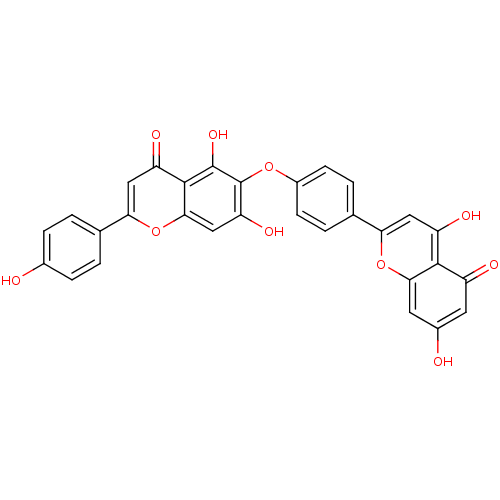 (6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50323212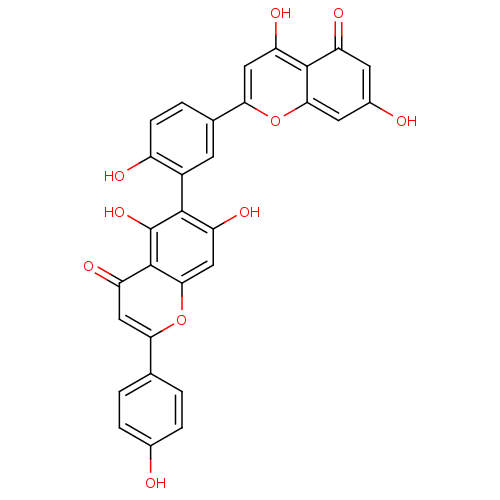 (6-[5-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478418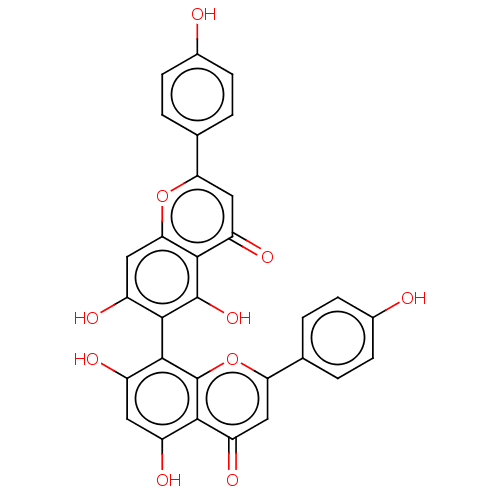 (Agathisflavone | CHEBI:2512) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478419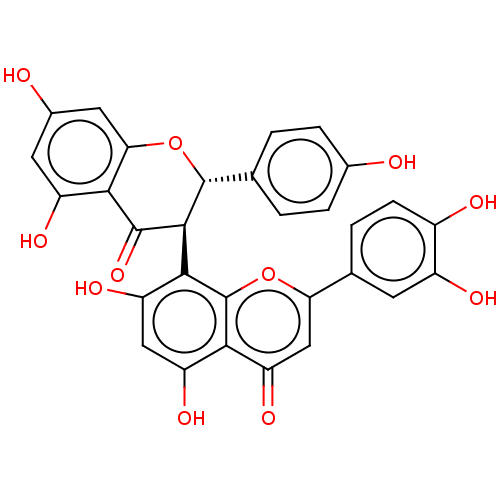 ((+)-Morelloflavone | Morelloflavone) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50129952 (2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50050431 ((-)-calanolide B | (10S,11R,12S)-12-Hydroxy-6,6,10...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against DNA -dependent DNA polymerase(DDDP) of HIV-1 RT Nucleic Acid polymerase | J Med Chem 39: 1303-13 (1996) Article DOI: 10.1021/jm950797i BindingDB Entry DOI: 10.7270/Q2WD3ZN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478421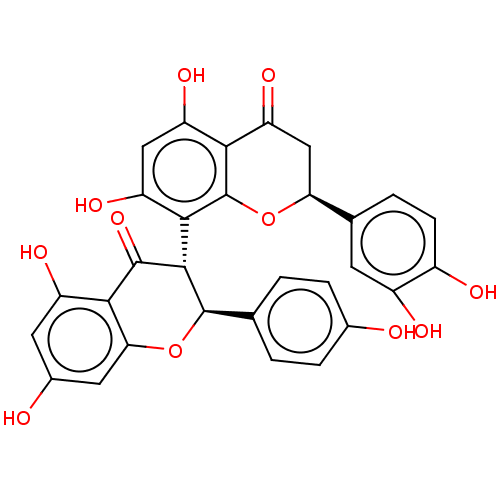 (CHEMBL445452) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50368352 (Cerubidine | DAUNORUBICIN) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478420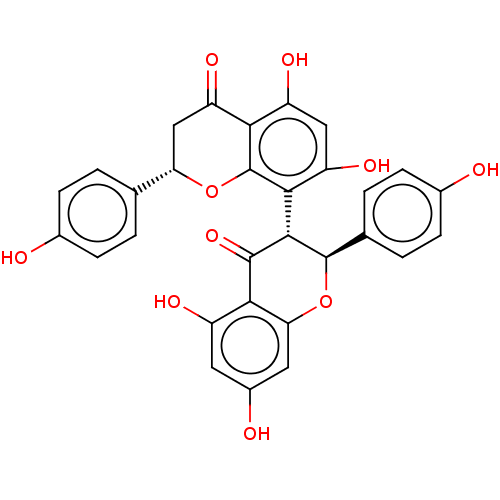 (CHEMBL456859) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant reverse transcriptase p66/p51 expressed in Escherichia coli | J Nat Prod 60: 884-8 (1997) Article DOI: 10.1021/np9700275 BindingDB Entry DOI: 10.7270/Q2JD50MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50002665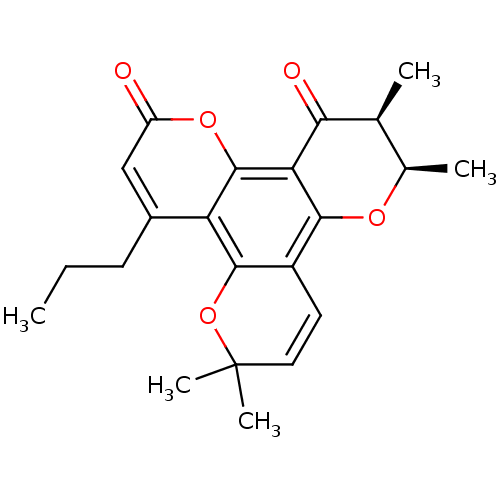 ((10R,11S)-6,6,10,11-Tetramethyl-4-propyl-10,11-dih...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase using a poly(rC):oligo(dG)12-18 template primer | J Med Chem 40: 1005-17 (1997) Article DOI: 10.1021/jm960355m BindingDB Entry DOI: 10.7270/Q2N015NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50002662 ((+)-calanolide A | (+)-calnolide A | (10R,11S,12S)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase using a poly(rC):oligo(dG)12-18 template primer | J Med Chem 40: 1005-17 (1997) Article DOI: 10.1021/jm960355m BindingDB Entry DOI: 10.7270/Q2N015NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057014 ((10R,12R)-12-Hydroxy-6,6,10,11,11-pentamethyl-4-pr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase using a poly(rC):oligo(dG)12-18 template primer | J Med Chem 40: 1005-17 (1997) Article DOI: 10.1021/jm960355m BindingDB Entry DOI: 10.7270/Q2N015NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50054452 ((10R,11R)-6,6,10,11-Tetramethyl-4-propyl-10,11-dih...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase using a poly(rC):oligo(dG)12-18 template primer | J Med Chem 40: 1005-17 (1997) Article DOI: 10.1021/jm960355m BindingDB Entry DOI: 10.7270/Q2N015NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057028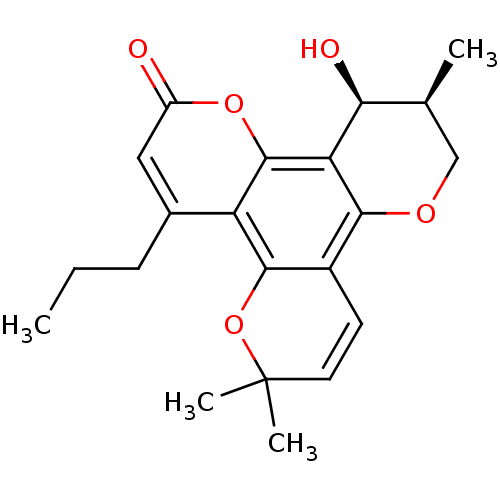 ((11S,12S)-12-Hydroxy-6,6,11-trimethyl-4-propyl-11,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase using a poly(rC):oligo(dG)12-18 template primer | J Med Chem 40: 1005-17 (1997) Article DOI: 10.1021/jm960355m BindingDB Entry DOI: 10.7270/Q2N015NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057037 ((11R,12R)-12-Hydroxy-6,6,10,10,11-pentamethyl-4-pr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase using a poly(rC):oligo(dG)12-18 template primer | J Med Chem 40: 1005-17 (1997) Article DOI: 10.1021/jm960355m BindingDB Entry DOI: 10.7270/Q2N015NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057029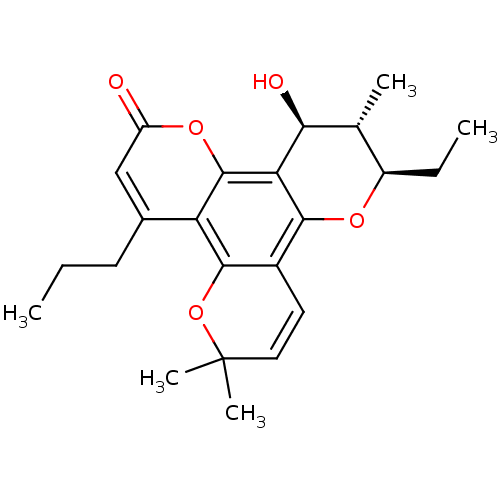 ((10R,11S,12S)-10-Ethyl-12-hydroxy-6,6,11-trimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase using a poly(rC):oligo(dG)12-18 template primer | J Med Chem 40: 1005-17 (1997) Article DOI: 10.1021/jm960355m BindingDB Entry DOI: 10.7270/Q2N015NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057030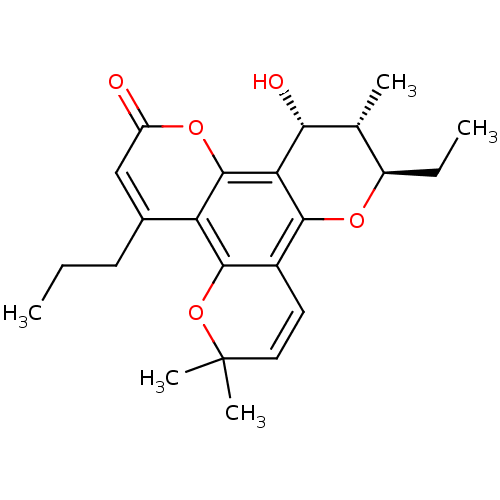 ((10R,11S,12R)-10-Ethyl-12-hydroxy-6,6,11-trimethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
MediChem Research, Inc. Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase using a poly(rC):oligo(dG)12-18 template primer | J Med Chem 40: 1005-17 (1997) Article DOI: 10.1021/jm960355m BindingDB Entry DOI: 10.7270/Q2N015NG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||