 Found 12 hits with Last Name = 'fotio' and Initial = 'y'
Found 12 hits with Last Name = 'fotio' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50151057

(CHEMBL3770726)Show InChI InChI=1S/C14H24N2O3/c17-13-12(10-15-13)16-14(18)19-9-5-4-8-11-6-2-1-3-7-11/h11-12H,1-10H2,(H,15,17)(H,16,18)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human NAAA |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Rattus norvegicus (Rat)) | BDBM50151057

(CHEMBL3770726)Show InChI InChI=1S/C14H24N2O3/c17-13-12(10-15-13)16-14(18)19-9-5-4-8-11-6-2-1-3-7-11/h11-12H,1-10H2,(H,15,17)(H,16,18)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat NAAA |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50539731
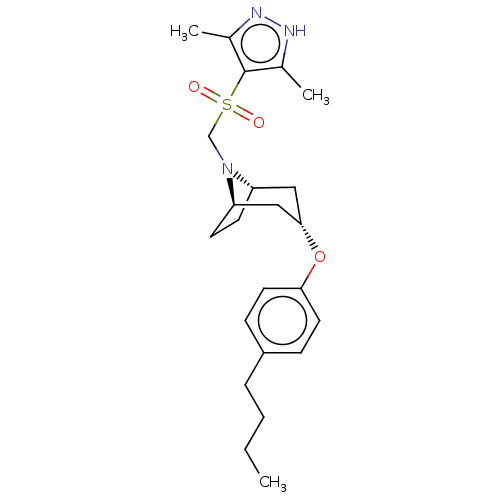
(CHEMBL4643312)Show SMILES [H][C@]12CC[C@]([H])(C[C@H](C1)Oc1ccc(CCCC)cc1)N2CS(=O)(=O)c1c(C)n[nH]c1C |r,TLB:9:7:20:3.2| Show InChI InChI=1S/C23H33N3O3S/c1-4-5-6-18-7-11-21(12-8-18)29-22-13-19-9-10-20(14-22)26(19)15-30(27,28)23-16(2)24-25-17(23)3/h7-8,11-12,19-20,22H,4-6,9-10,13-15H2,1-3H3,(H,24,25)/t19-,20+,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human NAAA |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50151057

(CHEMBL3770726)Show InChI InChI=1S/C14H24N2O3/c17-13-12(10-15-13)16-14(18)19-9-5-4-8-11-6-2-1-3-7-11/h11-12H,1-10H2,(H,15,17)(H,16,18)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human NAAA expressed in HEK cells |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM393301
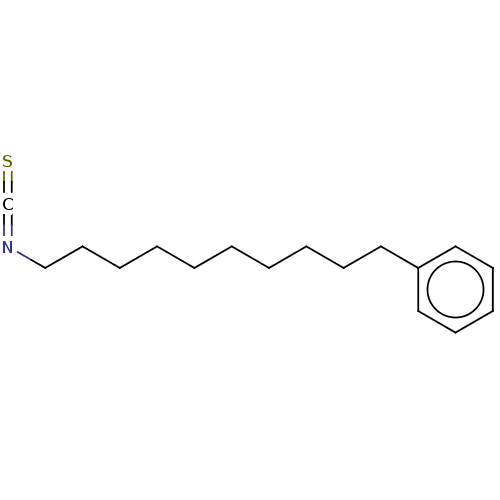
((10-Isothiocyanatodecyl)benzene | US9963444, Examp...)Show InChI InChI=1S/C17H25NS/c19-16-18-15-11-6-4-2-1-3-5-8-12-17-13-9-7-10-14-17/h7,9-10,13-14H,1-6,8,11-12,15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human NAAA |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM430282
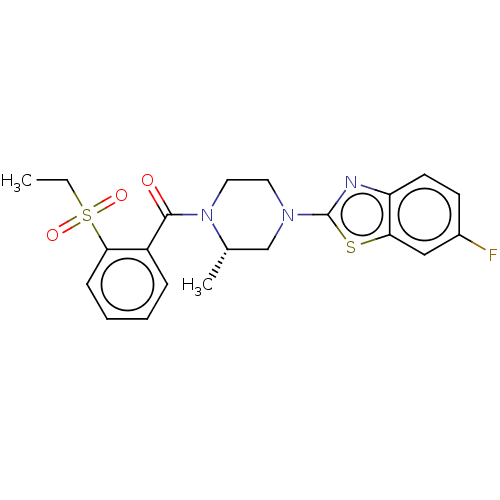
(US10556892, Compound 19 | US10556892, Compound 8)Show SMILES CCS(=O)(=O)c1ccccc1C(=O)N1CCN(C[C@@H]1C)c1nc2ccc(F)cc2s1 |r| Show InChI InChI=1S/C21H22FN3O3S2/c1-3-30(27,28)19-7-5-4-6-16(19)20(26)25-11-10-24(13-14(25)2)21-23-17-9-8-15(22)12-18(17)29-21/h4-9,12,14H,3,10-11,13H2,1-2H3/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human NAAA |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
N-acylethanolamine-hydrolyzing acid amidase
(Rattus norvegicus (Rat)) | BDBM50416509

(CHEMBL1214256 | US9321743, URB783)Show InChI InChI=1S/C12H13NO3/c14-11(13-10-8-16-12(10)15)7-6-9-4-2-1-3-5-9/h1-5,10H,6-8H2,(H,13,14)/t10-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat NAAA |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM32018
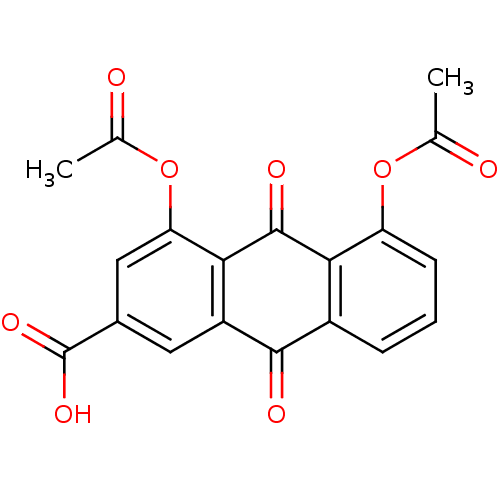
(1,8-DIACETOXY-3-CARBOXYANTHRAQUINONE | 4,5-diaceto...)Show SMILES CC(=O)Oc1cccc2C(=O)c3cc(cc(OC(C)=O)c3C(=O)c12)C(O)=O Show InChI InChI=1S/C19H12O8/c1-8(20)26-13-5-3-4-11-15(13)18(23)16-12(17(11)22)6-10(19(24)25)7-14(16)27-9(2)21/h3-7H,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of NAAA (unknown origin) |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Rattus norvegicus (Rat)) | BDBM50389007
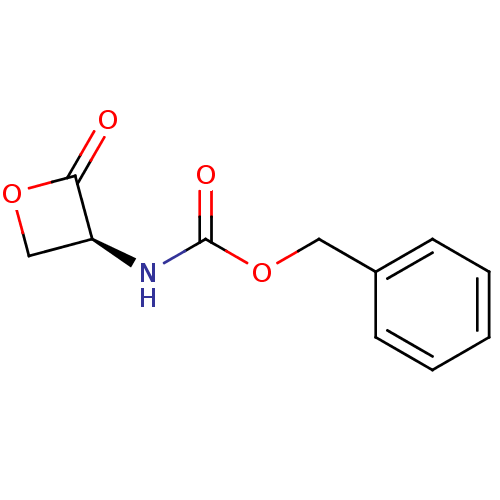
(CHEMBL1214255 | US9321743, SD41)Show InChI InChI=1S/C11H11NO4/c13-10-9(7-15-10)12-11(14)16-6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,12,14)/t9-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat NAAA |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50450753
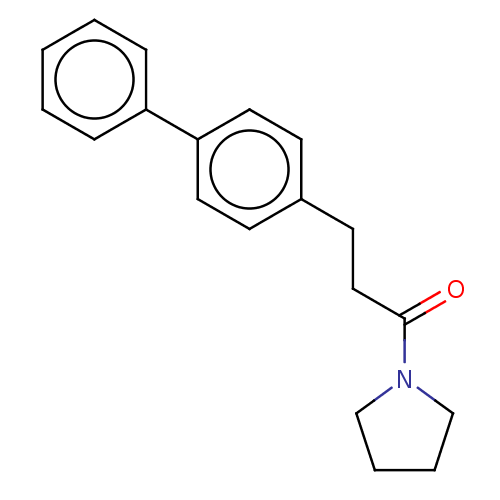
(CHEMBL4207570)Show InChI InChI=1S/C19H21NO/c21-19(20-14-4-5-15-20)13-10-16-8-11-18(12-9-16)17-6-2-1-3-7-17/h1-3,6-9,11-12H,4-5,10,13-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of NAAA (unknown origin) |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Rattus norvegicus (Rat)) | BDBM50416510
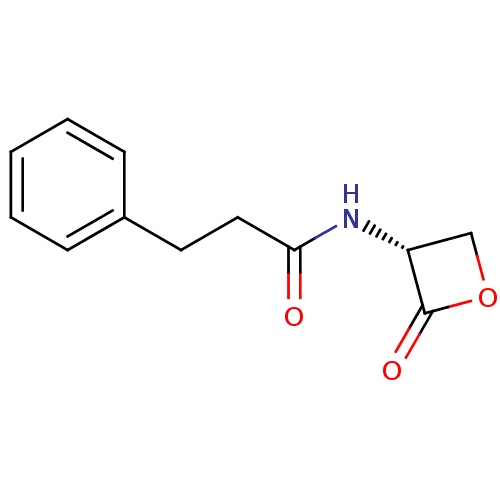
(CHEMBL1214257 | US9321743, URB818)Show InChI InChI=1S/C12H13NO3/c14-11(13-10-8-16-12(10)15)7-6-9-4-2-1-3-5-9/h1-5,10H,6-8H2,(H,13,14)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of rat NAAA |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |
N-acylethanolamine-hydrolyzing acid amidase
(Homo sapiens (Human)) | BDBM50309177

(CHEMBL590884 | cyclopentyl palmitate)Show InChI InChI=1S/C21H40O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-19-21(22)23-20-17-15-16-18-20/h20H,2-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of human NAAA expressed in HEK293 cell membranes using [3H]-N-palmitoylethanolamine as substrate after 30 mins by liquid scintillation cou... |
J Med Chem 63: 7475-7490 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00191
BindingDB Entry DOI: 10.7270/Q2V98CM4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data