 Found 104 hits with Last Name = 'fletcher' and Initial = 'ds'
Found 104 hits with Last Name = 'fletcher' and Initial = 'ds' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50281051

((2S,5R,6S)-6-Ethoxy-3,3-dimethyl-4,4,7-trioxo-4lam...)Show SMILES CCO[C@@H]1[C@@H]2N([C@@H](C(=O)OCc3ccc(cc3)C(O)=O)C(C)(C)S2(=O)=O)C1=O Show InChI InChI=1S/C18H21NO8S/c1-4-26-12-14(20)19-13(18(2,3)28(24,25)15(12)19)17(23)27-9-10-5-7-11(8-6-10)16(21)22/h5-8,12-13,15H,4,9H2,1-3H3,(H,21,22)/t12-,13-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate |
Bioorg Med Chem Lett 3: 2289-2294 (1993)
Article DOI: 10.1016/S0960-894X(01)80941-0
BindingDB Entry DOI: 10.7270/Q2NC614S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50281052

((2S,5R,6S)-3,3-Dimethyl-4,7-dioxo-6-(2,2,2-trifluo...)Show SMILES CC1(C)[C@@H](N2[C@@H]([C@@H](NC(=O)C(F)(F)F)C2=O)S1=O)C(=O)OCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C18H17F3N2O7S/c1-17(2)11(15(27)30-7-8-3-5-9(6-4-8)14(25)26)23-12(24)10(13(23)31(17)29)22-16(28)18(19,20)21/h3-6,10-11,13H,7H2,1-2H3,(H,22,28)(H,25,26)/t10-,11-,13+,31?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate |
Bioorg Med Chem Lett 3: 2289-2294 (1993)
Article DOI: 10.1016/S0960-894X(01)80941-0
BindingDB Entry DOI: 10.7270/Q2NC614S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50281055
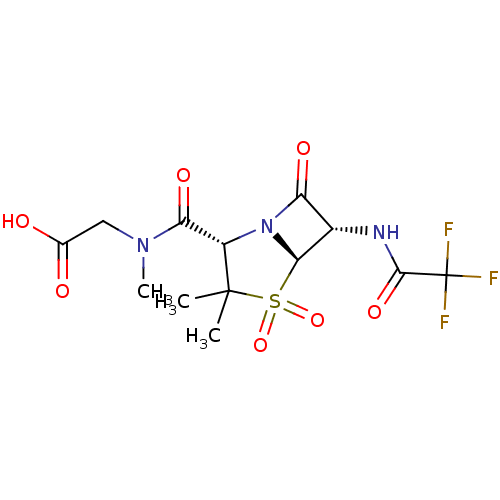
(CHEMBL73926 | {[(2S,5R,6S)-3,3-Dimethyl-4,4,7-trio...)Show SMILES CN(CC(O)=O)C(=O)[C@@H]1N2[C@@H]([C@@H](NC(=O)C(F)(F)F)C2=O)S(=O)(=O)C1(C)C Show InChI InChI=1S/C13H16F3N3O7S/c1-12(2)7(9(23)18(3)4-5(20)21)19-8(22)6(10(19)27(12,25)26)17-11(24)13(14,15)16/h6-7,10H,4H2,1-3H3,(H,17,24)(H,20,21)/t6-,7-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate |
Bioorg Med Chem Lett 3: 2289-2294 (1993)
Article DOI: 10.1016/S0960-894X(01)80941-0
BindingDB Entry DOI: 10.7270/Q2NC614S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50281054

((R)-1-[(2S,5R,6R)-3,3-Dimethyl-4,4,7-trioxo-6-(2,2...)Show SMILES CC1(C)[C@@H](N2[C@@H]([C@H](NC(=O)C(F)(F)F)C2=O)S1(=O)=O)C(=O)N1CCC[C@@H]1C(O)=O Show InChI InChI=1S/C15H18F3N3O7S/c1-14(2)8(10(23)20-5-3-4-6(20)12(24)25)21-9(22)7(11(21)29(14,27)28)19-13(26)15(16,17)18/h6-8,11H,3-5H2,1-2H3,(H,19,26)(H,24,25)/t6-,7-,8+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate |
Bioorg Med Chem Lett 3: 2289-2294 (1993)
Article DOI: 10.1016/S0960-894X(01)80941-0
BindingDB Entry DOI: 10.7270/Q2NC614S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50281056

(CHEMBL307622 | {[(2S,5R,6R)-3,3-Dimethyl-4,4,7-tri...)Show SMILES CN(CC(O)=O)C(=O)[C@@H]1N2[C@@H]([C@H](NC(=O)C(F)(F)F)C2=O)S(=O)(=O)C1(C)C Show InChI InChI=1S/C13H16F3N3O7S/c1-12(2)7(9(23)18(3)4-5(20)21)19-8(22)6(10(19)27(12,25)26)17-11(24)13(14,15)16/h6-7,10H,4H2,1-3H3,(H,17,24)(H,20,21)/t6-,7+,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate |
Bioorg Med Chem Lett 3: 2289-2294 (1993)
Article DOI: 10.1016/S0960-894X(01)80941-0
BindingDB Entry DOI: 10.7270/Q2NC614S |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50031664

(4-((2R,3R)-1-Benzylcarbamoyl-3-methyl-4-oxo-3-prop...)Show SMILES CCC[C@]1(C)[C@@H](Oc2ccc(cc2)C(O)=O)N(C(=O)NCc2ccccc2)C1=O Show InChI InChI=1S/C22H24N2O5/c1-3-13-22(2)19(27)24(21(28)23-14-15-7-5-4-6-8-15)20(22)29-17-11-9-16(10-12-17)18(25)26/h4-12,20H,3,13-14H2,1-2H3,(H,23,28)(H,25,26)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for the human leukocyte elastase (HLE) inhibition, and the Kobs[I] is the second-order rate constant for the time dep... |
J Med Chem 38: 2449-62 (1995)
BindingDB Entry DOI: 10.7270/Q24B30BX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50281053

(CHEMBL430991 | [((2S,5R,6S)-6-Methoxy-3,3-dimethyl...)Show SMILES CO[C@@H]1[C@@H]2N([C@@H](C(=O)N(C)CC(O)=O)C(C)(C)S2(=O)=O)C1=O Show InChI InChI=1S/C12H18N2O7S/c1-12(2)8(10(18)13(3)5-6(15)16)14-9(17)7(21-4)11(14)22(12,19)20/h7-8,11H,5H2,1-4H3,(H,15,16)/t7-,8-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested in vitro for its inhibitory effect against Elastase using Suc-Ala--Ala-Pro-Ala-pNA as substrate |
Bioorg Med Chem Lett 3: 2289-2294 (1993)
Article DOI: 10.1016/S0960-894X(01)80941-0
BindingDB Entry DOI: 10.7270/Q2NC614S |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164777
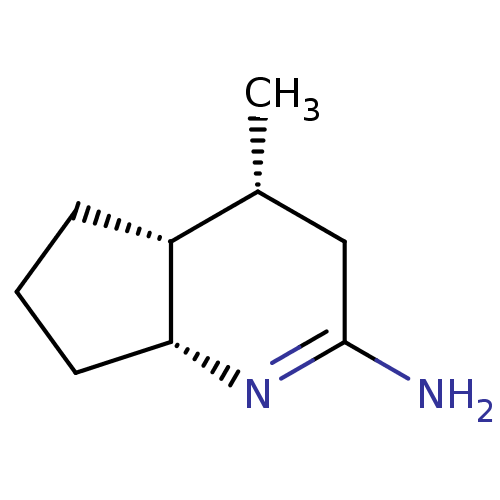
((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50062133
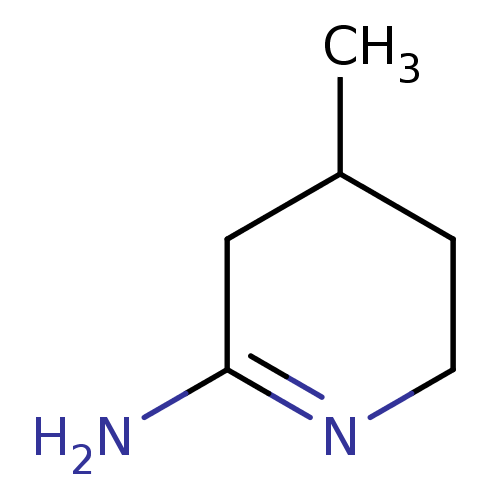
(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50077960

(4-[5-(3,4-Dichloro-phenyl)-2-phenyl-3H-imidazol-4-...)Show SMILES Clc1ccc(cc1Cl)-c1[nH]c(nc1-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C20H13Cl2N3/c21-16-7-6-15(12-17(16)22)19-18(13-8-10-23-11-9-13)24-20(25-19)14-4-2-1-3-5-14/h1-12H,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164782

((4R,4aR,5R)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50077961
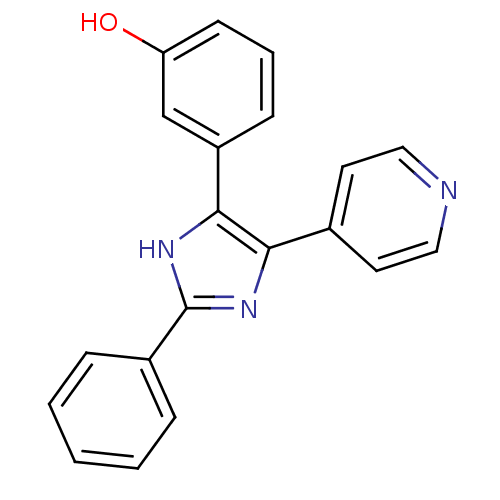
(3-(2-Phenyl-5-pyridin-4-yl-1H-imidazol-4-yl)-pheno...)Show InChI InChI=1S/C20H15N3O/c24-17-8-4-7-16(13-17)19-18(14-9-11-21-12-10-14)22-20(23-19)15-5-2-1-3-6-15/h1-13,24H,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50077957

(4-[2-Cyclohexyl-5-(4-fluoro-phenyl)-3H-imidazol-4-...)Show InChI InChI=1S/C20H20FN3/c21-17-8-6-14(7-9-17)18-19(15-10-12-22-13-11-15)24-20(23-18)16-4-2-1-3-5-16/h6-13,16H,1-5H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164784
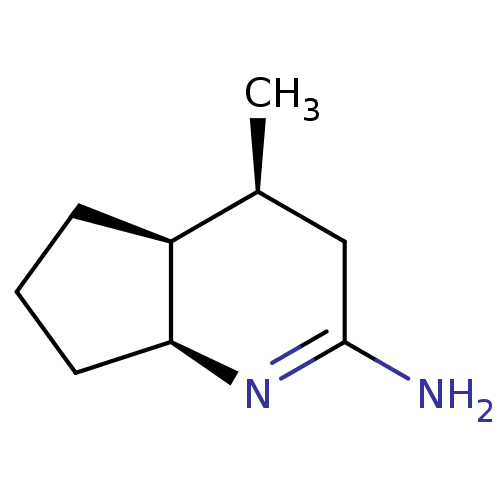
((4S,7S)-4-Methyl-octahydro-[1]pyrindin-(2E)-yliden...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50062133

(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164779
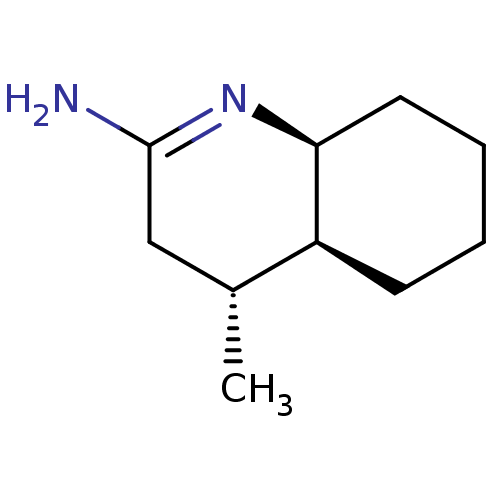
((4R,4aS,5S)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8+,9+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164782

((4R,4aR,5R)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50077974
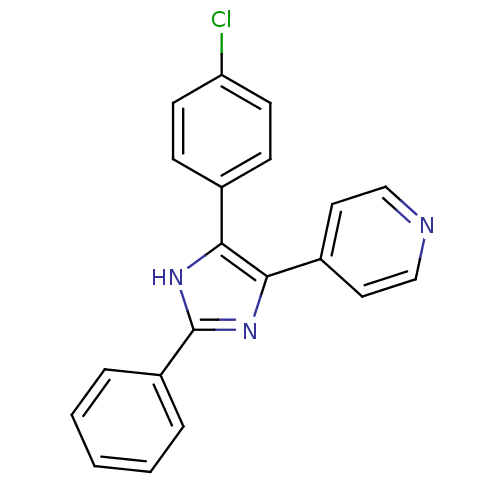
(4-(4-(4-chlorophenyl)-2-phenyl-1H-imidazol-5-yl)py...)Show SMILES Clc1ccc(cc1)-c1[nH]c(nc1-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C20H14ClN3/c21-17-8-6-14(7-9-17)18-19(15-10-12-22-13-11-15)24-20(23-18)16-4-2-1-3-5-16/h1-13H,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50077958
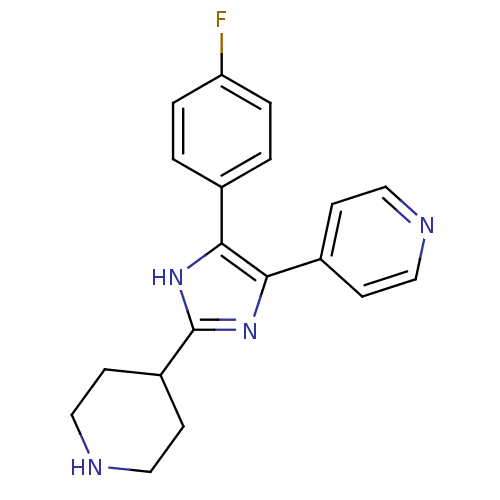
(4-[5-(4-Fluoro-phenyl)-2-piperidin-4-yl-3H-imidazo...)Show InChI InChI=1S/C19H19FN4/c20-16-3-1-13(2-4-16)17-18(14-5-9-21-10-6-14)24-19(23-17)15-7-11-22-12-8-15/h1-6,9-10,15,22H,7-8,11-12H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50077964
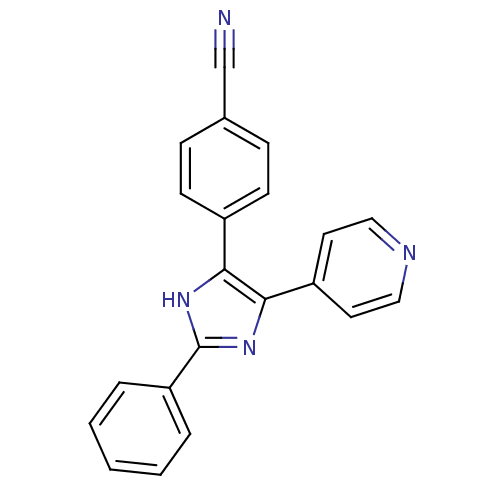
(4-(2-Phenyl-5-pyridin-4-yl-1H-imidazol-4-yl)-benzo...)Show SMILES N#Cc1ccc(cc1)-c1[nH]c(nc1-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C21H14N4/c22-14-15-6-8-16(9-7-15)19-20(17-10-12-23-13-11-17)25-21(24-19)18-4-2-1-3-5-18/h1-13H,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50237712

(4-(4-(3-chlorophenyl)-2-phenyl-1H-imidazol-5-yl)py...)Show SMILES Clc1cccc(c1)-c1[nH]c(nc1-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C20H14ClN3/c21-17-8-4-7-16(13-17)19-18(14-9-11-22-12-10-14)23-20(24-19)15-5-2-1-3-6-15/h1-13H,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50077951
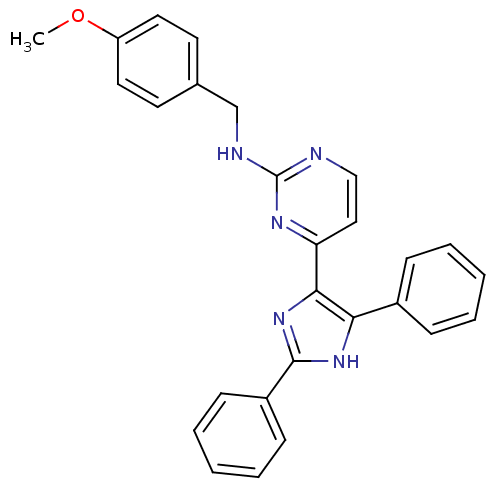
(CHEMBL68572 | [4-(2,5-Diphenyl-3H-imidazol-4-yl)-p...)Show SMILES COc1ccc(CNc2nccc(n2)-c2nc([nH]c2-c2ccccc2)-c2ccccc2)cc1 Show InChI InChI=1S/C27H23N5O/c1-33-22-14-12-19(13-15-22)18-29-27-28-17-16-23(30-27)25-24(20-8-4-2-5-9-20)31-26(32-25)21-10-6-3-7-11-21/h2-17H,18H2,1H3,(H,31,32)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50077979
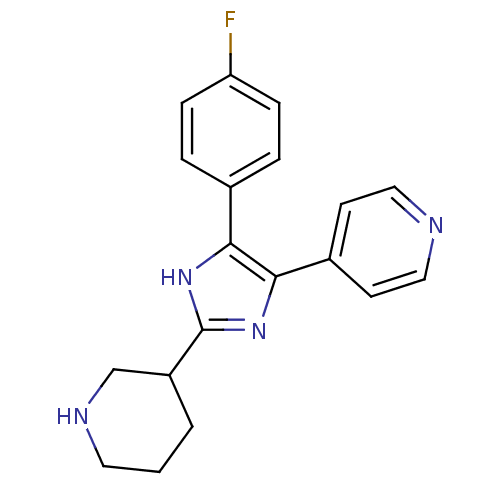
(4-[5-(4-Fluoro-phenyl)-2-piperidin-3-yl-3H-imidazo...)Show InChI InChI=1S/C19H19FN4/c20-16-5-3-13(4-6-16)17-18(14-7-10-21-11-8-14)24-19(23-17)15-2-1-9-22-12-15/h3-8,10-11,15,22H,1-2,9,12H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50077963

(4-(2,5-Diphenyl-3H-imidazol-4-yl)-pyridine | CHEMB...)Show SMILES c1ccc(cc1)-c1nc(c([nH]1)-c1ccccc1)-c1ccncc1 Show InChI InChI=1S/C20H15N3/c1-3-7-15(8-4-1)18-19(16-11-13-21-14-12-16)23-20(22-18)17-9-5-2-6-10-17/h1-14H,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164784

((4S,7S)-4-Methyl-octahydro-[1]pyrindin-(2E)-yliden...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50077957

(4-[2-Cyclohexyl-5-(4-fluoro-phenyl)-3H-imidazol-4-...)Show InChI InChI=1S/C20H20FN3/c21-17-8-6-14(7-9-17)18-19(15-10-12-22-13-11-15)24-20(23-18)16-4-2-1-3-5-16/h6-13,16H,1-5H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50077979

(4-[5-(4-Fluoro-phenyl)-2-piperidin-3-yl-3H-imidazo...)Show InChI InChI=1S/C19H19FN4/c20-16-5-3-13(4-6-16)17-18(14-7-10-21-11-8-14)24-19(23-17)15-2-1-9-22-12-15/h3-8,10-11,15,22H,1-2,9,12H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50062133

(4-Methyl-piperidin-(2E)-ylideneamine | 4-Methyl-pi...)Show InChI InChI=1S/C6H12N2/c1-5-2-3-8-6(7)4-5/h5H,2-4H2,1H3,(H2,7,8) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50077977
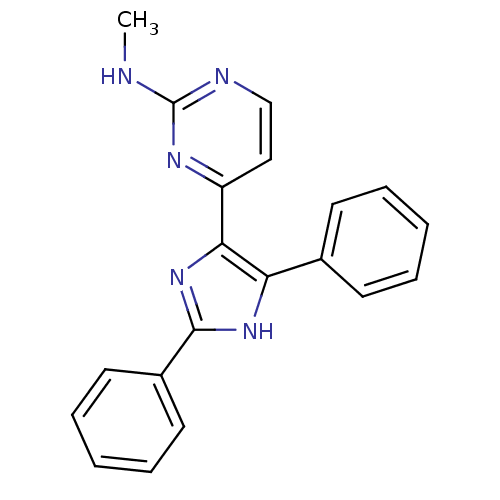
(4-(2,4-diphenyl-1H-imidazol-5-yl)-N-methylpyrimidi...)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H17N5/c1-21-20-22-13-12-16(23-20)18-17(14-8-4-2-5-9-14)24-19(25-18)15-10-6-3-7-11-15/h2-13H,1H3,(H,24,25)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50077962
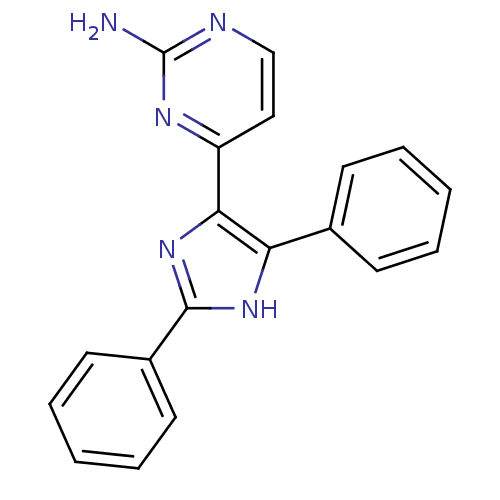
(4-(2,5-Diphenyl-3H-imidazol-4-yl)-pyrimidin-2-ylam...)Show InChI InChI=1S/C19H15N5/c20-19-21-12-11-15(22-19)17-16(13-7-3-1-4-8-13)23-18(24-17)14-9-5-2-6-10-14/h1-12H,(H,23,24)(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50164781
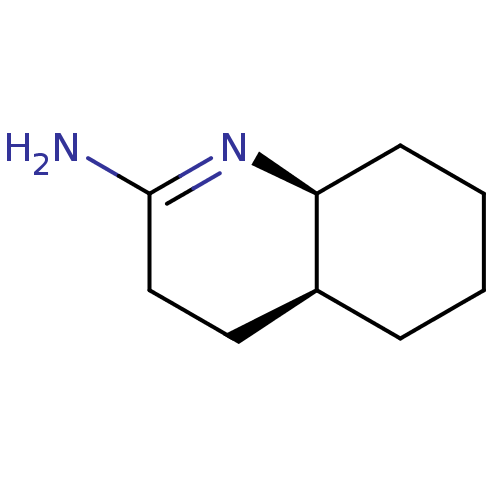
((5S,6S)-[Octahydro-quinolin-(2E)-ylidene]amine | C...)Show InChI InChI=1S/C9H16N2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h7-8H,1-6H2,(H2,10,11)/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164779

((4R,4aS,5S)-4-Methyl-octahydro-quinolin-(2E)-ylide...)Show InChI InChI=1S/C10H18N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h7-9H,2-6H2,1H3,(H2,11,12)/t7-,8+,9+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50053410
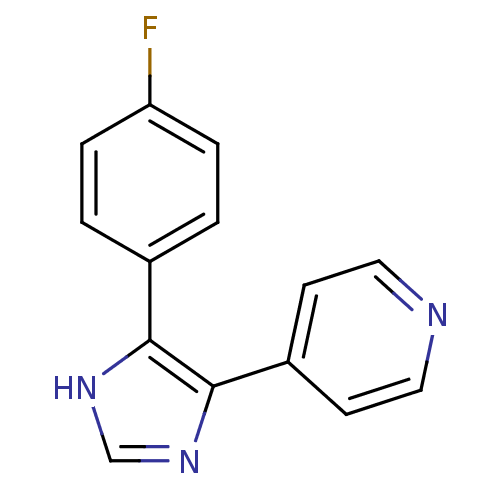
(4-(4-Fluorophenyl)-5-(pyridin-4-yl)-1H-imidazole |...)Show InChI InChI=1S/C14H10FN3/c15-12-3-1-10(2-4-12)13-14(18-9-17-13)11-5-7-16-8-6-11/h1-9H,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50077958

(4-[5-(4-Fluoro-phenyl)-2-piperidin-4-yl-3H-imidazo...)Show InChI InChI=1S/C19H19FN4/c20-16-3-1-13(2-4-16)17-18(14-5-9-21-10-6-14)24-19(23-17)15-7-11-22-12-8-15/h1-6,9-10,15,22H,7-8,11-12H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50077967

(4-(4-(3-methoxyphenyl)-2-phenyl-1H-imidazol-5-yl)p...)Show SMILES COc1cccc(c1)-c1[nH]c(nc1-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C21H17N3O/c1-25-18-9-5-8-17(14-18)20-19(15-10-12-22-13-11-15)23-21(24-20)16-6-3-2-4-7-16/h2-14H,1H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164780
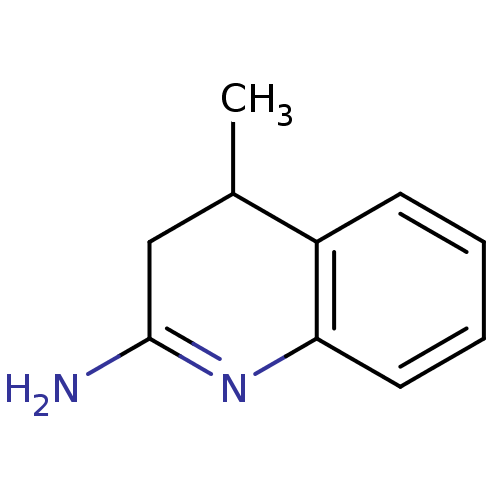
(4-Methyl-3,4-dihydro-1H-quinolin-(2E)-ylideneamine...)Show InChI InChI=1S/C10H12N2/c1-7-6-10(11)12-9-5-3-2-4-8(7)9/h2-5,7H,6H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50049255
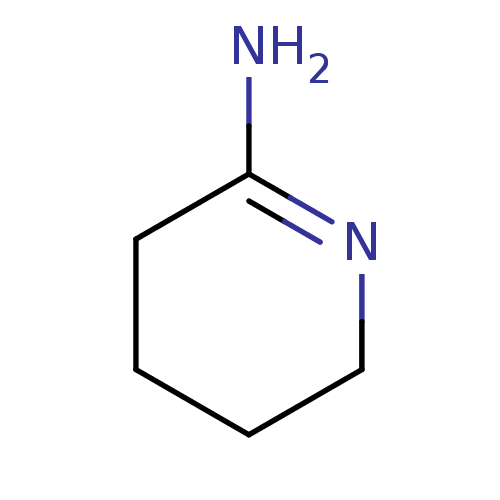
(CHEMBL269058 | PIPERIDIN-2-IMINE | Piperidin-(2E)-...)Show InChI InChI=1S/C5H10N2/c6-5-3-1-2-4-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50164781

((5S,6S)-[Octahydro-quinolin-(2E)-ylidene]amine | C...)Show InChI InChI=1S/C9H16N2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h7-8H,1-6H2,(H2,10,11)/t7-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Neuronal nitric oxide synthase (nNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM13336
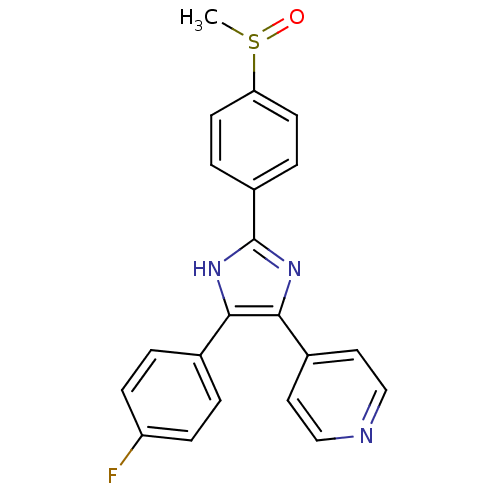
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50077969

(((R)-1-Phenyl-ethyl)-{4-[2-piperidin-4-yl-5-(3-tri...)Show SMILES C[C@@H](Nc1nccc(n1)-c1nc([nH]c1-c1cccc(c1)C(F)(F)F)C1CCNCC1)c1ccccc1 Show InChI InChI=1S/C27H27F3N6/c1-17(18-6-3-2-4-7-18)33-26-32-15-12-22(34-26)24-23(20-8-5-9-21(16-20)27(28,29)30)35-25(36-24)19-10-13-31-14-11-19/h2-9,12,15-17,19,31H,10-11,13-14H2,1H3,(H,35,36)(H,32,33,34)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
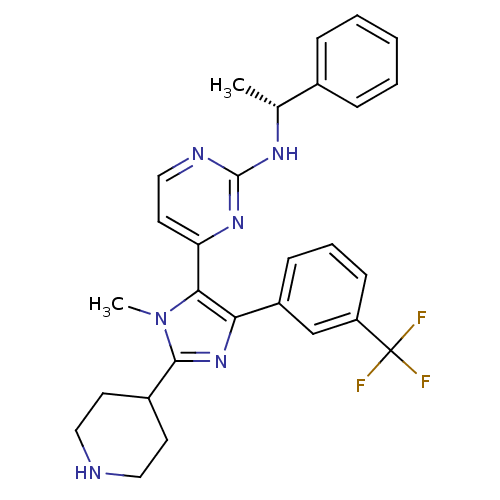
(Homo sapiens (Human)) | BDBM50077953
(4-(1-methyl-2-(piperidin-4-yl)-4-(3-(trifluorometh...)Show SMILES C[C@@H](Nc1nccc(n1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C28H29F3N6/c1-18(19-7-4-3-5-8-19)34-27-33-16-13-23(35-27)25-24(21-9-6-10-22(17-21)28(29,30)31)36-26(37(25)2)20-11-14-32-15-12-20/h3-10,13,16-18,20,32H,11-12,14-15H2,1-2H3,(H,33,34,35)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of c-Jun N-terminal kinase 2-alpha 1 |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50049255

(CHEMBL269058 | PIPERIDIN-2-IMINE | Piperidin-(2E)-...)Show InChI InChI=1S/C5H10N2/c6-5-3-1-2-4-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Inducible nitric oxide synthase (iNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
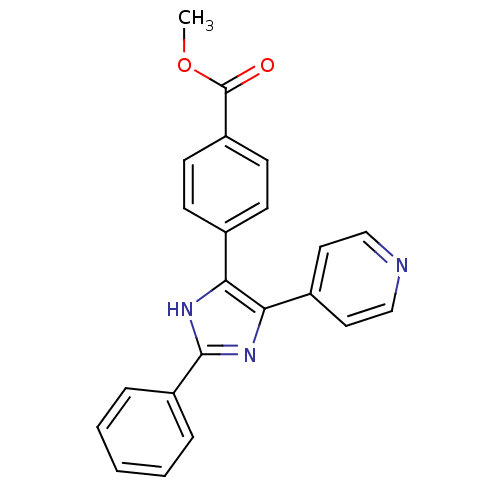
(Homo sapiens (Human)) | BDBM50077959
(4-(2-Phenyl-5-pyridin-4-yl-1H-imidazol-4-yl)-benzo...)Show SMILES COC(=O)c1ccc(cc1)-c1[nH]c(nc1-c1ccncc1)-c1ccccc1 Show InChI InChI=1S/C22H17N3O2/c1-27-22(26)18-9-7-15(8-10-18)19-20(16-11-13-23-14-12-16)25-21(24-19)17-5-3-2-4-6-17/h2-14H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human c-Raf kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM13336

(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human c-Raf kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50077963

(4-(2,5-Diphenyl-3H-imidazol-4-yl)-pyridine | CHEMB...)Show SMILES c1ccc(cc1)-c1nc(c([nH]1)-c1ccccc1)-c1ccncc1 Show InChI InChI=1S/C20H15N3/c1-3-7-15(8-4-1)18-19(16-11-13-21-14-12-16)23-20(22-18)17-9-5-2-6-10-17/h1-14H,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50077976
(4-(2-Phenyl-5-pyridin-4-yl-1H-imidazol-4-yl)-pheno...)Show InChI InChI=1S/C20H15N3O/c24-17-8-6-14(7-9-17)18-19(15-10-12-21-13-11-15)23-20(22-18)16-4-2-1-3-5-16/h1-13,24H,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human RAF proto-oncogene serine/threonine-protein kinase |
J Med Chem 42: 2180-90 (1999)
Article DOI: 10.1021/jm9805236
BindingDB Entry DOI: 10.7270/Q2Q81C8D |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50164777

((4R,4aR,7aR)-4-Methyl-octahydro-[1]pyrindin-(2E)-y...)Show InChI InChI=1S/C9H16N2/c1-6-5-9(10)11-8-4-2-3-7(6)8/h6-8H,2-5H2,1H3,(H2,10,11)/t6-,7-,8-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Endothelial nitric oxide synthase (eNOS) |
Bioorg Med Chem Lett 15: 1997-2001 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.067
BindingDB Entry DOI: 10.7270/Q2XK8F3M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data