

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM36514 (CID46912122 | MDW933, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | -48.4 | 1.24 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University | Assay Description Enzymatic activity assay using fluorescent activity-based labeling method. | Nat Chem Biol 6: 907-13 (2010) Article DOI: 10.1038/nchembio.466 BindingDB Entry DOI: 10.7270/Q2FX77ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM36515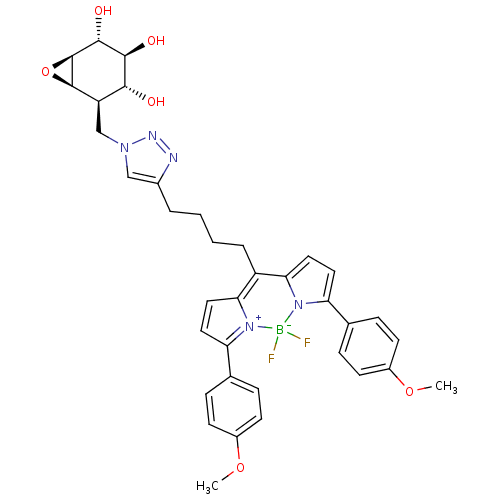 (CID46912120 | MDW941, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -48.1 | 1.94 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University | Assay Description Enzymatic activity assay using fluorescent activity-based labeling method. | Nat Chem Biol 6: 907-13 (2010) Article DOI: 10.1038/nchembio.466 BindingDB Entry DOI: 10.7270/Q2FX77ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM36513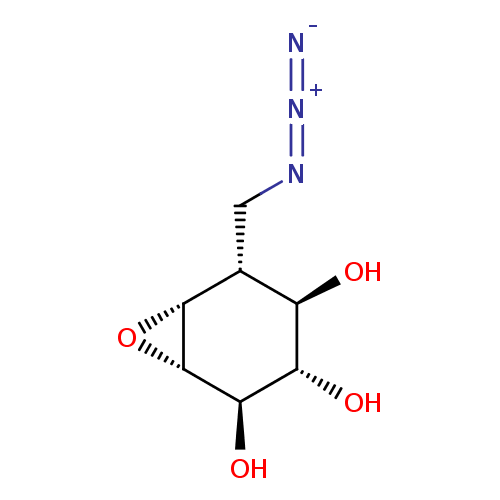 (CID46912128 | KY170, 4 | US9056847, Azido-cyclophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 44 | -43.7 | 120 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University | Assay Description Enzymatic activity assay using fluorescent activity-based labeling method. | Nat Chem Biol 6: 907-13 (2010) Article DOI: 10.1038/nchembio.466 BindingDB Entry DOI: 10.7270/Q2FX77ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM36512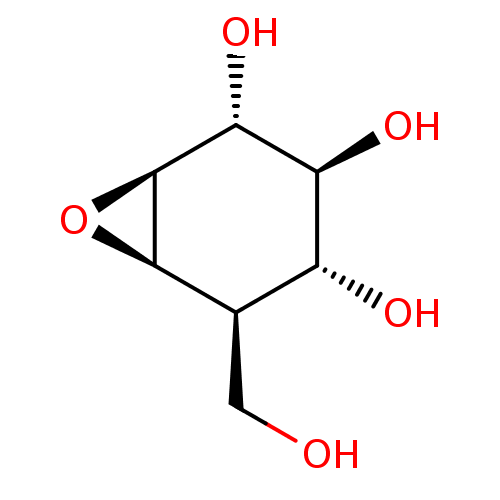 (CID164227 | Cylcophellitol, 3 | US11826435, Compou...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 152 | -40.5 | 150 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University | Assay Description Enzymatic activity assay using fluorescent activity-based labeling method. | Nat Chem Biol 6: 907-13 (2010) Article DOI: 10.1038/nchembio.466 BindingDB Entry DOI: 10.7270/Q2FX77ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50078114 ((1S,2R,3S,4S,5R,6R)-7-Oxa-bicyclo[4.1.0]heptane-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.30E+4 | -25.4 | 9.49E+3 | n/a | n/a | n/a | n/a | 5.2 | 37 |
Leiden University | Assay Description Enzymatic activity assay using fluorescent activity-based labeling method. | Nat Chem Biol 6: 907-13 (2010) Article DOI: 10.1038/nchembio.466 BindingDB Entry DOI: 10.7270/Q2FX77ST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of 26S proteasome beta 5 using LLVY as substrate | J Med Chem 53: 2319-23 (2010) Article DOI: 10.1021/jm9015685 BindingDB Entry DOI: 10.7270/Q2WS8TCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536438 (CHEMBL4534488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536438 (CHEMBL4534488) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307482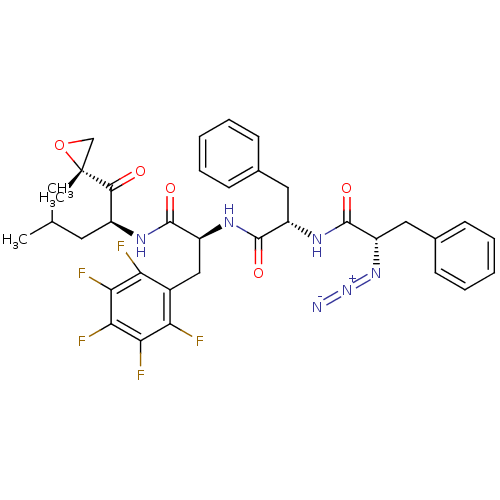 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of 26S proteasome beta 5 using LLVY as substrate | J Med Chem 53: 2319-23 (2010) Article DOI: 10.1021/jm9015685 BindingDB Entry DOI: 10.7270/Q2WS8TCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536425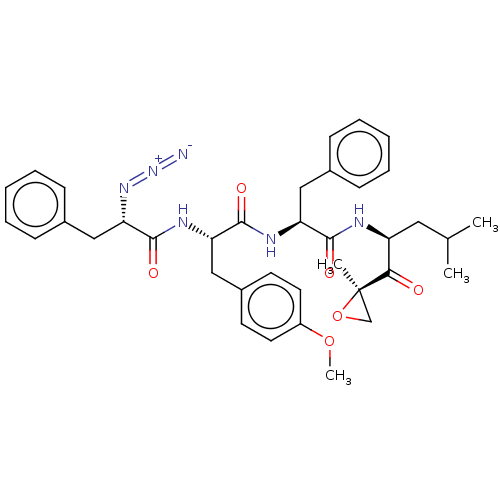 (CHEMBL4563298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536429 (CHEMBL4581491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536425 (CHEMBL4563298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536429 (CHEMBL4581491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307480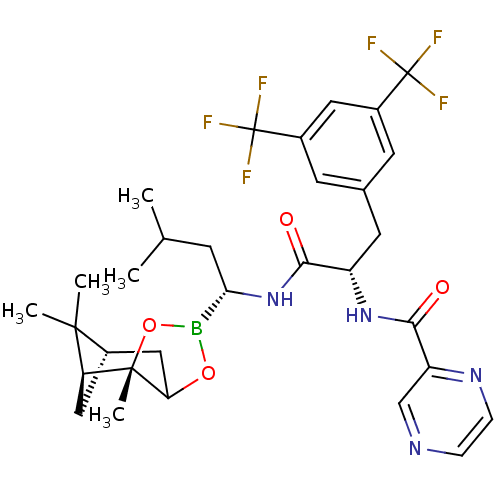 (CHEMBL579296 | Pyrazine-2-carboxylic acid(1-[3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of 26S proteasome beta 5 using LLVY as substrate | J Med Chem 53: 2319-23 (2010) Article DOI: 10.1021/jm9015685 BindingDB Entry DOI: 10.7270/Q2WS8TCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536442 (CHEMBL4570216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536439 (CHEMBL4581001) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50517643 (CHEMBL4451682) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of 20S constitutive proteasome beta 2 trypsin-like activity in human Raji cell lysates after 1 hr by competitive ABPP assay | J Med Chem 62: 1626-1642 (2019) Article DOI: 10.1021/acs.jmedchem.8b01884 BindingDB Entry DOI: 10.7270/Q28P63XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536431 (CHEMBL4519899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50307481 ((S)-2-azido-N-((S)-1-((S)-1-((S)-4-methyl-1-((R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50517609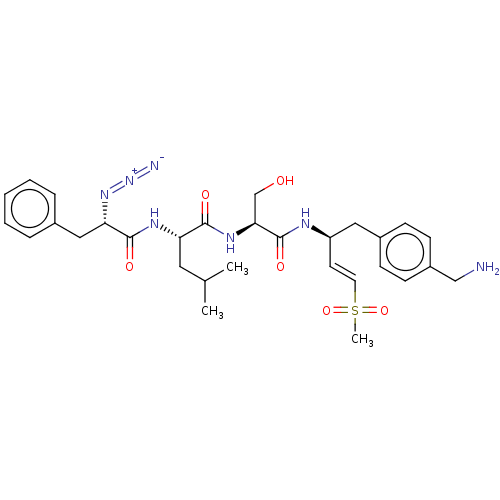 (CHEMBL4440581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of 20S constitutive proteasome beta 2 trypsin-like activity in human Raji cell lysates after 1 hr by competitive ABPP assay | J Med Chem 62: 1626-1642 (2019) Article DOI: 10.1021/acs.jmedchem.8b01884 BindingDB Entry DOI: 10.7270/Q28P63XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50517607 (CHEMBL4590398) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of 20S constitutive proteasome beta 2 trypsin-like activity in human Raji cell lysates after 1 hr by competitive ABPP assay | J Med Chem 62: 1626-1642 (2019) Article DOI: 10.1021/acs.jmedchem.8b01884 BindingDB Entry DOI: 10.7270/Q28P63XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536425 (CHEMBL4563298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536425 (CHEMBL4563298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50517607 (CHEMBL4590398) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of 20S constitutive proteasome beta 2 trypsin-like activity (unknown origin) by cell based ABPP assay | J Med Chem 62: 1626-1642 (2019) Article DOI: 10.1021/acs.jmedchem.8b01884 BindingDB Entry DOI: 10.7270/Q28P63XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50517617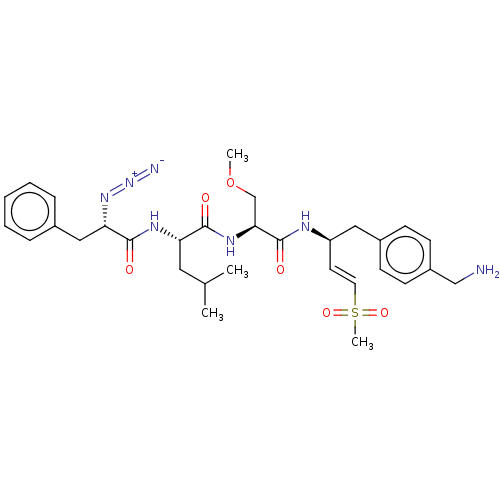 (CHEMBL4458756) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of 20S constitutive proteasome beta 2 trypsin-like activity (unknown origin) by cell based ABPP assay | J Med Chem 62: 1626-1642 (2019) Article DOI: 10.1021/acs.jmedchem.8b01884 BindingDB Entry DOI: 10.7270/Q28P63XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536451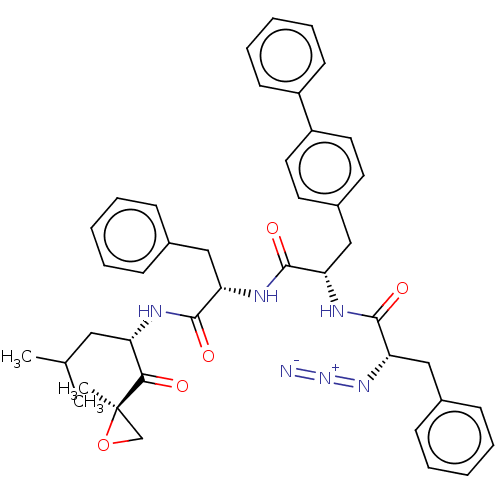 (CHEMBL4528476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536452 (CHEMBL4574722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536453 (CHEMBL4587878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536450 (CHEMBL4522697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536451 (CHEMBL4528476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536453 (CHEMBL4587878) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536437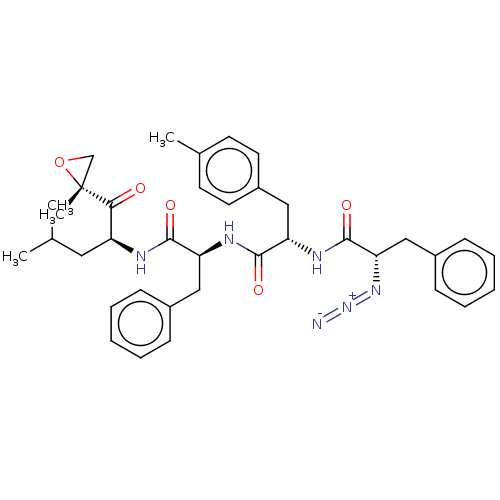 (CHEMBL4588350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-2 (Homo sapiens (Human)) | BDBM50517643 (CHEMBL4451682) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry and Netherlands Proteomics Centre Curated by ChEMBL | Assay Description Inhibition of 20S constitutive proteasome beta 2 trypsin-like activity (unknown origin) by cell based ABPP assay | J Med Chem 62: 1626-1642 (2019) Article DOI: 10.1021/acs.jmedchem.8b01884 BindingDB Entry DOI: 10.7270/Q28P63XM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536457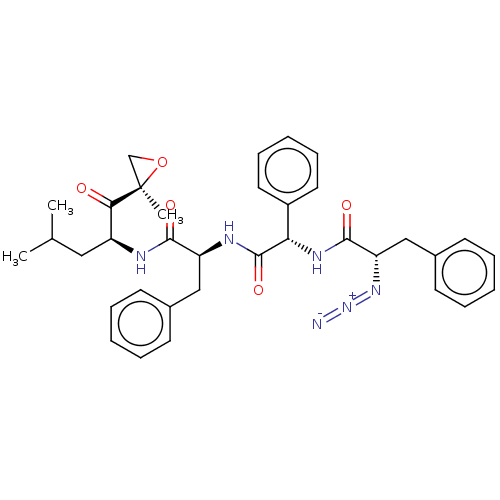 (CHEMBL4555159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536452 (CHEMBL4574722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536440 (CHEMBL4560006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536458 (CHEMBL4556673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536436 (CHEMBL4556581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536458 (CHEMBL4556673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536457 (CHEMBL4555159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of immunoproteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe based co... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536428 (CHEMBL4556631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536430 (CHEMBL4543267) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536436 (CHEMBL4556581) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50536437 (CHEMBL4588350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden Institute of Chemistry Curated by ChEMBL | Assay Description Inhibition of catalytic activity of constitutive proteasome 20s subunit beta5 in human Raji cells incubated for 1 hr by BODIPY(TMR)-NC-005-VS probe b... | J Med Chem 59: 7177-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00705 BindingDB Entry DOI: 10.7270/Q2Z60SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 583 total ) | Next | Last >> |