

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50405683 (CHEMBL174909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Apparent inhibition constant (Ki) for cytochrome P450 19A1 with androstenedione | J Med Chem 31: 971-6 (1988) BindingDB Entry DOI: 10.7270/Q2154J7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011767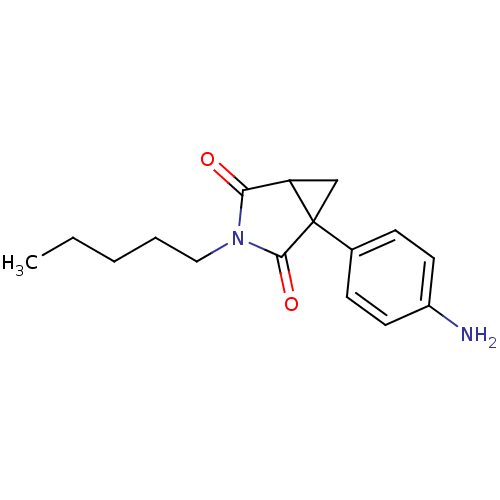 (1-(4-Amino-phenyl)-3-pentyl-3-aza-bicyclo[3.1.0]he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 androstenedione | J Med Chem 31: 971-6 (1988) BindingDB Entry DOI: 10.7270/Q2154J7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50405688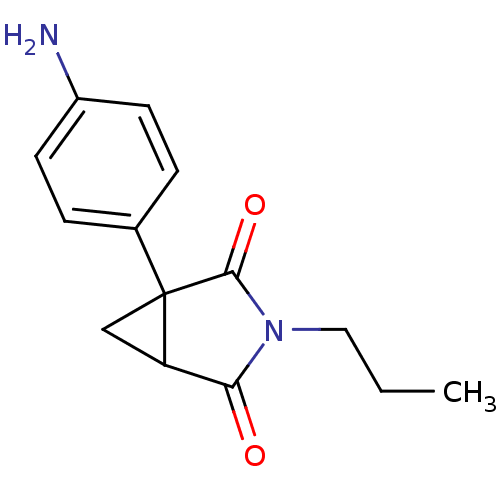 (CHEMBL174407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 androstenedione | J Med Chem 31: 971-6 (1988) BindingDB Entry DOI: 10.7270/Q2154J7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50250422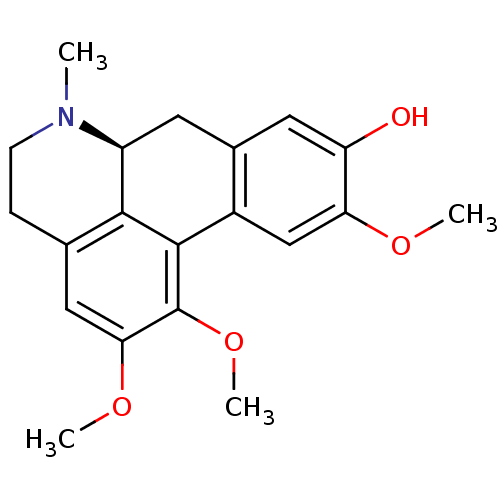 ((+)-N-methyl-laurotetanine | CHEMBL464099 | N-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in CHO cells | J Nat Prod 69: 432-5 (2006) Article DOI: 10.1021/np058114h BindingDB Entry DOI: 10.7270/Q2V98907 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50024544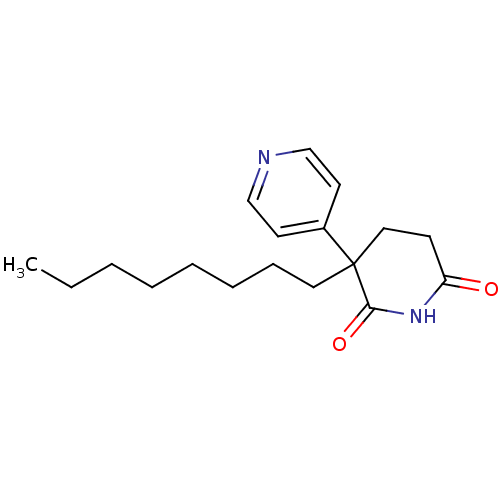 (3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with androstenedione | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50015983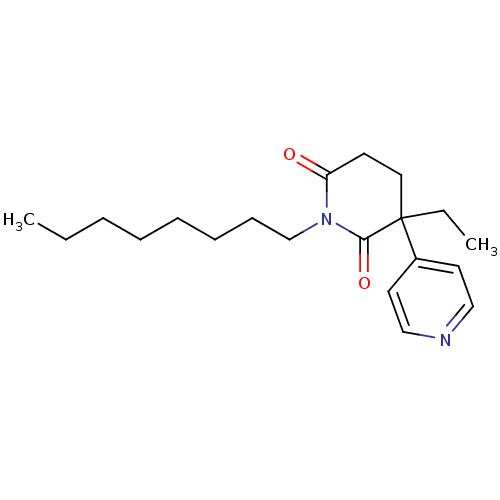 ((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with testosterone | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50024544 (3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with androstenedione | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50015983 ((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with androstenedione | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8885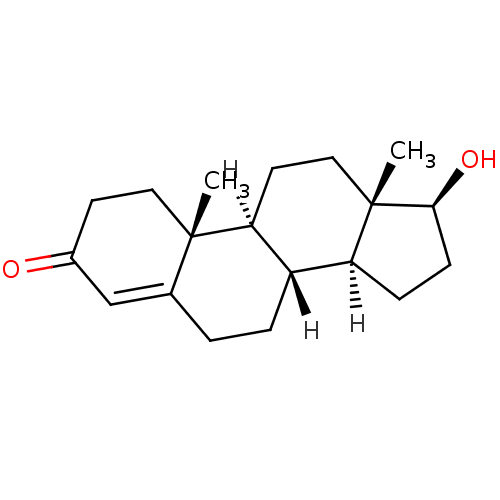 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental aromatase Cytochrome P450 19A1 | J Med Chem 26: 50-4 (1983) BindingDB Entry DOI: 10.7270/Q2V125CG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human placental cytochrome P450 19A1 | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant (Ki) for Cytochrome P450 19A1 | J Med Chem 28: 200-4 (1985) BindingDB Entry DOI: 10.7270/Q2WM1FK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50015985 (3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory constant (Ki) for Cytochrome P450 19A1 | J Med Chem 28: 200-4 (1985) BindingDB Entry DOI: 10.7270/Q2WM1FK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50015985 (3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with androstenedione | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50011760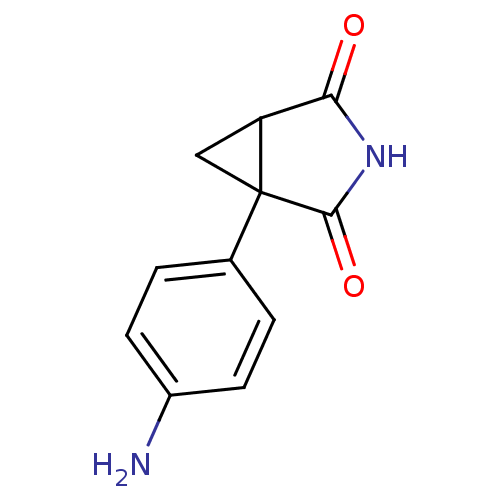 (1-(4-Amino-phenyl)-3-aza-bicyclo[3.1.0]hexane-2,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 androstenedione | J Med Chem 31: 971-6 (1988) BindingDB Entry DOI: 10.7270/Q2154J7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with testosterone | J Med Chem 31: 971-6 (1988) BindingDB Entry DOI: 10.7270/Q2154J7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9460 (3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with androstenedione | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50259677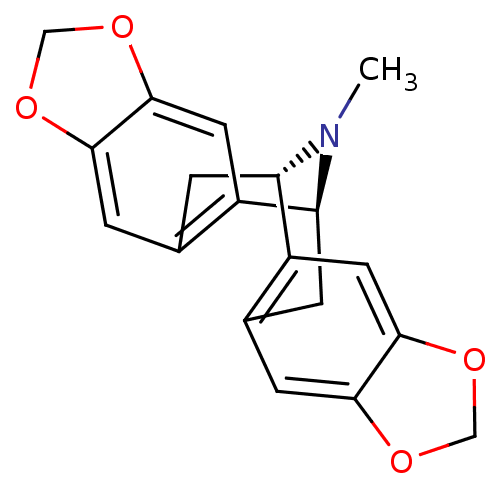 (CHEMBL481839 | escholtzine) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tom's of Maine Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor expressed in CHO cells | J Nat Prod 69: 432-5 (2006) Article DOI: 10.1021/np058114h BindingDB Entry DOI: 10.7270/Q2V98907 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50015985 (3-Ethyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with androstenedione | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24922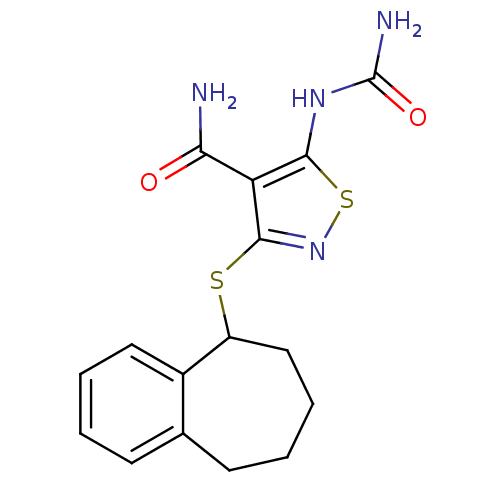 (5-(carbamoylamino)-3-{6,7,8,9-tetrahydro-5H-benzo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24923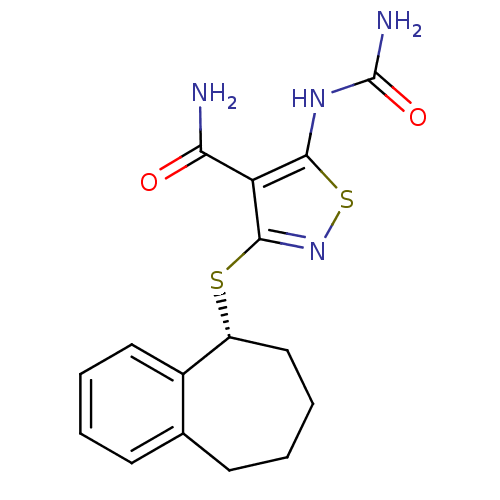 (5-(carbamoylamino)-3-[(5R)-6,7,8,9-tetrahydro-5H-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24899 (3-{[1-(4-chlorophenyl)ethyl]sulfanyl}-5-[(methylca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24901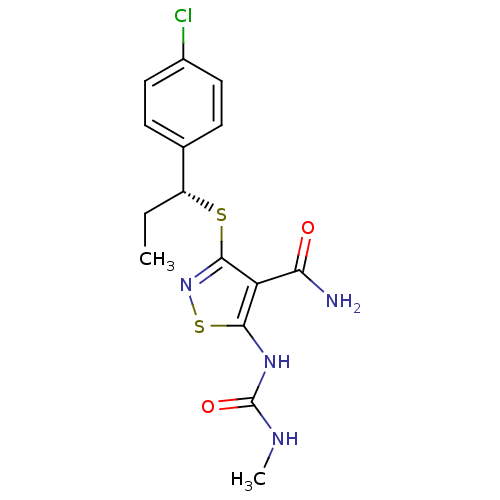 (3-{[(1R)-1-(4-chlorophenyl)propyl]sulfanyl}-5-[(me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24898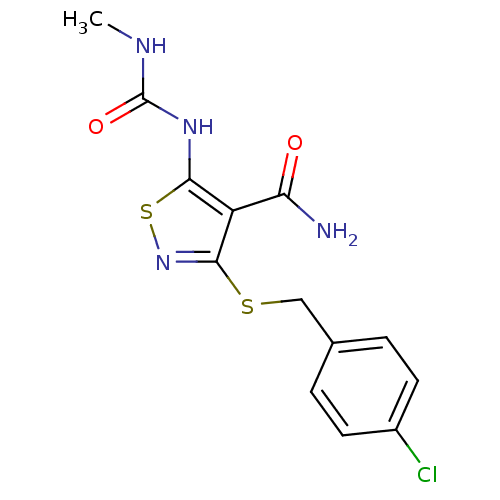 (3-{[(4-chlorophenyl)methyl]sulfanyl}-5-[(methylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24919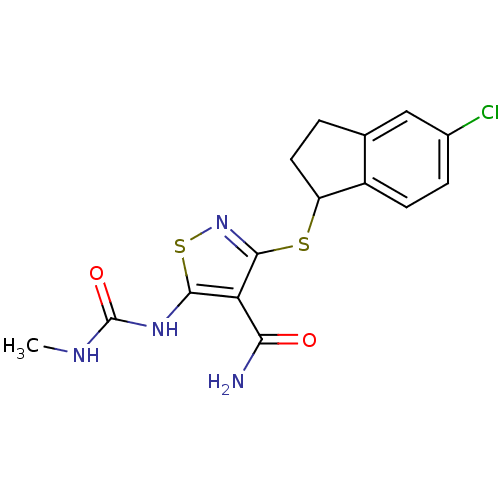 (3-[(5-chloro-2,3-dihydro-1H-inden-1-yl)sulfanyl]-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24900 (3-{[1-(4-chlorophenyl)propyl]sulfanyl}-5-[(methylc...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24918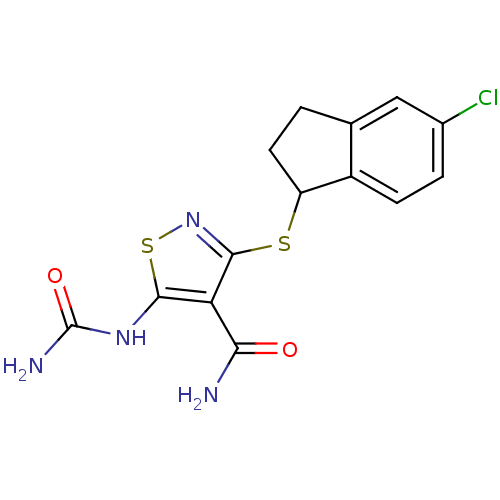 (5-(carbamoylamino)-3-[(5-chloro-2,3-dihydro-1H-ind...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24903 (3-{[1-(4-chlorophenyl)-2-methylpropyl]sulfanyl}-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24910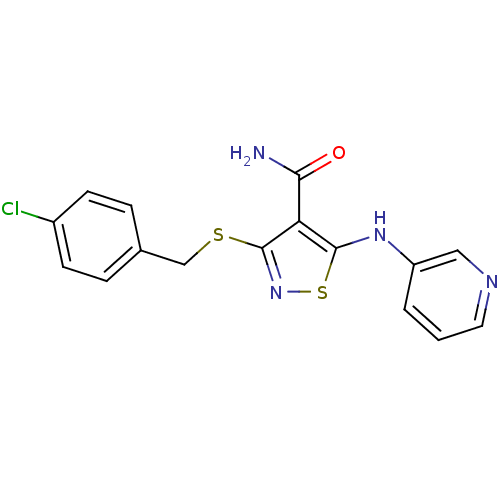 (3-{[(4-chlorophenyl)methyl]sulfanyl}-5-(pyridin-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24914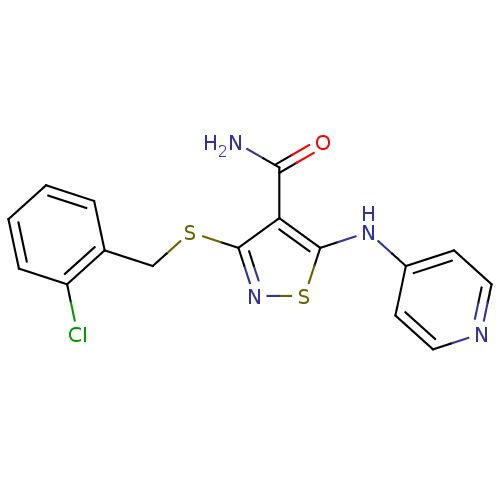 (3-{[(2-chlorophenyl)methyl]sulfanyl}-5-(pyridin-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24911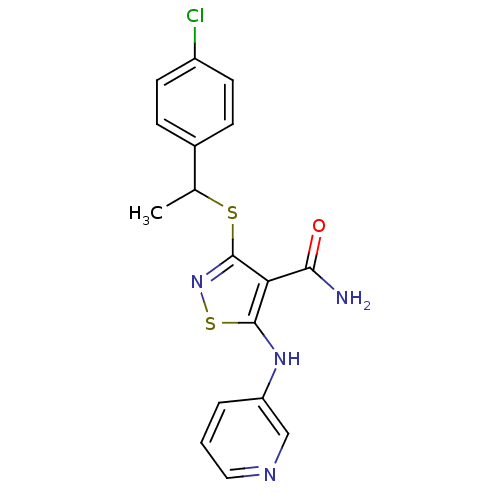 (3-{[1-(4-chlorophenyl)ethyl]sulfanyl}-5-(pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24909 (3-{[(4-chlorophenyl)methyl]sulfanyl}-5-(pyridin-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24904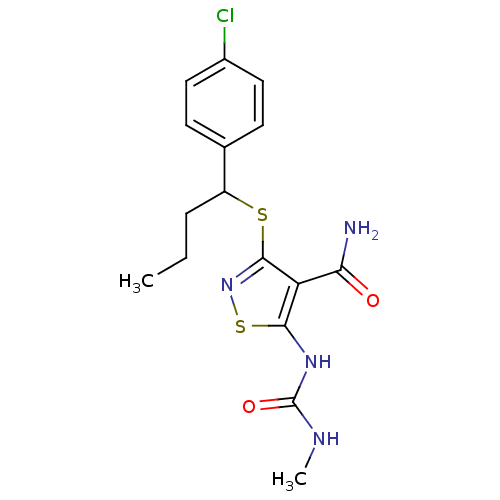 (3-{[1-(4-chlorophenyl)butyl]sulfanyl}-5-[(methylca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24913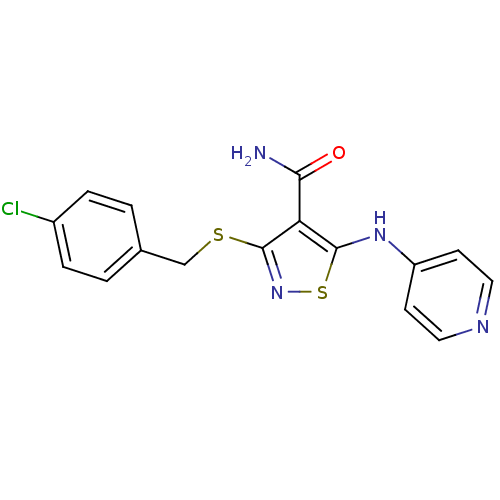 (3-{[(4-chlorophenyl)methyl]sulfanyl}-5-(pyridin-4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24915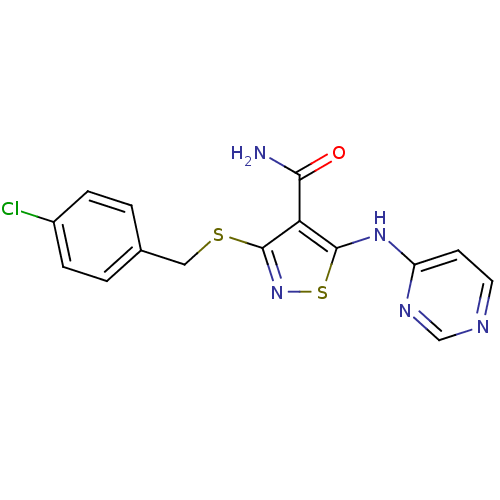 (3-{[(4-chlorophenyl)methyl]sulfanyl}-5-(pyrimidin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24902 (3-{[(1S)-1-(4-chlorophenyl)propyl]sulfanyl}-5-[(me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24917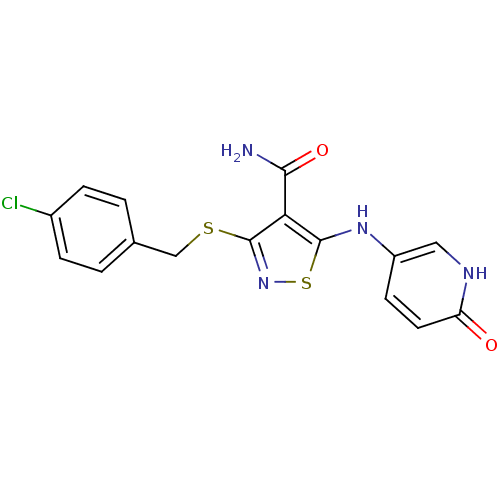 (3-{[(4-chlorophenyl)methyl]sulfanyl}-5-[(6-oxo-1,6...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24924 (5-(carbamoylamino)-3-[(5S)-6,7,8,9-tetrahydro-5H-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24920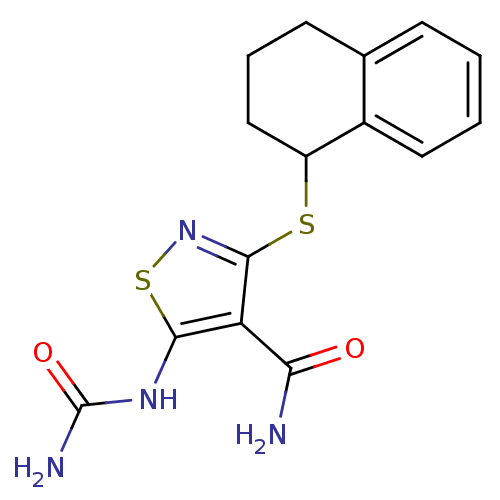 (5-(carbamoylamino)-3-(1,2,3,4-tetrahydronaphthalen...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24905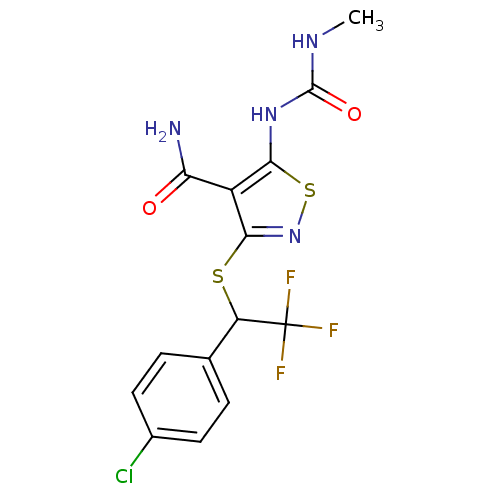 (3-{[1-(4-chlorophenyl)-2,2,2-trifluoroethyl]sulfan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24921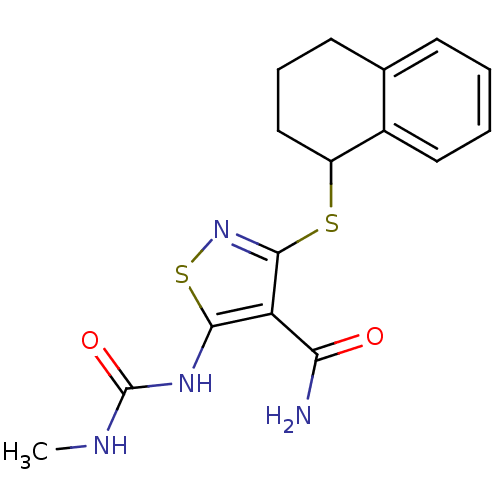 (5-[(methylcarbamoyl)amino]-3-(1,2,3,4-tetrahydrona...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24916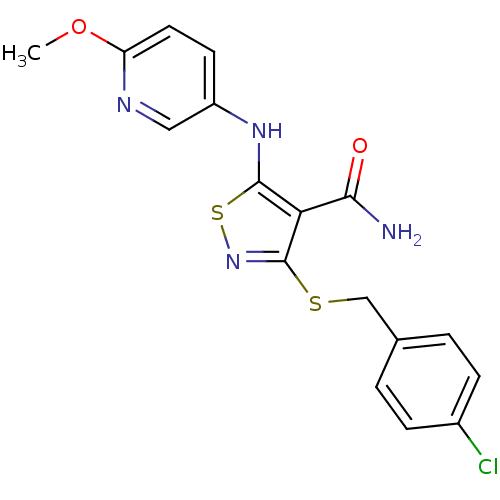 (3-{[(4-chlorophenyl)methyl]sulfanyl}-5-[(6-methoxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50024544 (3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human placental cytochrome P450 19A1 | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50024544 (3-Octyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with testosterone | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24912 (3-[(cyclohexylmethyl)sulfanyl]-5-(pyridin-3-ylamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50015983 ((S)-3-Ethyl-1-octyl-4,5-dihydro-3H-[3,4']bipyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human placental cytochrome P450 19A1 with testosterone | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50024550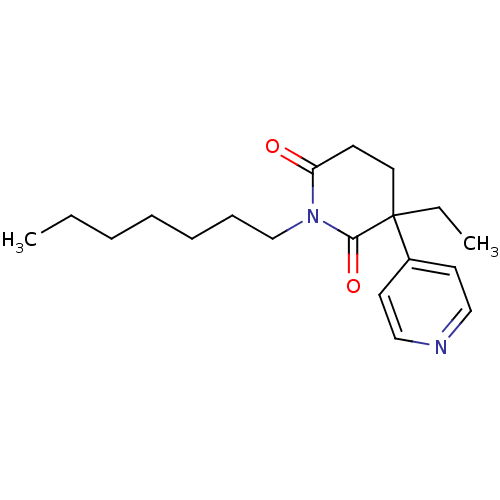 (3-Ethyl-1-heptyl-4,5-dihydro-3H-[3,4']bipyridinyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human placental cytochrome P450 19A1 | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50024555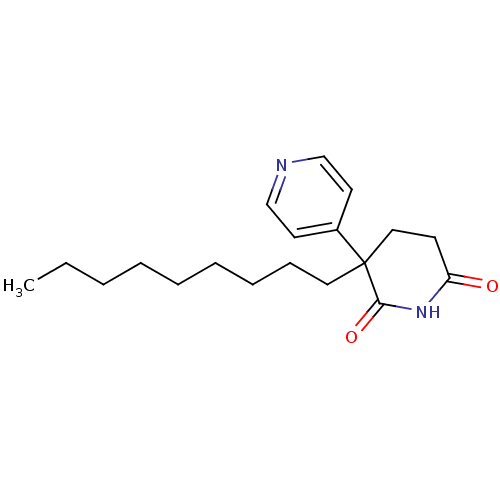 (3-Nonyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dione...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human placental cytochrome P450 19A1 | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50024557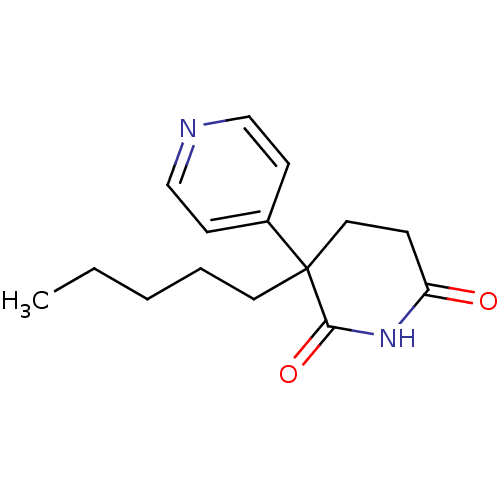 (3-Pentyl-4,5-dihydro-3H-[3,4']bipyridinyl-2,6-dion...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human placental cytochrome P450 19A1 | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM24906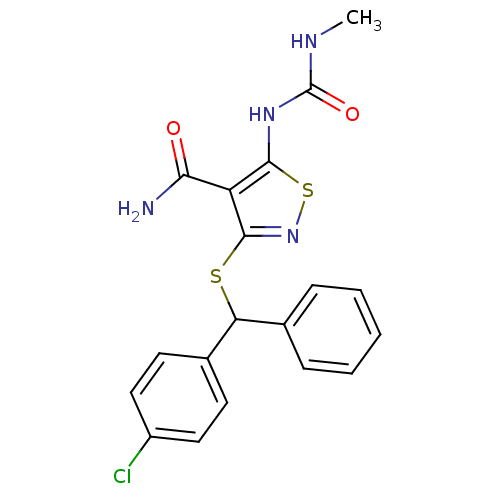 (3-{[(4-chlorophenyl)(phenyl)methyl]sulfanyl}-5-[(m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pfizer | Assay Description The kinase domain of the human TrkA receptor in phosphorylation buffer with 0.5 uM ATP is incubated in plates coated with PGT substrate. Compounds ar... | Bioorg Med Chem Lett 16: 3444-8 (2006) Article DOI: 10.1016/j.bmcl.2006.04.003 BindingDB Entry DOI: 10.7270/Q26Q1VH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50024553 (3-Ethyl-1-nonyl-4,5-dihydro-3H-[3,4']bipyridinyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human placental cytochrome P450 19A1 | J Med Chem 30: 1550-4 (1987) BindingDB Entry DOI: 10.7270/Q2GM869Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 95 total ) | Next | Last >> |