 Found 229 hits with Last Name = 'forrest' and Initial = 'ak'
Found 229 hits with Last Name = 'forrest' and Initial = 'ak' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172639
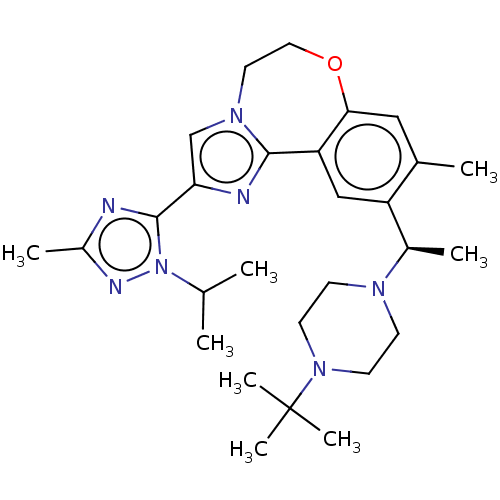
(US9090628, 299)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(C)c(cc3-c2n1)[C@@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C28H41N7O/c1-18(2)35-27(29-21(5)31-35)24-17-33-13-14-36-25-15-19(3)22(16-23(25)26(33)30-24)20(4)32-9-11-34(12-10-32)28(6,7)8/h15-18,20H,9-14H2,1-8H3/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172634
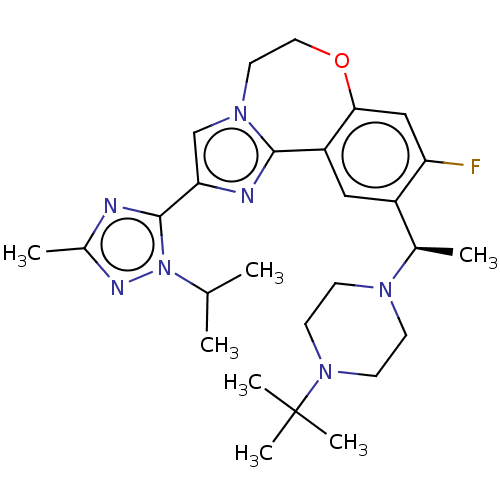
(US9090628, 294)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(F)c(cc3-c2n1)[C@@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C27H38FN7O/c1-17(2)35-26(29-19(4)31-35)23-16-33-12-13-36-24-15-22(28)20(14-21(24)25(33)30-23)18(3)32-8-10-34(11-9-32)27(5,6)7/h14-18H,8-13H2,1-7H3/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50278665
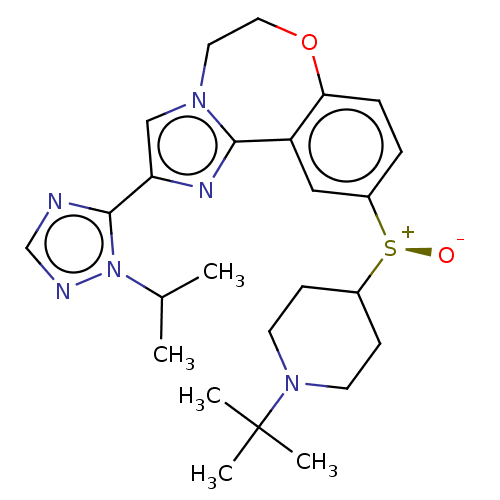
(CHEMBL4175041)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(cc3-c2n1)[S@@+]([O-])C1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C25H34N6O2S/c1-17(2)31-24(26-16-27-31)21-15-29-12-13-33-22-7-6-19(14-20(22)23(29)28-21)34(32)18-8-10-30(11-9-18)25(3,4)5/h6-7,14-18H,8-13H2,1-5H3/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172640
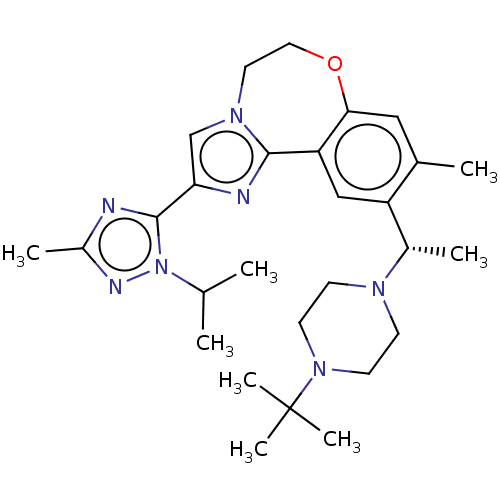
(US9090628, 300)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(C)c(cc3-c2n1)[C@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C28H41N7O/c1-18(2)35-27(29-21(5)31-35)24-17-33-13-14-36-25-15-19(3)22(16-23(25)26(33)30-24)20(4)32-9-11-34(12-10-32)28(6,7)8/h15-18,20H,9-14H2,1-8H3/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50278667

(CHEMBL4167171)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(cc3-c2n1)S(=O)(=O)N1CCN(CC1)C(C)(C)C Show InChI InChI=1S/C24H33N7O3S/c1-17(2)31-23(25-16-26-31)20-15-28-12-13-34-21-7-6-18(14-19(21)22(28)27-20)35(32,33)30-10-8-29(9-11-30)24(3,4)5/h6-7,14-17H,8-13H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172467

(US9090628, 110)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(cc3-c2n1)C(=O)N1CCN(CC1)C(C)(C)C Show InChI InChI=1S/C25H33N7O2/c1-17(2)32-23(26-16-27-32)20-15-30-12-13-34-21-7-6-18(14-19(21)22(30)28-20)24(33)29-8-10-31(11-9-29)25(3,4)5/h6-7,14-17H,8-13H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172643
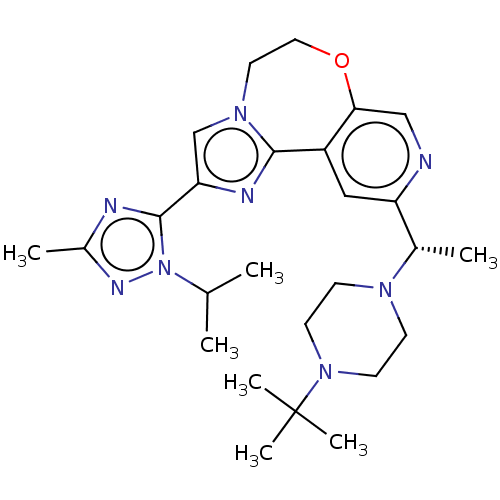
(US9090628, 303)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cnc(cc3-c2n1)[C@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C26H38N8O/c1-17(2)34-25(28-19(4)30-34)22-16-32-12-13-35-23-15-27-21(14-20(23)24(32)29-22)18(3)31-8-10-33(11-9-31)26(5,6)7/h14-18H,8-13H2,1-7H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172637

(US9090628, 297)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(F)c(cc3-c2n1)[C@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C27H38FN7O/c1-17(2)35-26(29-19(4)31-35)23-16-33-12-13-36-24-15-22(28)20(14-21(24)25(33)30-23)18(3)32-8-10-34(11-9-32)27(5,6)7/h14-18H,8-13H2,1-7H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172465
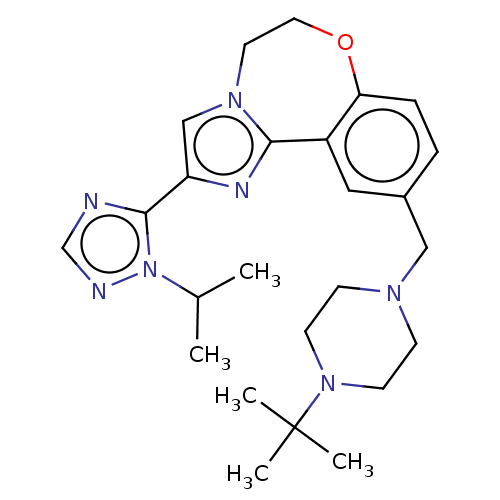
(US9090628, 108)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(CN4CCN(CC4)C(C)(C)C)cc3-c2n1 Show InChI InChI=1S/C25H35N7O/c1-18(2)32-24(26-17-27-32)21-16-30-12-13-33-22-7-6-19(14-20(22)23(30)28-21)15-29-8-10-31(11-9-29)25(3,4)5/h6-7,14,16-18H,8-13,15H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM172645
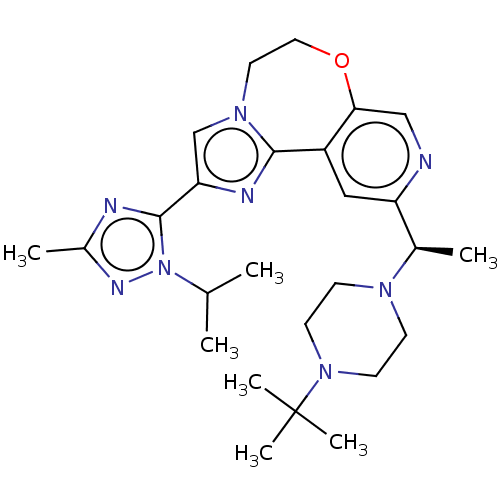
(US9090628, 305)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cnc(cc3-c2n1)[C@@H](C)N1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C26H38N8O/c1-17(2)34-25(28-19(4)30-34)22-16-32-12-13-35-23-15-27-21(14-20(23)24(32)29-22)18(3)31-8-10-33(11-9-31)26(5,6)7/h14-18H,8-13H2,1-7H3/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50278666
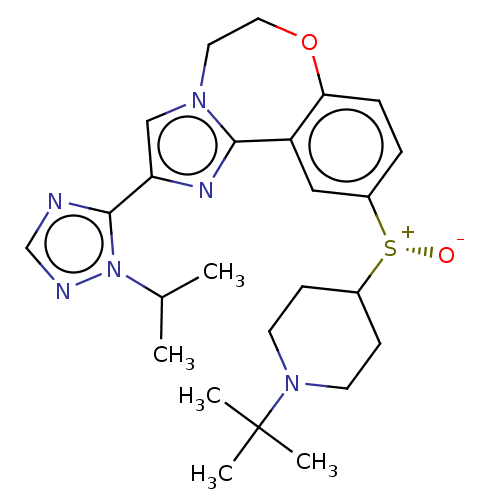
(CHEMBL4170565)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(cc3-c2n1)[S@+]([O-])C1CCN(CC1)C(C)(C)C |r| Show InChI InChI=1S/C25H34N6O2S/c1-17(2)31-24(26-16-27-31)21-15-29-12-13-33-22-7-6-19(14-20(22)23(29)28-21)34(32)18-8-10-30(11-9-18)25(3,4)5/h6-7,14-18H,8-13H2,1-5H3/t34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50278664
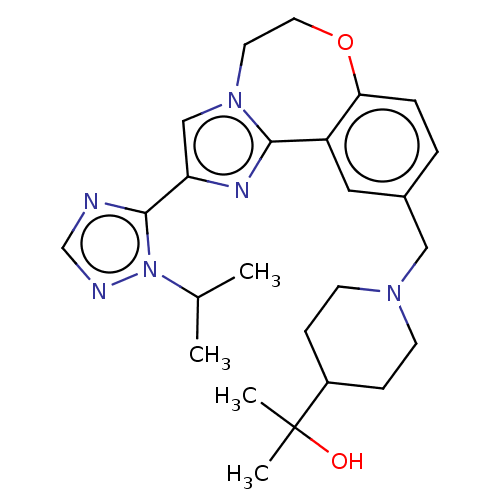
(CHEMBL4168260)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3ccc(CN4CCC(CC4)C(C)(C)O)cc3-c2n1 Show InChI InChI=1S/C25H34N6O2/c1-17(2)31-24(26-16-27-31)21-15-30-11-12-33-22-6-5-18(13-20(22)23(30)28-21)14-29-9-7-19(8-10-29)25(3,4)32/h5-6,13,15-17,19,32H,7-12,14H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta (unknown origin) |
ACS Med Chem Lett 8: 936-940 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00170
BindingDB Entry DOI: 10.7270/Q2CV4M7R |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559562

(CHEMBL4762834)Show SMILES Cn1c(CNC(=O)C2(CC(O)=O)Cc3ccccc3C2)nc2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559561
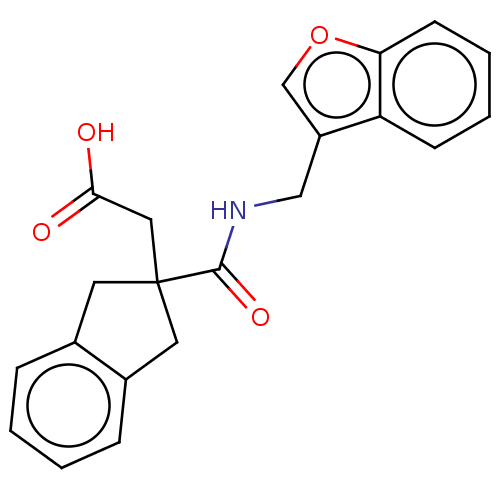
(CHEMBL4760006) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559558

(CHEMBL4764936)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCCc1cc2ccccc2s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559569

(CHEMBL4761199) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559570

(CHEMBL4760832) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559571

(CHEMBL4793389)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1ccc2ccccc2n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559572

(CHEMBL4747047) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559573
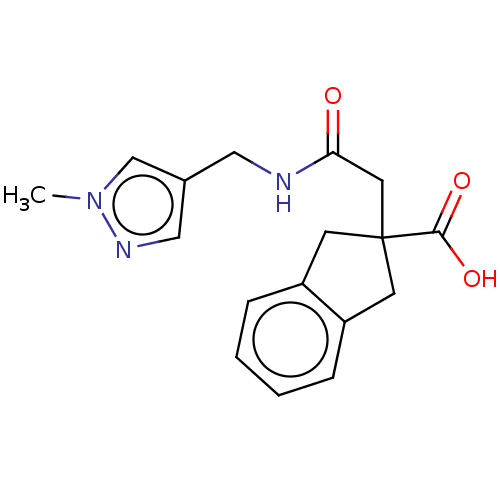
(CHEMBL4741361) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559574

(CHEMBL4785438)Show SMILES Cc1ccc2oc(CNC(=O)C3(CC(O)=O)Cc4ccccc4C3)cc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559575

(CHEMBL4798439)Show SMILES Cc1ccc2oc(CNC(=O)CC3(Cc4ccccc4C3)C(O)=O)cc2c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559576

(CHEMBL4794850)Show SMILES CC(NC(=O)CC1(Cc2ccccc2C1)C(O)=O)c1cc2ccccc2o1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559577

(CHEMBL4797456) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559568
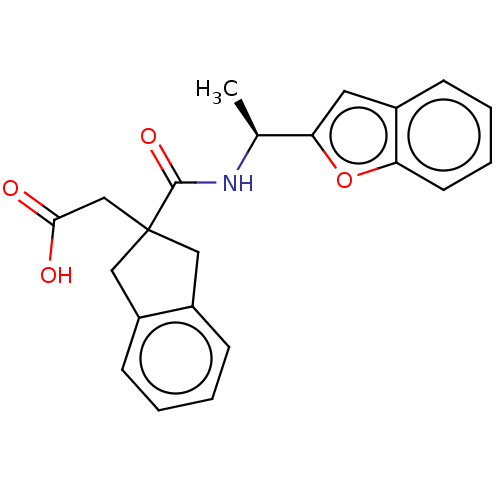
(CHEMBL4748733)Show SMILES C[C@H](NC(=O)C1(CC(O)=O)Cc2ccccc2C1)c1cc2ccccc2o1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559567
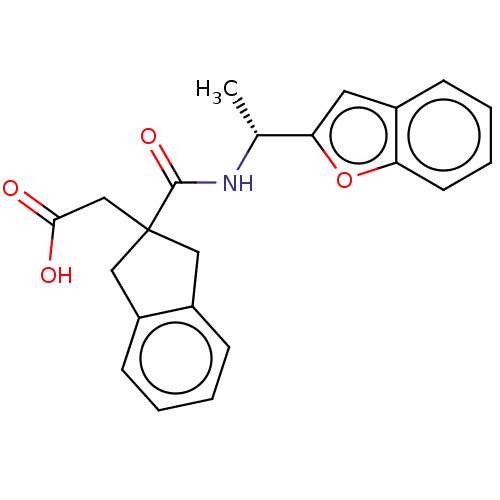
(CHEMBL4760989)Show SMILES C[C@@H](NC(=O)C1(CC(O)=O)Cc2ccccc2C1)c1cc2ccccc2o1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559566

(CHEMBL4749721) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559565
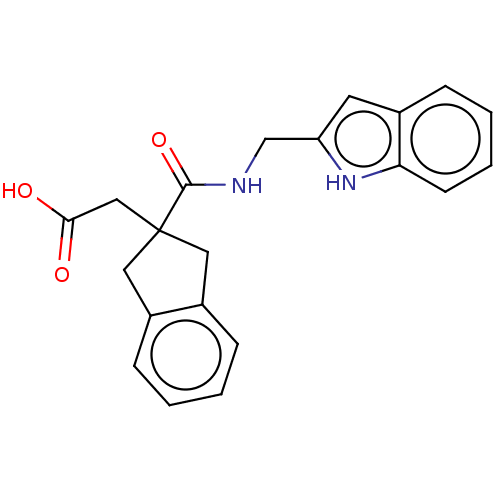
(CHEMBL4776453)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1cc2ccccc2[nH]1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559564
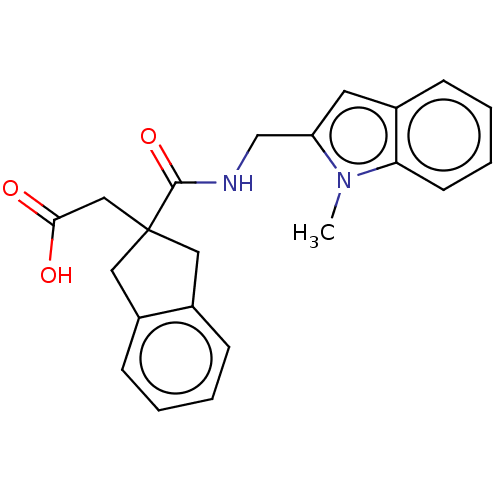
(CHEMBL4746167)Show SMILES Cn1c(CNC(=O)C2(CC(O)=O)Cc3ccccc3C2)cc2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559563

(CHEMBL4776454)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1nc2ccccc2[nH]1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559552

(CHEMBL4776002) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559560
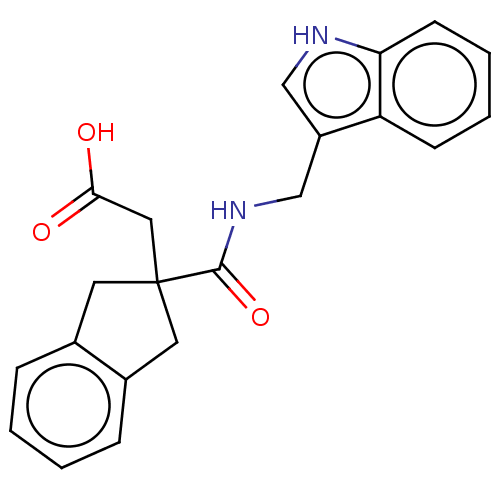
(CHEMBL4756804)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1c[nH]c2ccccc12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559578
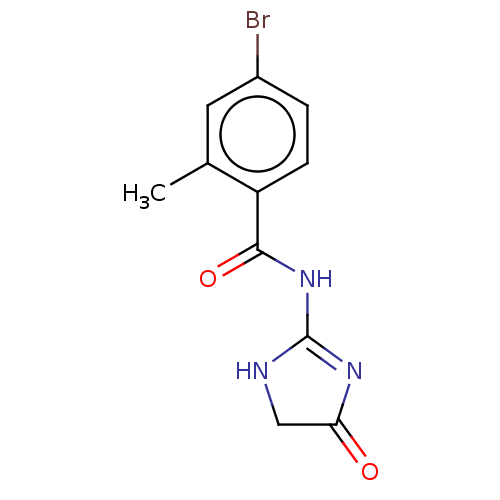
(CHEMBL4751618) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559557

(CHEMBL4794689)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCCc1cc2ccccc2[nH]1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559556
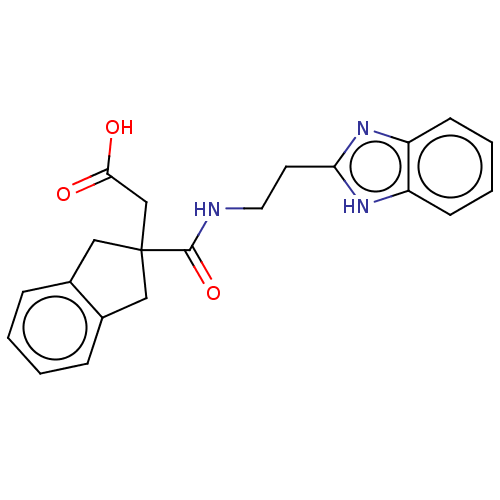
(CHEMBL4749889)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCCc1nc2ccccc2[nH]1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559555

(CHEMBL4745988)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCCc1nc2ccccc2s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559554
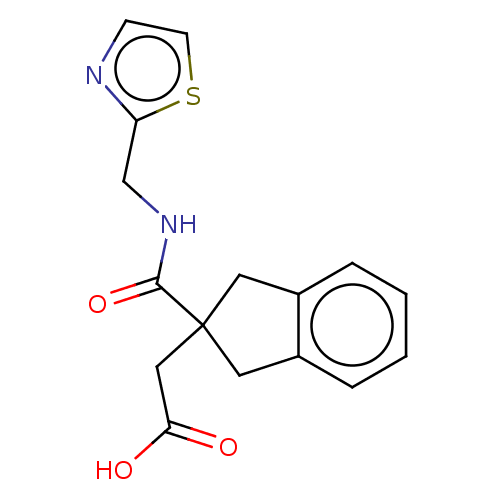
(CHEMBL4751454) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559553
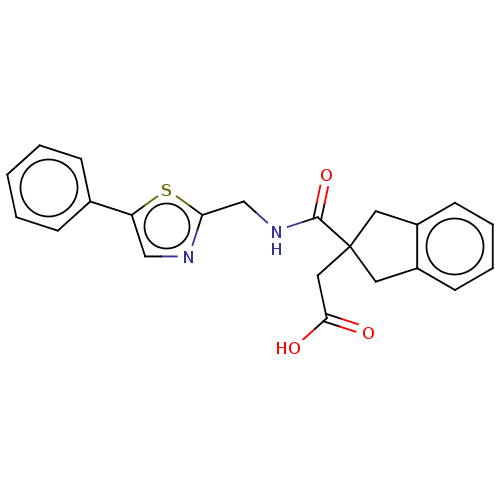
(CHEMBL4745918)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1ncc(s1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559551
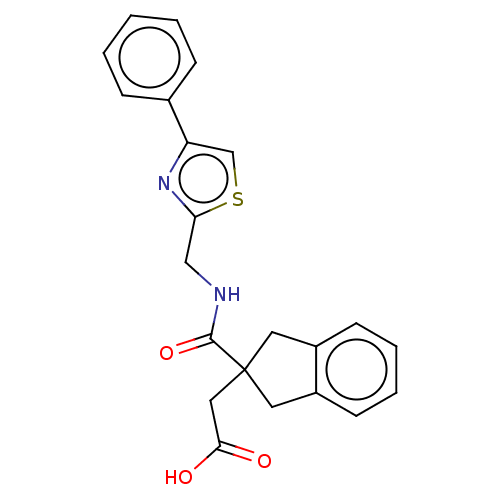
(CHEMBL4758612)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCc1nc(cs1)-c1ccccc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50559559
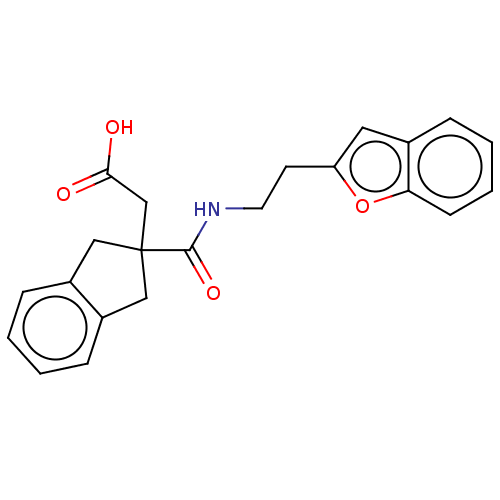
(CHEMBL4746129)Show SMILES OC(=O)CC1(Cc2ccccc2C1)C(=O)NCCc1cc2ccccc2o1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rabbit ACE using Abz-FRK(DNP)-P as substrate measured for every 30 sec for 5 mins by UV-fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00554
BindingDB Entry DOI: 10.7270/Q2X63RNT |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50112575
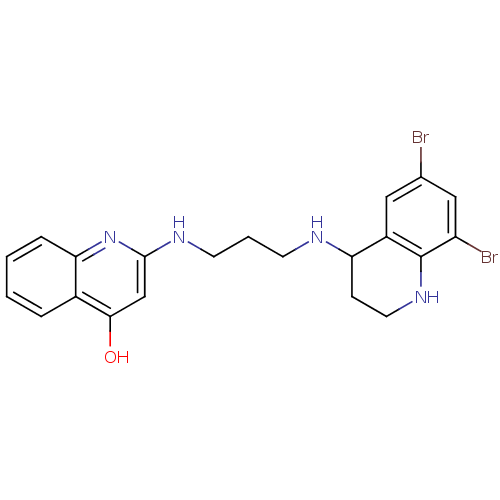
(2-[3-(6,8-Dibromo-1,2,3,4-tetrahydro-quinolin-4-yl...)Show SMILES Oc1cc(NCCCNC2CCNc3c(Br)cc(Br)cc23)nc2ccccc12 Show InChI InChI=1S/C21H22Br2N4O/c22-13-10-15-17(6-9-26-21(15)16(23)11-13)24-7-3-8-25-20-12-19(28)14-4-1-2-5-18(14)27-20/h1-2,4-5,10-12,17,24,26H,3,6-9H2,(H2,25,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against Methionyl-tRNA synthetase in a pyrophosphate exchange assay |
J Med Chem 45: 1959-62 (2002)
BindingDB Entry DOI: 10.7270/Q2HQ3Z6X |
More data for this
Ligand-Target Pair | |
Isoleucyl-tRNA synthetase
(Rattus norvegicus) | BDBM50093001
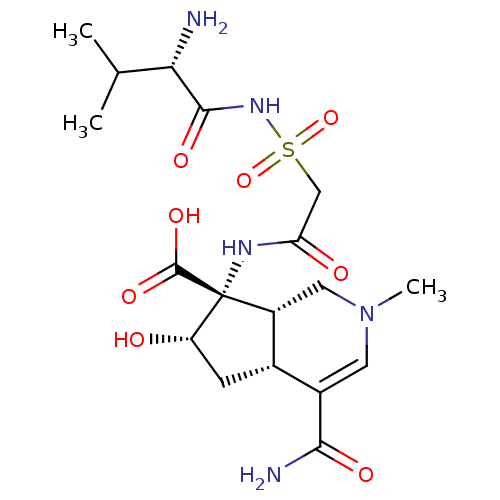
((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...)Show SMILES CC(C)[C@H](N)C(=O)NS(=O)(=O)CC(=O)N[C@]1([C@@H](O)C[C@@H]2[C@H]1CN(C)C=C2C(N)=O)C(O)=O |c:25| Show InChI InChI=1S/C18H29N5O8S/c1-8(2)14(19)16(27)22-32(30,31)7-13(25)21-18(17(28)29)11-6-23(3)5-10(15(20)26)9(11)4-12(18)24/h5,8-9,11-12,14,24H,4,6-7,19H2,1-3H3,(H2,20,26)(H,21,25)(H,22,27)(H,28,29)/t9-,11+,12-,14-,18+/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver |
Bioorg Med Chem Lett 10: 2263-6 (2001)
BindingDB Entry DOI: 10.7270/Q2PN94W3 |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50222701
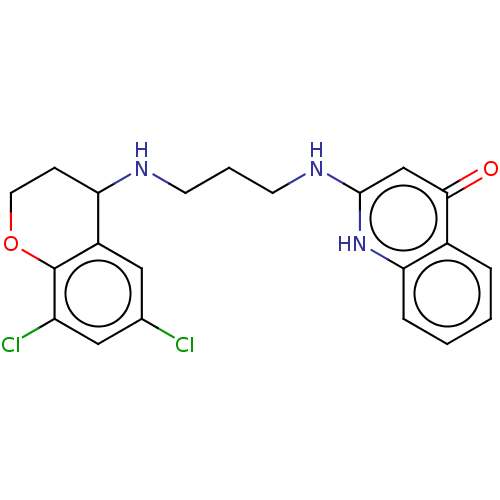
(CHEMBL158051)Show SMILES Clc1cc(Cl)c2OCCC(NCCCNc3cc(=O)c4ccccc4[nH]3)c2c1 Show InChI InChI=1S/C21H21Cl2N3O2/c22-13-10-15-17(6-9-28-21(15)16(23)11-13)24-7-3-8-25-20-12-19(27)14-4-1-2-5-18(14)26-20/h1-2,4-5,10-12,17,24H,3,6-9H2,(H2,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay |
Bioorg Med Chem Lett 13: 665-8 (2003)
BindingDB Entry DOI: 10.7270/Q2KP81JD |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50222700
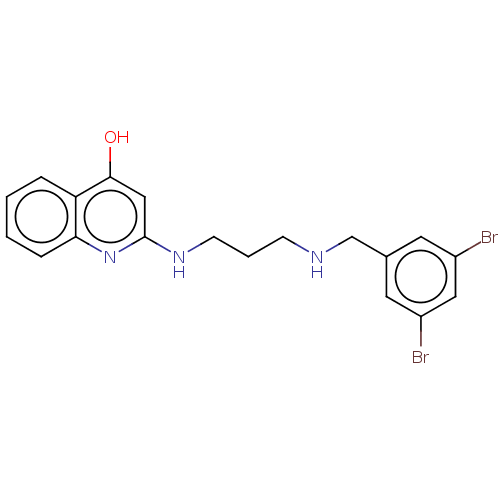
(CHEMBL161864)Show InChI InChI=1S/C19H19Br2N3O/c20-14-8-13(9-15(21)10-14)12-22-6-3-7-23-19-11-18(25)16-4-1-2-5-17(16)24-19/h1-2,4-5,8-11,22H,3,6-7,12H2,(H2,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay |
Bioorg Med Chem Lett 13: 665-8 (2003)
BindingDB Entry DOI: 10.7270/Q2KP81JD |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50222703
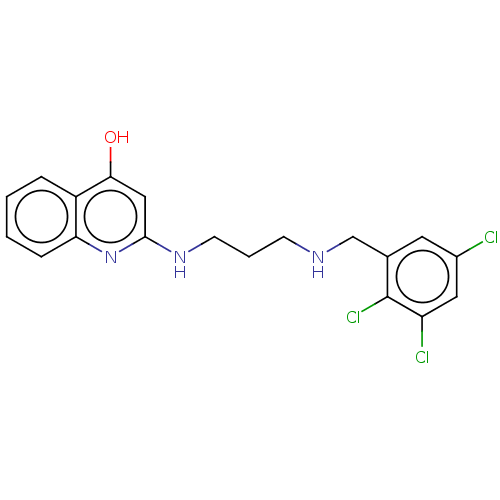
(CHEMBL160841)Show InChI InChI=1S/C19H18Cl3N3O/c20-13-8-12(19(22)15(21)9-13)11-23-6-3-7-24-18-10-17(26)14-4-1-2-5-16(14)25-18/h1-2,4-5,8-10,23H,3,6-7,11H2,(H2,24,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay |
Bioorg Med Chem Lett 13: 665-8 (2003)
BindingDB Entry DOI: 10.7270/Q2KP81JD |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50222702

(CHEMBL161347)Show SMILES Oc1cc(NCCCNC2CCNc3c(Cl)cc(Br)cc23)nc2ccccc12 Show InChI InChI=1S/C21H22BrClN4O/c22-13-10-15-17(6-9-26-21(15)16(23)11-13)24-7-3-8-25-20-12-19(28)14-4-1-2-5-18(14)27-20/h1-2,4-5,10-12,17,24,26H,3,6-9H2,(H2,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay |
Bioorg Med Chem Lett 13: 665-8 (2003)
BindingDB Entry DOI: 10.7270/Q2KP81JD |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50222705
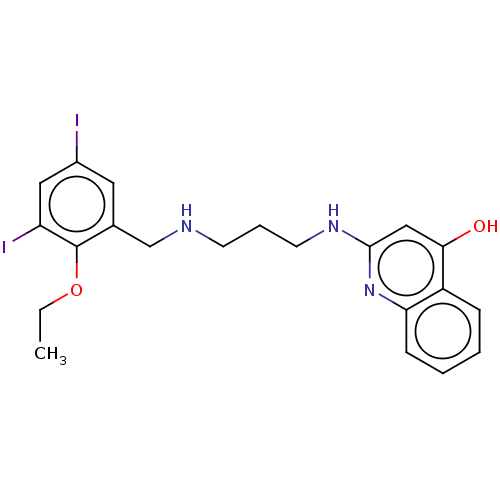
(CHEMBL161312)Show InChI InChI=1S/C21H23I2N3O2/c1-2-28-21-14(10-15(22)11-17(21)23)13-24-8-5-9-25-20-12-19(27)16-6-3-4-7-18(16)26-20/h3-4,6-7,10-12,24H,2,5,8-9,13H2,1H3,(H2,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay |
Bioorg Med Chem Lett 13: 665-8 (2003)
BindingDB Entry DOI: 10.7270/Q2KP81JD |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50222704

(CHEMBL345835)Show SMILES CCOc1c(CNCCCNc2cc(O)c3ccccc3n2)cc(I)cc1CC=C Show InChI InChI=1S/C24H28IN3O2/c1-3-8-17-13-19(25)14-18(24(17)30-4-2)16-26-11-7-12-27-23-15-22(29)20-9-5-6-10-21(20)28-23/h3,5-6,9-10,13-15,26H,1,4,7-8,11-12,16H2,2H3,(H2,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay |
Bioorg Med Chem Lett 13: 665-8 (2003)
BindingDB Entry DOI: 10.7270/Q2KP81JD |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50124821

(2-[3-(3,5-Dichloro-benzylamino)-propylamino]-1H-qu...)Show InChI InChI=1S/C19H19Cl2N3O/c20-14-8-13(9-15(21)10-14)12-22-6-3-7-23-19-11-18(25)16-4-1-2-5-17(16)24-19/h1-2,4-5,8-11,22H,3,6-7,12H2,(H2,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Compound tested for inhibition of Staphylococcus aureus MRS(methionyl tRNA synthetase) in aminoacylation assay |
Bioorg Med Chem Lett 13: 665-8 (2003)
BindingDB Entry DOI: 10.7270/Q2KP81JD |
More data for this
Ligand-Target Pair | |
Methionine--tRNA ligase
(Escherichia coli (strain K12)) | BDBM50222699

(CHEMBL161300)Show SMILES Oc1cc(NCCCNC2CCNc3c(Br)cc(Cl)cc23)nc2ccccc12 Show InChI InChI=1S/C21H22BrClN4O/c22-16-11-13(23)10-15-17(6-9-26-21(15)16)24-7-3-8-25-20-12-19(28)14-4-1-2-5-18(14)27-20/h1-2,4-5,10-12,17,24,26H,3,6-9H2,(H2,25,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Compound tested for inhibition of Staphylococcus aureus MRS (methionyl tRNA synthetase) in aminoacylation assay |
Bioorg Med Chem Lett 13: 665-8 (2003)
BindingDB Entry DOI: 10.7270/Q2KP81JD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data