

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244360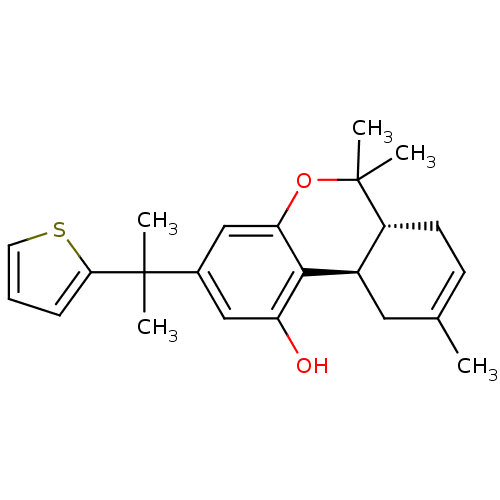 ((6aR,10aR)-6,6,9-trimethyl-3-(2-(thiophen-2-yl)pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem 17: 2598-606 (2009) Article DOI: 10.1016/j.bmc.2008.11.059 BindingDB Entry DOI: 10.7270/Q2DJ5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075926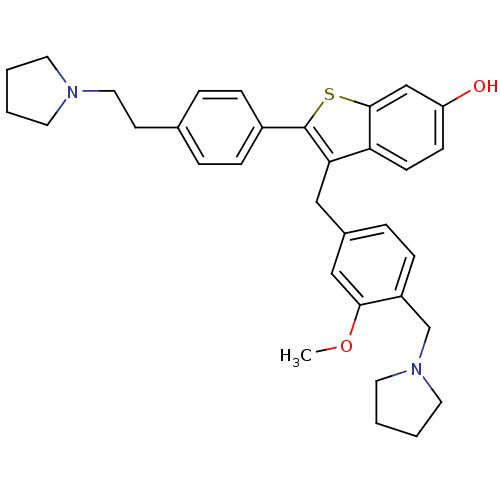 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050511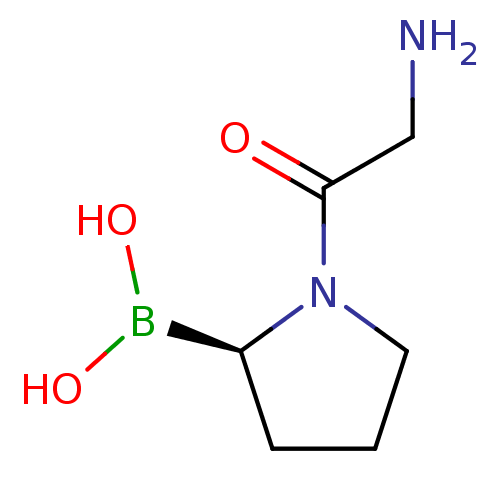 ((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075934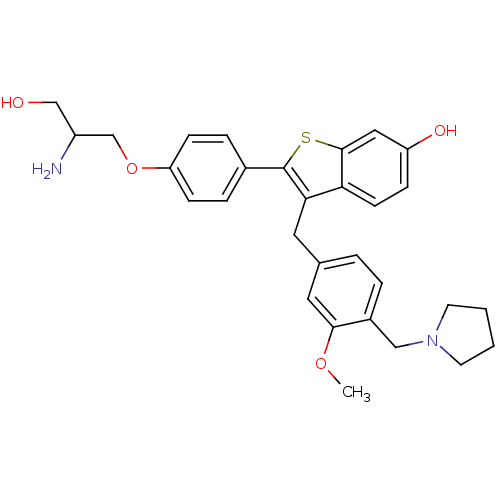 (2-[4-(2-Amino-3-hydroxy-propoxy)-phenyl]-3-(3-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50050511 ((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075937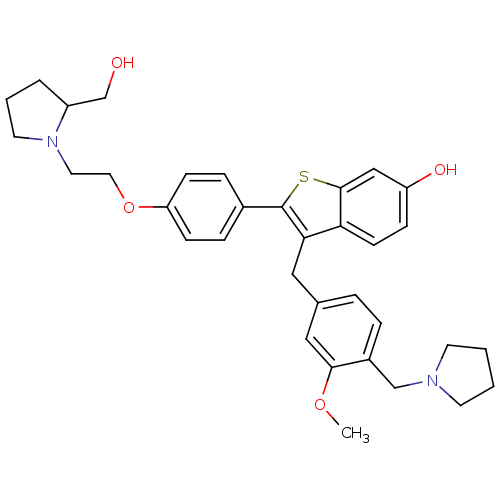 (2-{4-[2-(2-Hydroxymethyl-pyrrolidin-1-yl)-ethoxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075928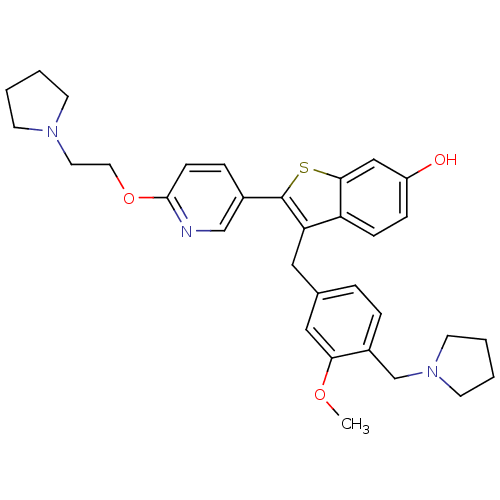 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244359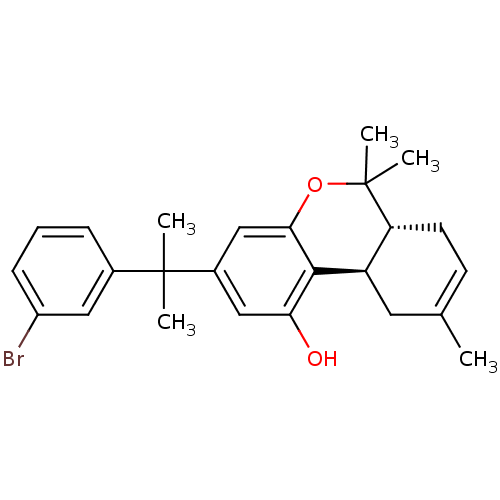 ((6aR,10aR)-3-(2-(3-bromophenyl)propan-2-yl)-6,6,9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem 17: 2598-606 (2009) Article DOI: 10.1016/j.bmc.2008.11.059 BindingDB Entry DOI: 10.7270/Q2DJ5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109106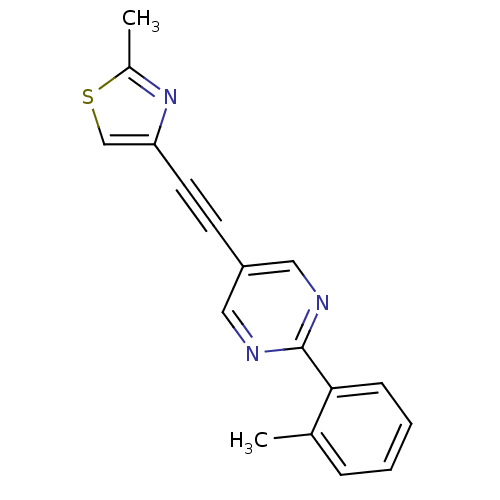 (US8609852, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109136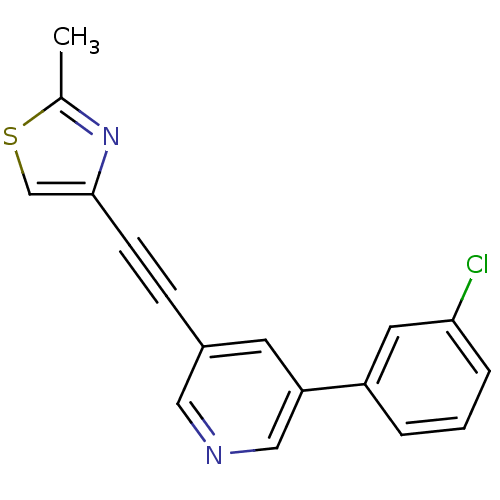 (US8609852, 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.650 | -52.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50172117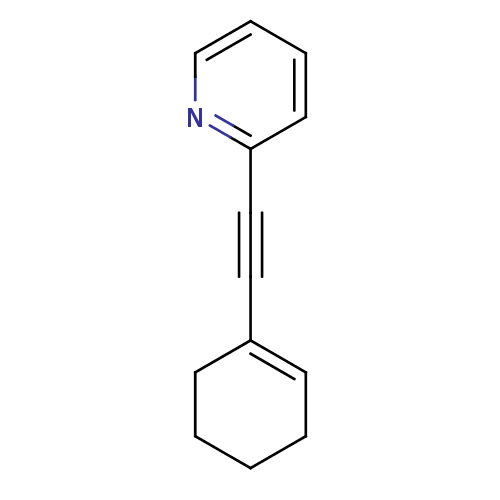 (2-Cyclohex-1-enylethynyl-pyridine | CHEMBL196643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5 receptor of rat cortical membrane | Bioorg Med Chem Lett 15: 4589-93 (2005) Article DOI: 10.1016/j.bmcl.2005.06.099 BindingDB Entry DOI: 10.7270/Q2DB81C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244284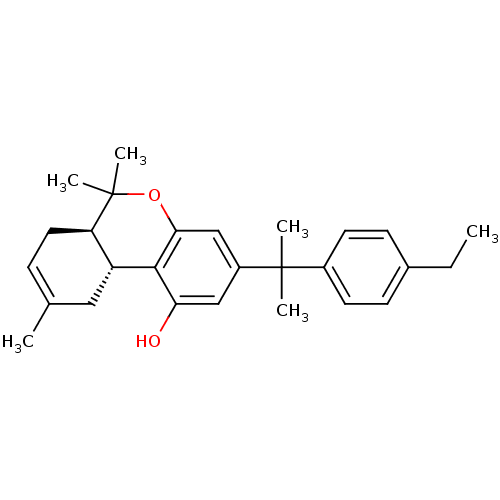 ((6aR,10aR)-3-(2-(4-ethylphenyl)propan-2-yl)-6,6,9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem 17: 2598-606 (2009) Article DOI: 10.1016/j.bmc.2008.11.059 BindingDB Entry DOI: 10.7270/Q2DJ5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109101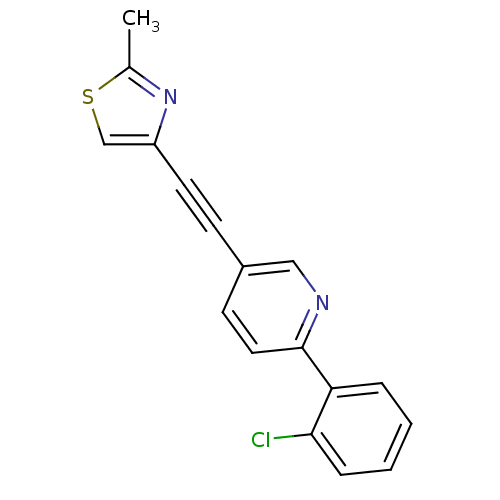 (US8609852, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431227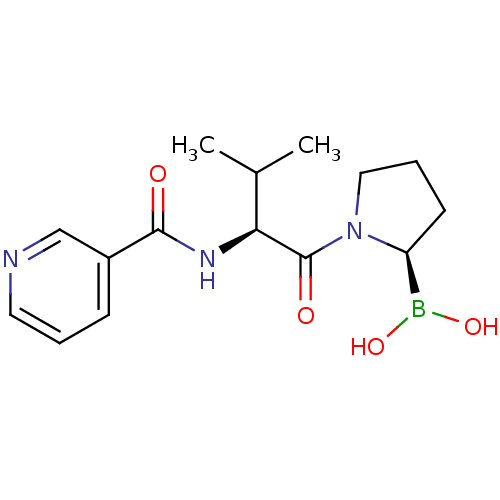 (CHEMBL2333024) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of human PREP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22170 ((2S)-1-(4-{2-[bis(4-fluorophenyl)methoxy]ethyl}pip...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity for dopamine transporter | J Med Chem 45: 1321-9 (2002) BindingDB Entry DOI: 10.7270/Q2K073KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109157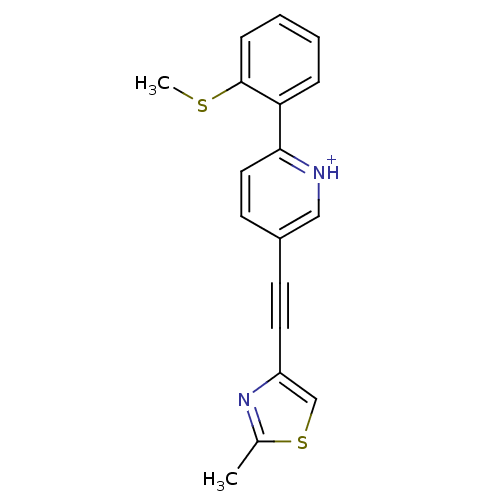 (US8609852, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50172136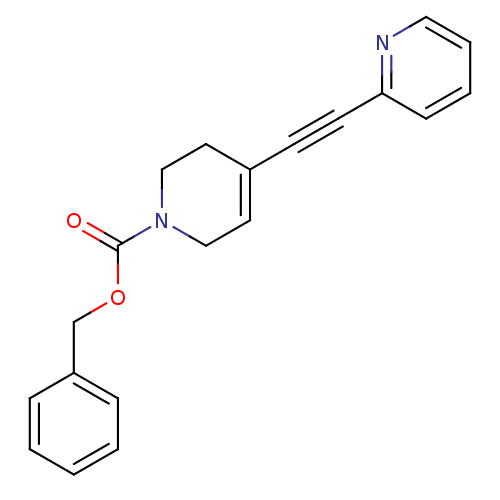 (4-Pyridin-2-ylethynyl-3,6-dihydro-2H-pyridine-1-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5 receptor of rat cortical membrane | Bioorg Med Chem Lett 15: 4589-93 (2005) Article DOI: 10.1016/j.bmcl.2005.06.099 BindingDB Entry DOI: 10.7270/Q2DB81C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244252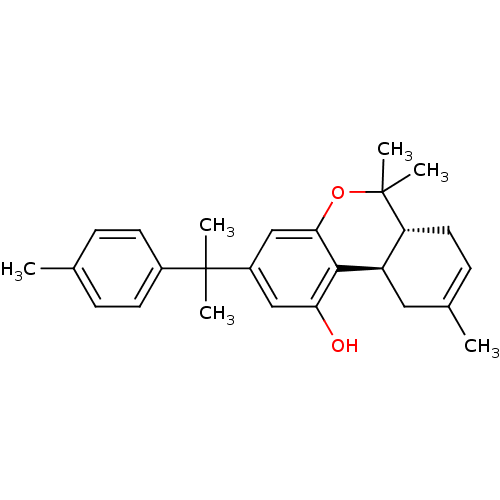 ((6aR,10aR)-6,6,9-trimethyl-3-(2-p-tolylpropan-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem 17: 2598-606 (2009) Article DOI: 10.1016/j.bmc.2008.11.059 BindingDB Entry DOI: 10.7270/Q2DJ5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075938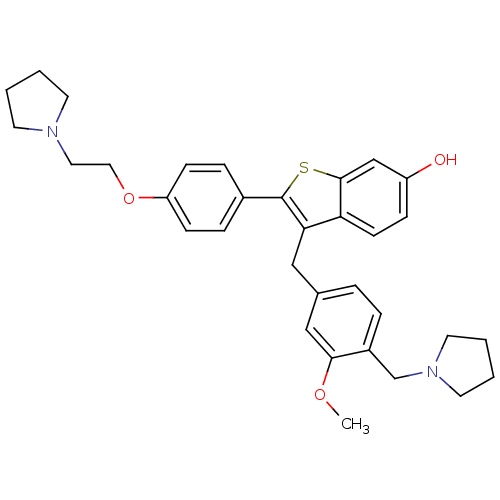 (3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50075932 (2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-N-{4-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition | Bioorg Med Chem Lett 9: 775-80 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244322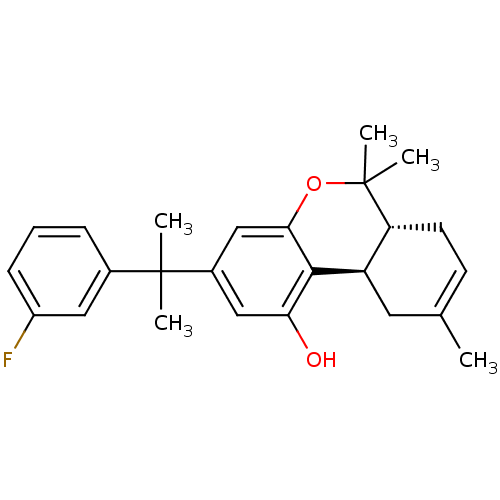 ((6aR,10aR)-3-(2-(3-fluorophenyl)propan-2-yl)-6,6,9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem 17: 2598-606 (2009) Article DOI: 10.1016/j.bmc.2008.11.059 BindingDB Entry DOI: 10.7270/Q2DJ5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM22199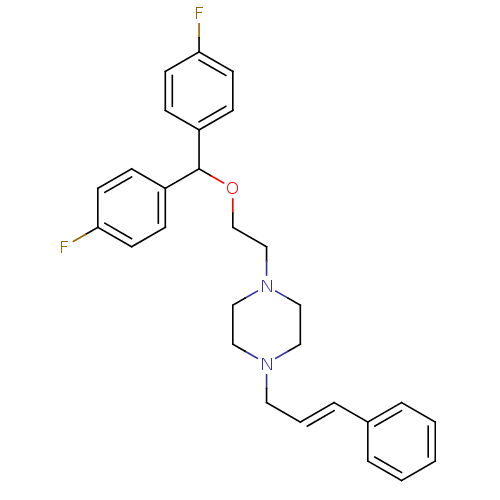 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-[(2E)-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity against dopamine transporter labelled with [125I]- RTI-55 in rat. | J Med Chem 42: 5029-42 (2000) BindingDB Entry DOI: 10.7270/Q2R49PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109121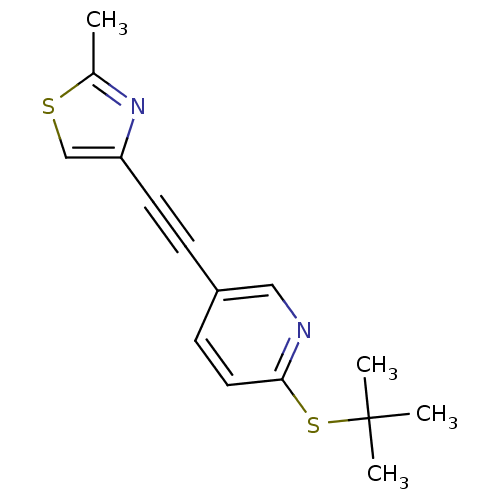 (US8609852, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109115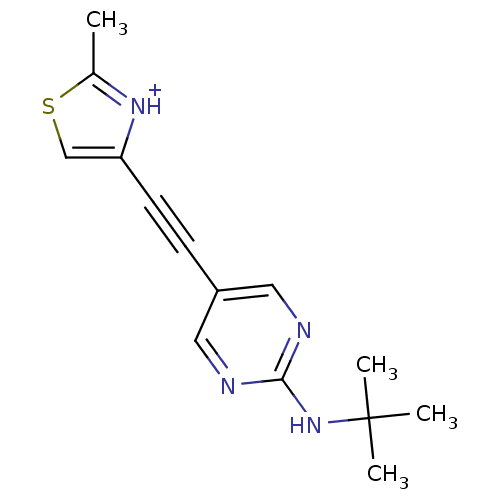 (US8609852, 117 | US8609852, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109102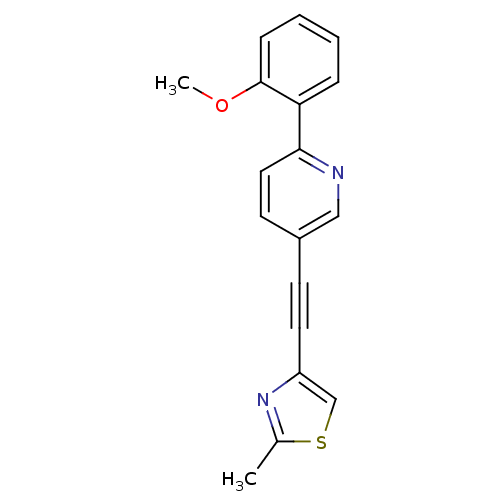 (US8609852, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50133546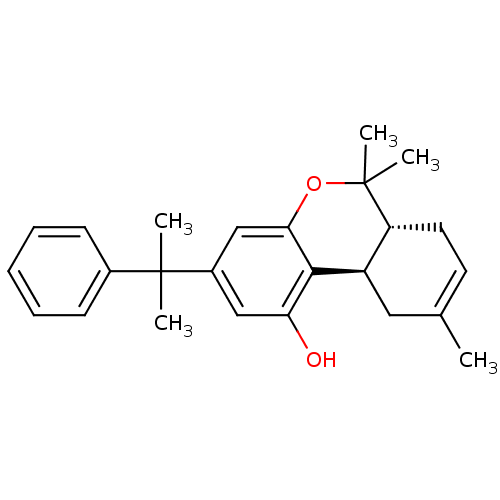 ((6aR,10aR)-6,6,9-Trimethyl-3-(1-methyl-1-phenyl-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem 17: 2598-606 (2009) Article DOI: 10.1016/j.bmc.2008.11.059 BindingDB Entry DOI: 10.7270/Q2DJ5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50243718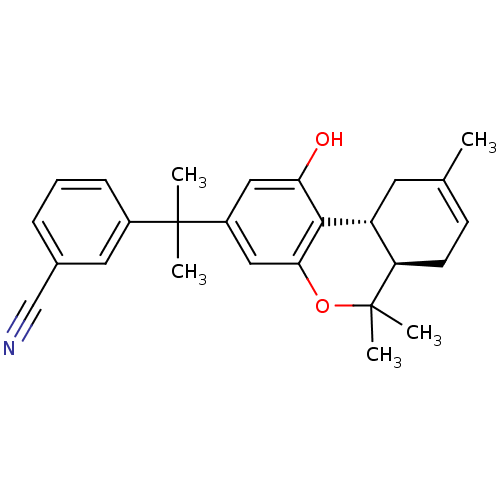 (3-(2-((6aR,10aR)-1-hydroxy-6,6,9-trimethyl-6a,7,10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem 17: 2598-606 (2009) Article DOI: 10.1016/j.bmc.2008.11.059 BindingDB Entry DOI: 10.7270/Q2DJ5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Rattus norvegicus (rat)) | BDBM50083214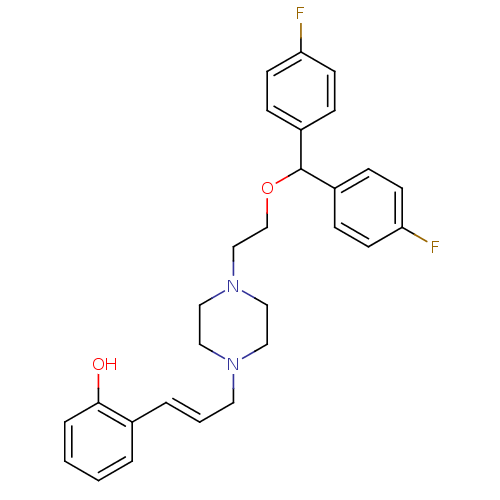 (2-[(E)-3-(4-{2-[Bis-(4-fluoro-phenyl)-methoxy]-eth...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity against dopamine transporter labelled with [125I]- RTI-55 in rat. | J Med Chem 42: 5029-42 (2000) BindingDB Entry DOI: 10.7270/Q2R49PZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109115 (US8609852, 117 | US8609852, 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50149792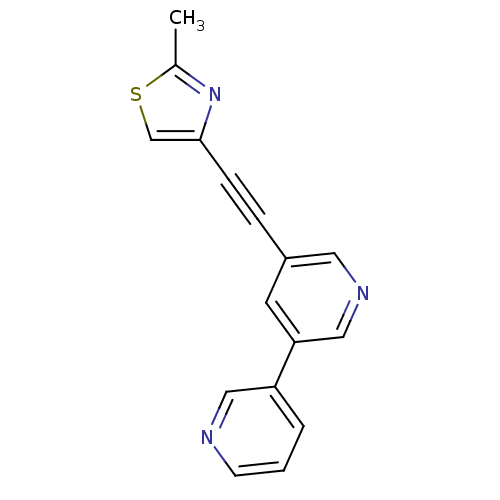 (5-(2-Methyl-thiazol-4-ylethynyl)-[3,3'']bipyridiny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement by compound of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat cortical membranes | Bioorg Med Chem Lett 14: 3993-6 (2004) Article DOI: 10.1016/j.bmcl.2004.05.037 BindingDB Entry DOI: 10.7270/Q2T43SKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109182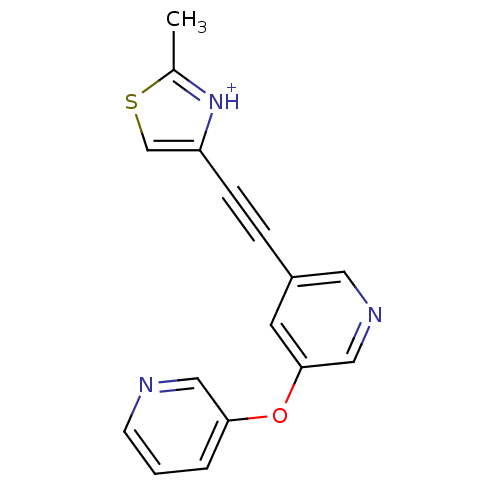 (US8609852, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109176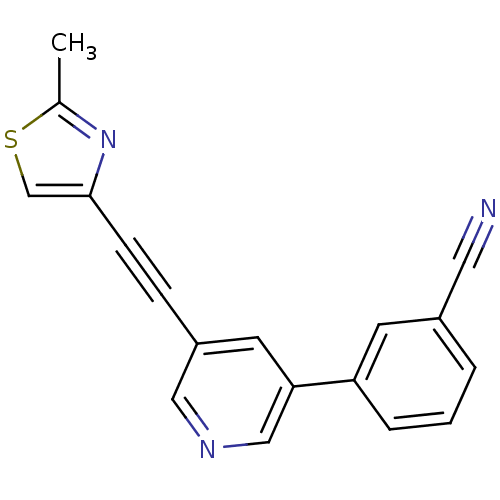 (US8609852, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109120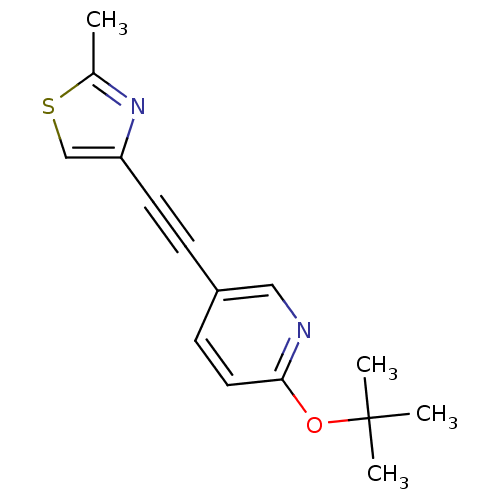 (US8609852, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109099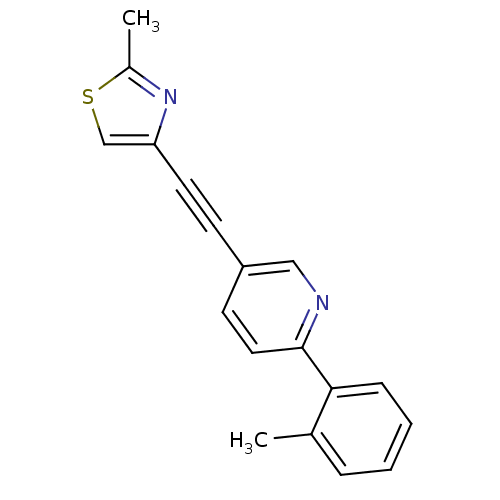 (US8609852, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50244360 ((6aR,10aR)-6,6,9-trimethyl-3-(2-(thiophen-2-yl)pro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor (unknown origin) | Bioorg Med Chem 17: 2598-606 (2009) Article DOI: 10.1016/j.bmc.2008.11.059 BindingDB Entry DOI: 10.7270/Q2DJ5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109105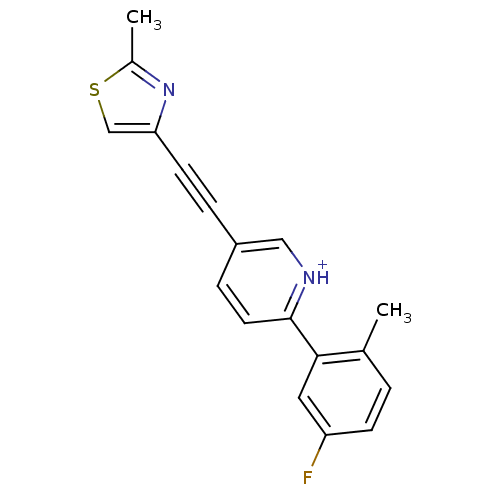 (US8609852, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50172123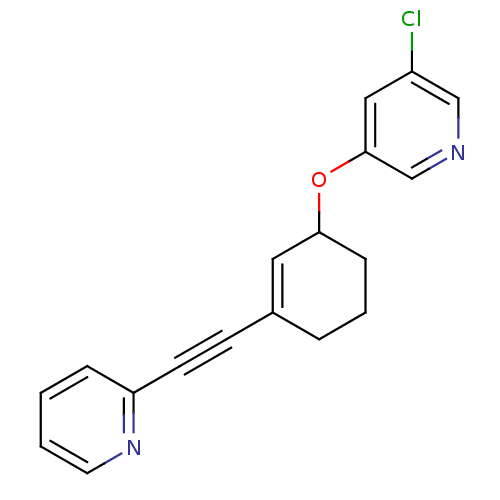 (3-Chloro-5-(3-pyridin-2-ylethynyl-cyclohex-2-enylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5 receptor of rat cortical membrane | Bioorg Med Chem Lett 15: 4589-93 (2005) Article DOI: 10.1016/j.bmcl.2005.06.099 BindingDB Entry DOI: 10.7270/Q2DB81C5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244281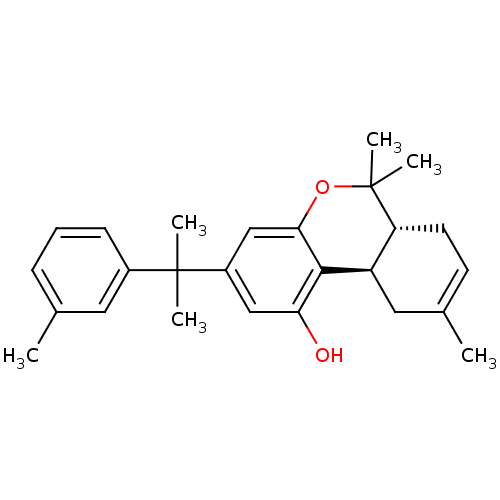 ((6aR,10aR)-6,6,9-trimethyl-3-(2-m-tolylpropan-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem 17: 2598-606 (2009) Article DOI: 10.1016/j.bmc.2008.11.059 BindingDB Entry DOI: 10.7270/Q2DJ5FHV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109100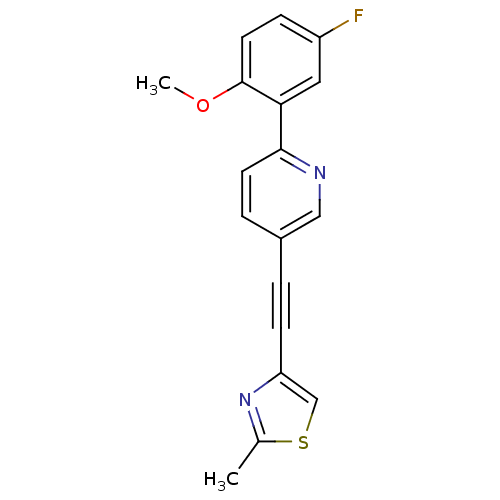 (US8609852, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50181753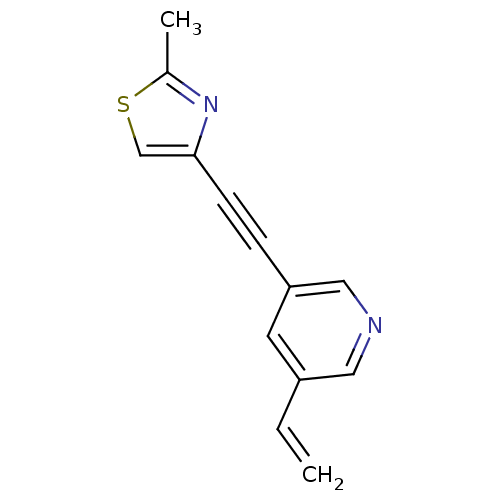 (3-[(2-methyl-4-thiazolyl)ethynyl]-5-vinylpyridine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109113 (US8609852, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109151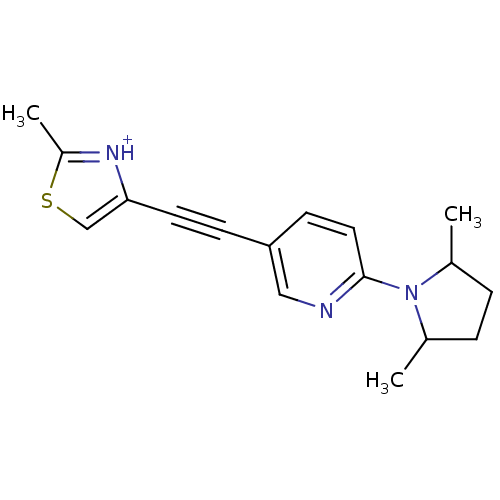 (US8609852, 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109147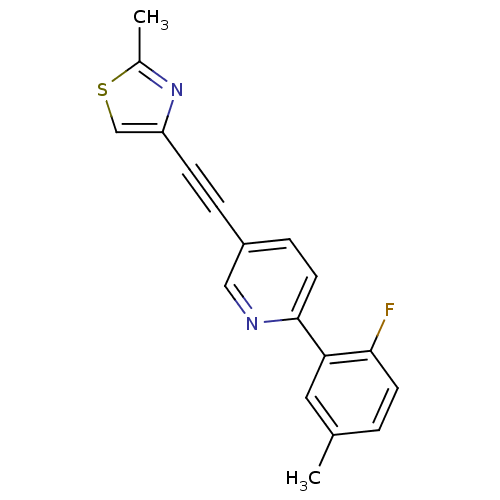 (US8609852, 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM109114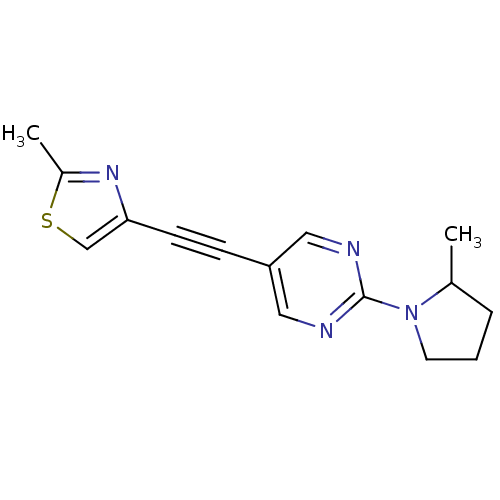 (US8609852, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.5 | -50.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Binding assays were performed as described in Schaffhauser H, Richards J G, Cartmell J, Chaboz S, Kemp J A, Klingelschmidt A, Messer J, Stadler H, Wo... | US Patent US8609852 (2013) BindingDB Entry DOI: 10.7270/Q27M06K3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7830 total ) | Next | Last >> |