

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359261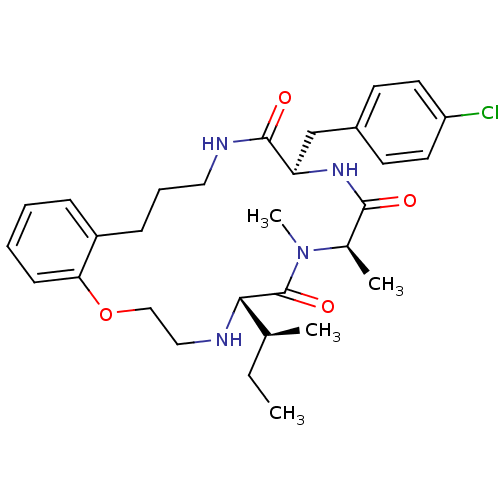 (CHEMBL1923617) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Homo sapiens (Human)) | BDBM50002694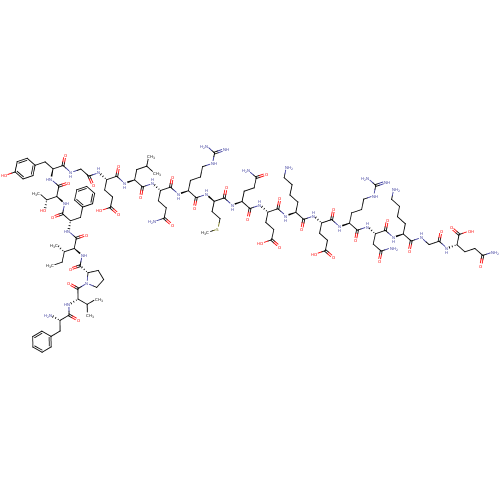 (MOTILIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I]motilin from human MOTR | J Med Chem 49: 7190-7 (2006) Article DOI: 10.1021/jm0606600 BindingDB Entry DOI: 10.7270/Q2251JZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359239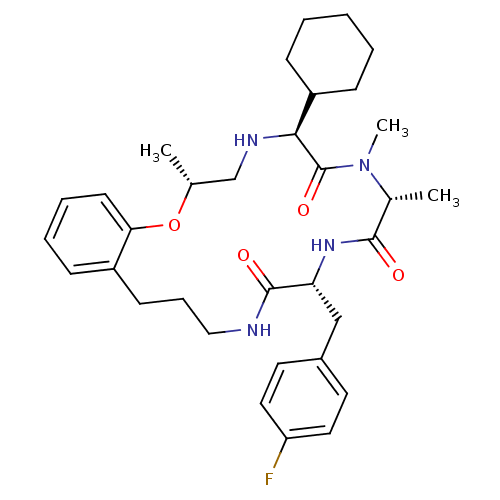 (CHEMBL1923629) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50079412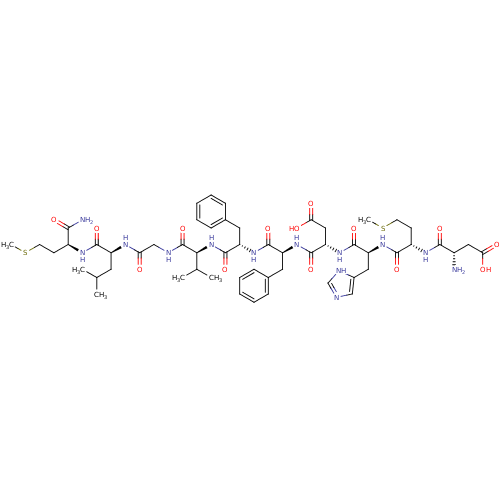 ((NKB)Asp-Met-His-Asp-Phe-Phe-Val-Gly-Leu-Met-NH2 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [125I][MePhe7]NKB from human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359250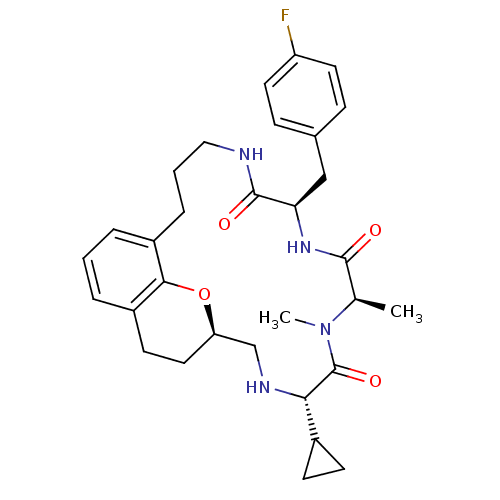 (CHEMBL1923640) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50291261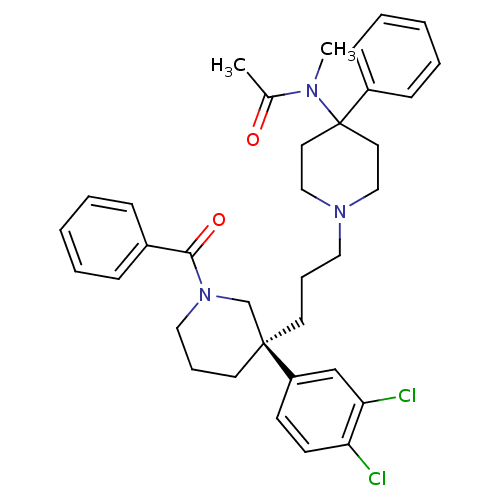 ((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081194 (CHEMBL3422009 | US10544150, Compound 156) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081194 (CHEMBL3422009 | US10544150, Compound 156) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Binding affinity to human recombinant NK3R by radioligand binding assay | ACS Med Chem Lett 6: 736-40 (2015) Article DOI: 10.1021/acsmedchemlett.5b00117 BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081396 (CHEMBL3422014) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359252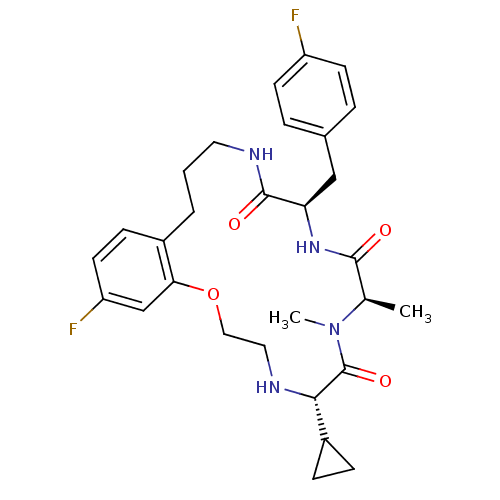 (CHEMBL1923642) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081196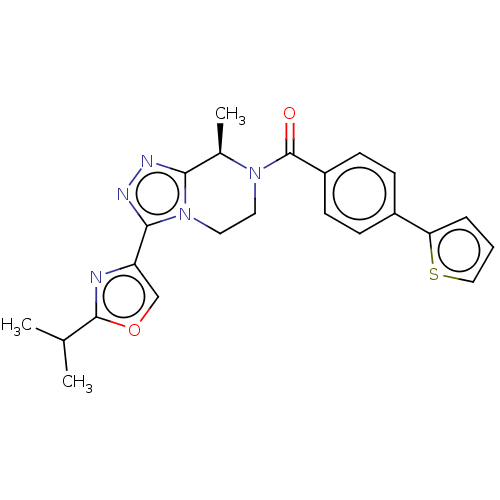 (CHEMBL3422015 | US10065961, Compound 13 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10941151 (2021) BindingDB Entry DOI: 10.7270/Q2PC35G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251923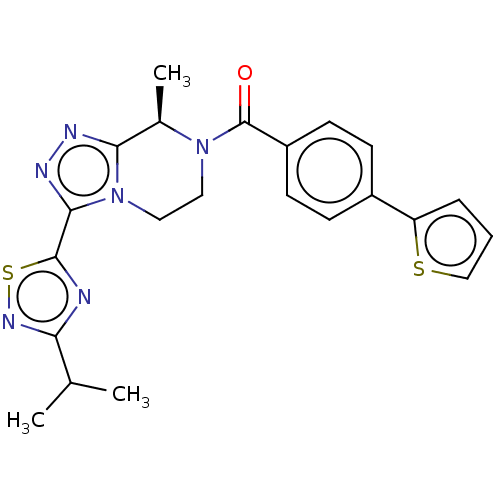 (BDBM272562 | US10683295, Compound 25 | US10941151,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA. US Patent | Assay Description The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad... | US Patent US10065961 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251923 (BDBM272562 | US10683295, Compound 25 | US10941151,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Euroscreen S.A. US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251923 (BDBM272562 | US10683295, Compound 25 | US10941151,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10941151 (2021) BindingDB Entry DOI: 10.7270/Q2PC35G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081196 (CHEMBL3422015 | US10065961, Compound 13 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Euroscreen S.A. US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081196 (CHEMBL3422015 | US10065961, Compound 13 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA. US Patent | Assay Description The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad... | US Patent US10065961 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251923 (BDBM272562 | US10683295, Compound 25 | US10941151,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10683295 (2020) BindingDB Entry DOI: 10.7270/Q2S75KC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081196 (CHEMBL3422015 | US10065961, Compound 13 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10683295 (2020) BindingDB Entry DOI: 10.7270/Q2S75KC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50052524 ((S)-N-((S)-1-{[(S)-1-({[(S)-1-((S)-1-Carbamoyl-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [125I][MePhe7]NKB from human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081198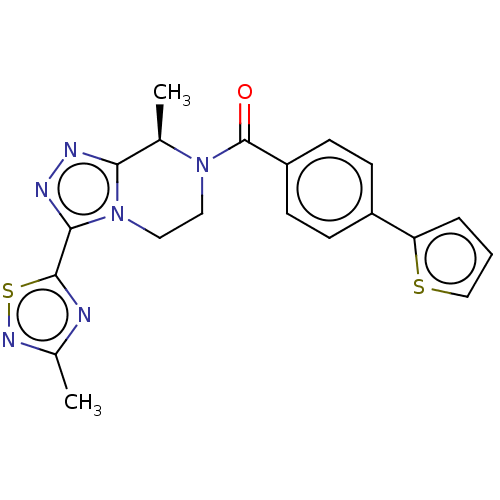 (CHEMBL3422018 | US10065961, Compound 24 | US947581...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Binding affinity to human recombinant NK3R by radioligand binding assay | ACS Med Chem Lett 6: 736-40 (2015) Article DOI: 10.1021/acsmedchemlett.5b00117 BindingDB Entry DOI: 10.7270/Q2TQ639V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081196 (CHEMBL3422015 | US10065961, Compound 13 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081198 (CHEMBL3422018 | US10065961, Compound 24 | US947581...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251909 (US10065961, Compound 7 | US10683295, Compound 7 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10941151 (2021) BindingDB Entry DOI: 10.7270/Q2PC35G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251909 (US10065961, Compound 7 | US10683295, Compound 7 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10683295 (2020) BindingDB Entry DOI: 10.7270/Q2S75KC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251909 (US10065961, Compound 7 | US10683295, Compound 7 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA. US Patent | Assay Description The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad... | US Patent US10065961 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251909 (US10065961, Compound 7 | US10683295, Compound 7 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Euroscreen S.A. US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Macaca mulatta) | BDBM50081194 (CHEMBL3422009 | US10544150, Compound 156) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from monkey NK3R after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359259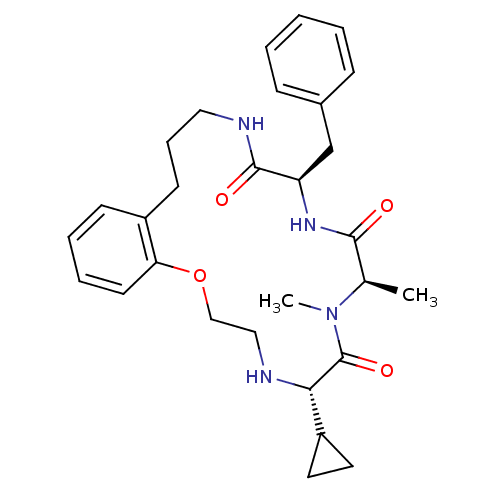 (CHEMBL1923610) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359238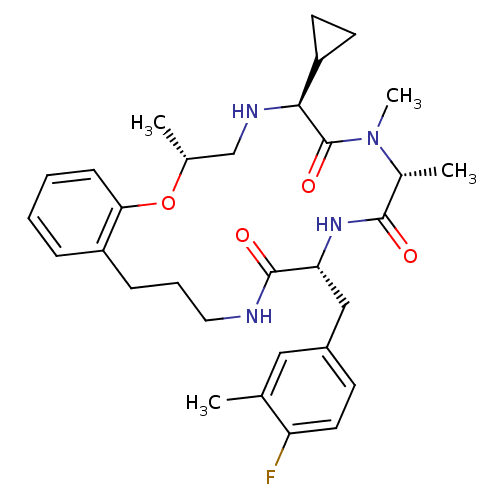 (CHEMBL1923628) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359238 (CHEMBL1923628) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359237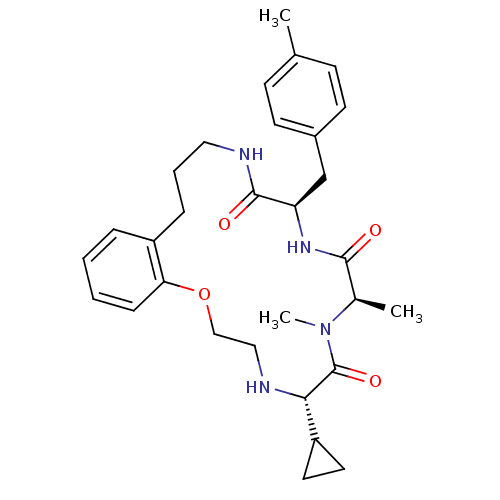 (CHEMBL1923627) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251907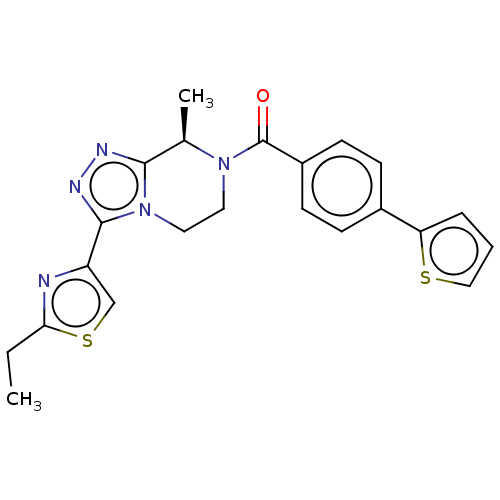 (US10065961, Compound 5 | US10683295, Compound 5 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10683295 (2020) BindingDB Entry DOI: 10.7270/Q2S75KC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251907 (US10065961, Compound 5 | US10683295, Compound 5 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10941151 (2021) BindingDB Entry DOI: 10.7270/Q2PC35G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251907 (US10065961, Compound 5 | US10683295, Compound 5 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA. US Patent | Assay Description The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad... | US Patent US10065961 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251907 (US10065961, Compound 5 | US10683295, Compound 5 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Euroscreen S.A. US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081399 (CHEMBL3422016 | US10065961, Compound 19 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Euroscreen S.A. US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081399 (CHEMBL3422016 | US10065961, Compound 19 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA. US Patent | Assay Description The antagonist activity of compounds of the invention is measured following pre-incubation (3 minutes) of the compound with the cells, followed by ad... | US Patent US10065961 (2018) BindingDB Entry DOI: 10.7270/Q2MW2K4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081399 (CHEMBL3422016 | US10065961, Compound 19 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10683295 (2020) BindingDB Entry DOI: 10.7270/Q2S75KC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081399 (CHEMBL3422016 | US10065961, Compound 19 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10941151 (2021) BindingDB Entry DOI: 10.7270/Q2PC35G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359233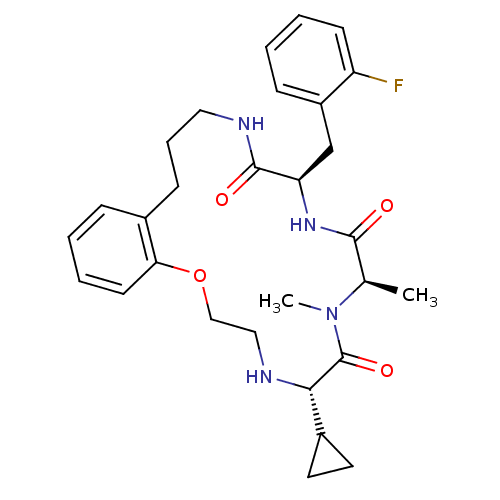 (CHEMBL1923623) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Macaca mulatta) | BDBM50081196 (CHEMBL3422015 | US10065961, Compound 13 | US106832...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from monkey NK3R after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Macaca mulatta) | BDBM50081198 (CHEMBL3422018 | US10065961, Compound 24 | US947581...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from monkey NK3R after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50081399 (CHEMBL3422016 | US10065961, Compound 19 | US106832...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Euroscreen SA Curated by ChEMBL | Assay Description Displacement of [3H]-SB222200 from recombinant human NK3R expressed in CHO cell membranes after 90 mins by scintillation counting analysis | J Med Chem 58: 3060-82 (2015) Article DOI: 10.1021/jm5017413 BindingDB Entry DOI: 10.7270/Q2ZP47TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Appetite-regulating hormone (Homo sapiens (Human)) | BDBM50359282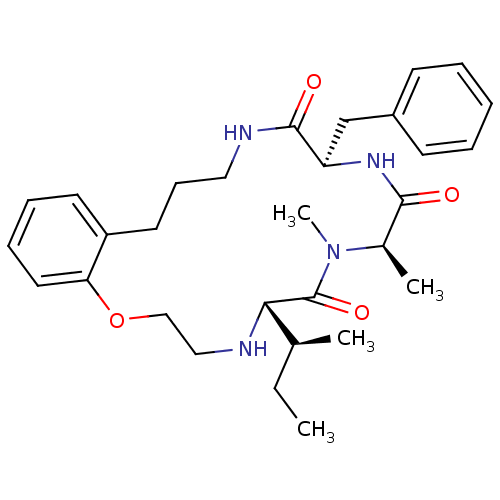 (CHEMBL1923609) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tranzyme Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay | J Med Chem 54: 8305-20 (2011) Article DOI: 10.1021/jm2007062 BindingDB Entry DOI: 10.7270/Q2930TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251910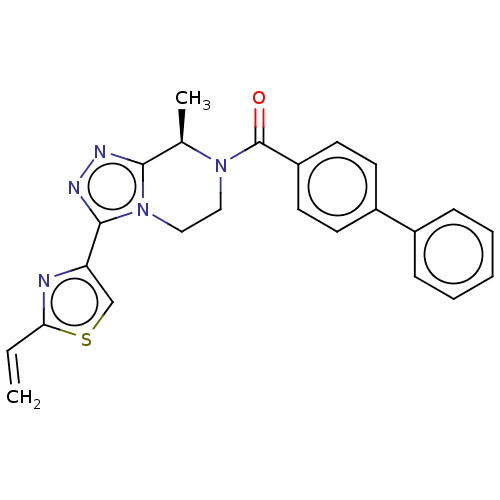 (US10065961, Compound 8 | US10683295, Compound 8 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Euroscreen S.A. US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251910 (US10065961, Compound 8 | US10683295, Compound 8 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10941151 (2021) BindingDB Entry DOI: 10.7270/Q2PC35G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251918 (US10065961, Compound 17 | US10683295, Compound 17 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10683295 (2020) BindingDB Entry DOI: 10.7270/Q2S75KC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251918 (US10065961, Compound 17 | US10683295, Compound 17 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10683295 (2020) BindingDB Entry DOI: 10.7270/Q2S75KC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251918 (US10065961, Compound 17 | US10683295, Compound 17 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Euroscreen S.A. US Patent | Assay Description The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra... | US Patent US9475814 (2016) BindingDB Entry DOI: 10.7270/Q2B8571Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM251918 (US10065961, Compound 17 | US10683295, Compound 17 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ogeda SA US Patent | Assay Description NK3: The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vit... | US Patent US10941151 (2021) BindingDB Entry DOI: 10.7270/Q2PC35G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 997 total ) | Next | Last >> |