 Found 502 hits with Last Name = 'frennesson' and Initial = 'd'
Found 502 hits with Last Name = 'frennesson' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin-like growth factor 1 receptor
(Rattus norvegicus) | BDBM50318112

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCN1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H33ClN6O3/c1-18-14-21(35-10-8-34(9-11-35)12-13-38-2)16-23-26(18)33-27(32-23)25-22(6-7-30-28(25)37)31-17-24(36)19-4-3-5-20(29)15-19/h3-7,14-16,24,36H,8-13,17H2,1-2H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of rat IGF1R |
Bioorg Med Chem Lett 20: 3182-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.057
BindingDB Entry DOI: 10.7270/Q2GX4BQB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252094
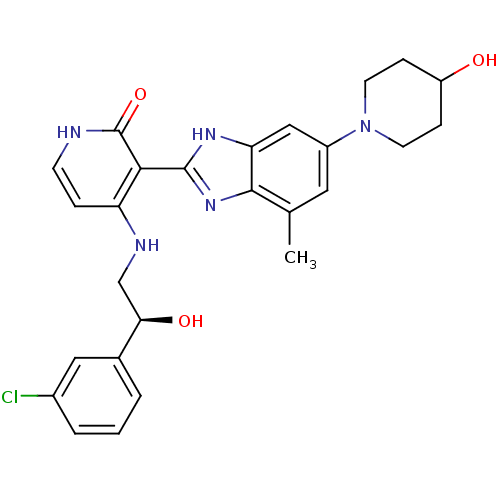
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(O)CC1 |r| Show InChI InChI=1S/C26H28ClN5O3/c1-15-11-18(32-9-6-19(33)7-10-32)13-21-24(15)31-25(30-21)23-20(5-8-28-26(23)35)29-14-22(34)16-3-2-4-17(27)12-16/h2-5,8,11-13,19,22,33-34H,6-7,9-10,14H2,1H3,(H,30,31)(H2,28,29,35)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252295

(3-(6-(4-((1R,4S)-5-oxa-2-aza-bicyclo[2.2.1]heptan-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1C[C@@H]2C[C@@H]1CO2 |r,THB:31:34:39.40:37| Show InChI InChI=1S/C31H35ClN6O3/c1-18-11-22(37-9-6-21(7-10-37)38-16-24-13-23(38)17-41-24)14-26-29(18)36-30(35-26)28-25(5-8-33-31(28)40)34-15-27(39)19-3-2-4-20(32)12-19/h2-5,8,11-12,14,21,23-24,27,39H,6-7,9-10,13,15-17H2,1H3,(H,35,36)(H2,33,34,40)/t23-,24+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252237

((S)-methyl 1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...)Show SMILES COC(=O)NC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H31ClN6O4/c1-16-12-20(35-10-7-19(8-11-35)32-28(38)39-2)14-22-25(16)34-26(33-22)24-21(6-9-30-27(24)37)31-15-23(36)17-4-3-5-18(29)13-17/h3-6,9,12-14,19,23,36H,7-8,10-11,15H2,1-2H3,(H,32,38)(H,33,34)(H2,30,31,37)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM27879

(4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCOCC1 |r| Show InChI InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252193
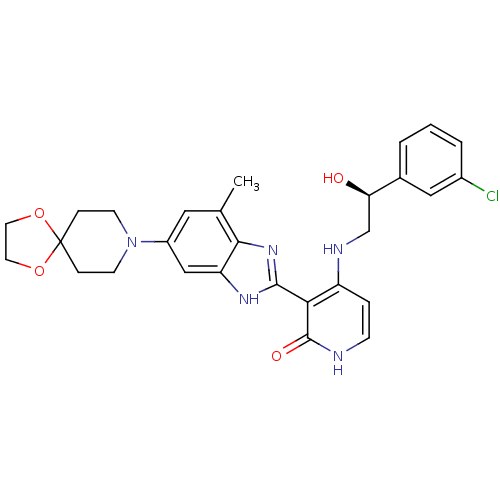
(4-((S)-2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC2(CC1)OCCO2 |r| Show InChI InChI=1S/C28H30ClN5O4/c1-17-13-20(34-9-6-28(7-10-34)37-11-12-38-28)15-22-25(17)33-26(32-22)24-21(5-8-30-27(24)36)31-16-23(35)18-3-2-4-19(29)14-18/h2-5,8,13-15,23,35H,6-7,9-12,16H2,1H3,(H,32,33)(H2,30,31,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252236
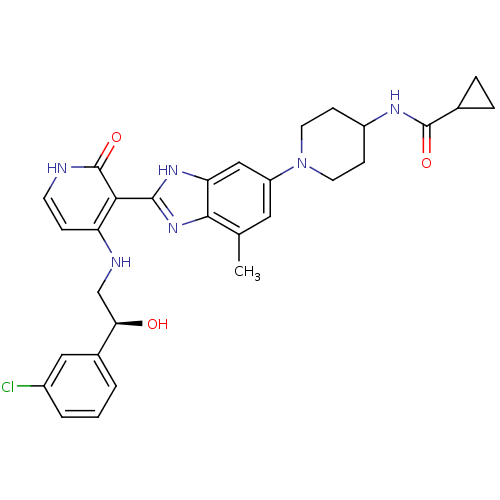
((S)-N-(1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyethyla...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)NC(=O)C1CC1 |r| Show InChI InChI=1S/C30H33ClN6O3/c1-17-13-22(37-11-8-21(9-12-37)34-29(39)18-5-6-18)15-24-27(17)36-28(35-24)26-23(7-10-32-30(26)40)33-16-25(38)19-3-2-4-20(31)14-19/h2-4,7,10,13-15,18,21,25,38H,5-6,8-9,11-12,16H2,1H3,(H,34,39)(H,35,36)(H2,32,33,40)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252297
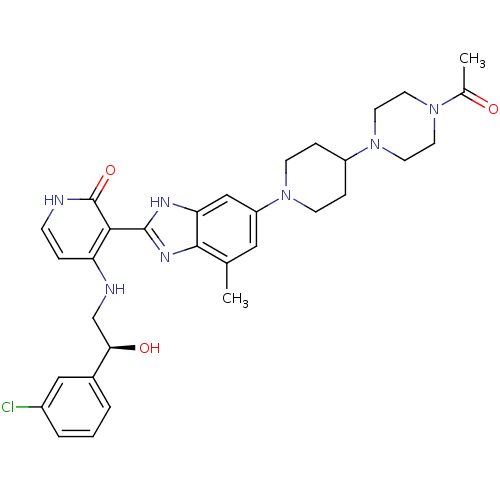
((S)-3-(6-(4-(4-acetylpiperazin-1-yl)piperidin-1-yl...)Show SMILES CC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H38ClN7O3/c1-20-16-25(39-10-7-24(8-11-39)40-14-12-38(13-15-40)21(2)41)18-27-30(20)37-31(36-27)29-26(6-9-34-32(29)43)35-19-28(42)22-4-3-5-23(33)17-22/h3-6,9,16-18,24,28,42H,7-8,10-15,19H2,1-2H3,(H,36,37)(H2,34,35,43)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252143
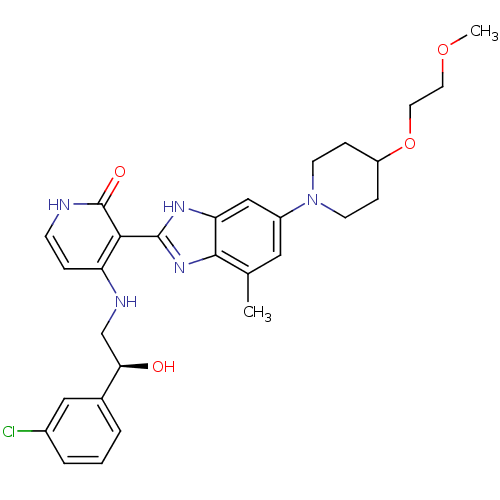
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCOC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C29H34ClN5O4/c1-18-14-21(35-10-7-22(8-11-35)39-13-12-38-2)16-24-27(18)34-28(33-24)26-23(6-9-31-29(26)37)32-17-25(36)19-4-3-5-20(30)15-19/h3-6,9,14-16,22,25,36H,7-8,10-13,17H2,1-2H3,(H,33,34)(H2,31,32,37)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252142

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H30ClN5O3/c1-16-12-19(33-10-7-20(36-2)8-11-33)14-22-25(16)32-26(31-22)24-21(6-9-29-27(24)35)30-15-23(34)17-4-3-5-18(28)13-17/h3-6,9,12-14,20,23,34H,7-8,10-11,15H2,1-2H3,(H,31,32)(H2,29,30,35)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107016
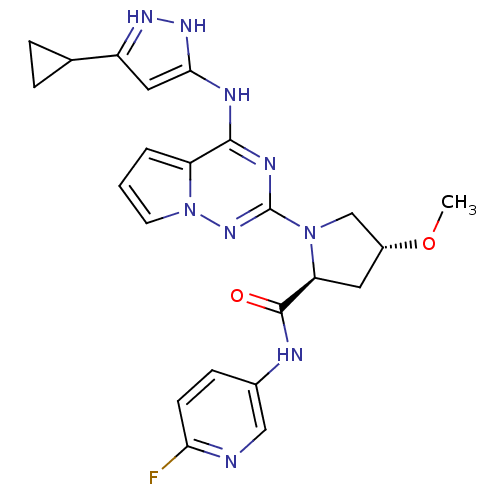
(US8592579, 219)Show SMILES CO[C@@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O2/c1-35-15-9-18(22(34)26-14-6-7-19(24)25-11-14)32(12-15)23-28-21(17-3-2-8-33(17)31-23)27-20-10-16(29-30-20)13-4-5-13/h2-3,6-8,10-11,13,15,18H,4-5,9,12H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t15-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM144614
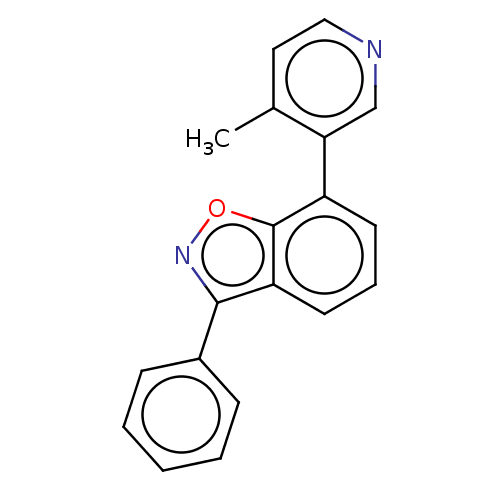
(US8969586, 1 | US9598436, 1)Show InChI InChI=1S/C19H14N2O/c1-13-10-11-20-12-17(13)15-8-5-9-16-18(21-22-19(15)16)14-6-3-2-4-7-14/h2-12H,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107013

(US8592579, 211)Show SMILES O[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1cccnc1 |r| Show InChI InChI=1S/C22H23N9O2/c32-15-9-18(21(33)24-14-3-1-7-23-11-14)30(12-15)22-26-20(17-4-2-8-31(17)29-22)25-19-10-16(27-28-19)13-5-6-13/h1-4,7-8,10-11,13,15,18,32H,5-6,9,12H2,(H,24,33)(H2,25,26,27,28,29)/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107018

(US8592579, 254)Show SMILES CO[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O2/c1-35-15-9-18(22(34)26-14-6-7-19(24)25-11-14)32(12-15)23-28-21(17-3-2-8-33(17)31-23)27-20-10-16(29-30-20)13-4-5-13/h2-3,6-8,10-11,13,15,18H,4-5,9,12H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107023

(US8592579, 288)Show SMILES CC1(CC1)c1cc(Nc2nc(nn3cccc23)N2C[C@@H](O)C[C@H]2C(=O)Nc2ncns2)[nH]n1 |r| Show InChI InChI=1S/C20H22N10O2S/c1-20(4-5-20)14-8-15(27-26-14)23-16-12-3-2-6-30(12)28-18(24-16)29-9-11(31)7-13(29)17(32)25-19-21-10-22-33-19/h2-3,6,8,10-11,13,31H,4-5,7,9H2,1H3,(H,21,22,25,32)(H2,23,24,26,27,28)/t11-,13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252238

((S)-methyl 1-(2-(4-(2-(3-chlorophenyl)-2-hydroxyet...)Show SMILES COCCN(CCOC)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H41ClN6O4/c1-21-17-25(38-11-8-24(9-12-38)39(13-15-42-2)14-16-43-3)19-27-30(21)37-31(36-27)29-26(7-10-34-32(29)41)35-20-28(40)22-5-4-6-23(33)18-22/h4-7,10,17-19,24,28,40H,8-9,11-16,20H2,1-3H3,(H,36,37)(H2,34,35,41)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252144

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)OCCO |r| Show InChI InChI=1S/C28H32ClN5O4/c1-17-13-20(34-9-6-21(7-10-34)38-12-11-35)15-23-26(17)33-27(32-23)25-22(5-8-30-28(25)37)31-16-24(36)18-3-2-4-19(29)14-18/h2-5,8,13-15,21,24,35-36H,6-7,9-12,16H2,1H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252300
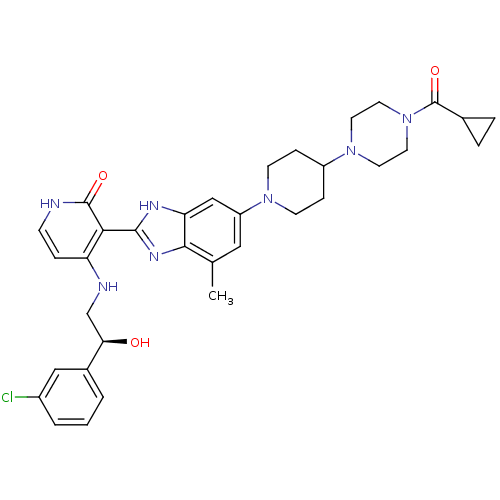
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1CCN(CC1)C(=O)C1CC1 |r| Show InChI InChI=1S/C34H40ClN7O3/c1-21-17-26(40-11-8-25(9-12-40)41-13-15-42(16-14-41)34(45)22-5-6-22)19-28-31(21)39-32(38-28)30-27(7-10-36-33(30)44)37-20-29(43)23-3-2-4-24(35)18-23/h2-4,7,10,17-19,22,25,29,43H,5-6,8-9,11-16,20H2,1H3,(H,38,39)(H2,36,37,44)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252303
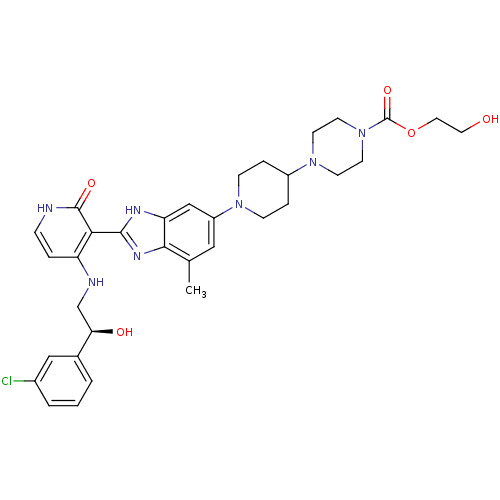
((S)-2-hydroxyethyl 4-(1-(2-(4-(2-(3-chlorophenyl)-...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1CCN(CC1)C(=O)OCCO |r| Show InChI InChI=1S/C33H40ClN7O5/c1-21-17-25(39-9-6-24(7-10-39)40-11-13-41(14-12-40)33(45)46-16-15-42)19-27-30(21)38-31(37-27)29-26(5-8-35-32(29)44)36-20-28(43)22-3-2-4-23(34)18-22/h2-5,8,17-19,24,28,42-43H,6-7,9-16,20H2,1H3,(H,37,38)(H2,35,36,44)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107003
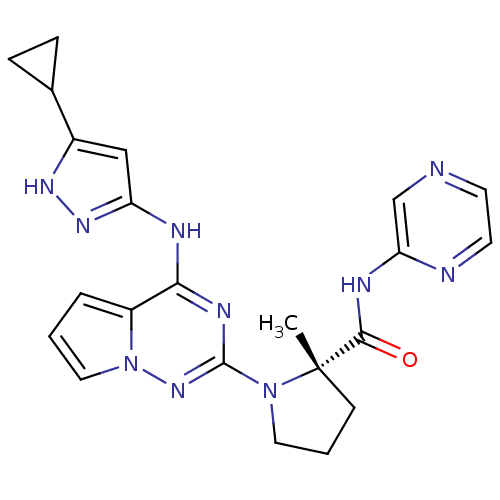
(CHEMBL575447 | US8592579, 110)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1cnccn1 |r| Show InChI InChI=1S/C22H24N10O/c1-22(20(33)26-18-13-23-8-9-24-18)7-3-10-31(22)21-27-19(16-4-2-11-32(16)30-21)25-17-12-15(28-29-17)14-5-6-14/h2,4,8-9,11-14H,3,5-7,10H2,1H3,(H,24,26,33)(H2,25,27,28,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50405216
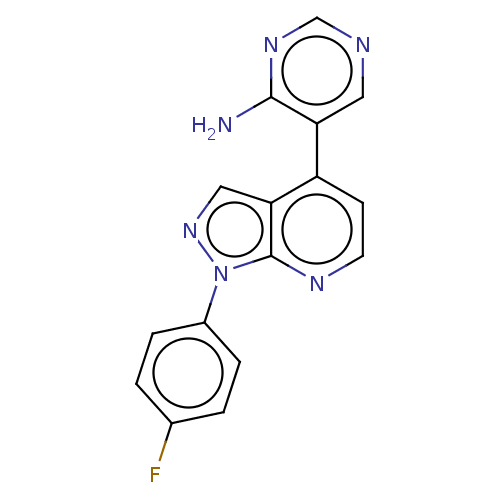
(CHEMBL5290029)Show InChI InChI=1S/C8H13N5O2/c1-3-13(4-2)6-5(12-15)7(14)11-8(9)10-6/h3-4H2,1-2H3,(H3,9,10,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against cell free dihydrofolate reductase (DHFR) from rat |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Macaca fascicularis) | BDBM50405216

(CHEMBL5290029)Show InChI InChI=1S/C8H13N5O2/c1-3-13(4-2)6-5(12-15)7(14)11-8(9)10-6/h3-4H2,1-2H3,(H3,9,10,11,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufu |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50299138
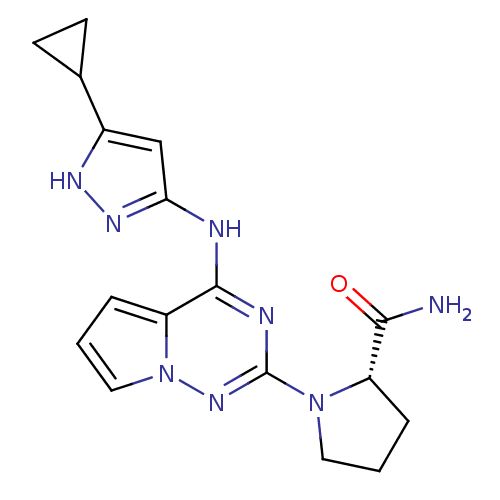
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES NC(=O)[C@@H]1CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1 |r| Show InChI InChI=1S/C17H20N8O/c18-15(26)12-3-1-7-24(12)17-20-16(13-4-2-8-25(13)23-17)19-14-9-11(21-22-14)10-5-6-10/h2,4,8-10,12H,1,3,5-7H2,(H2,18,26)(H2,19,20,21,22,23)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299148
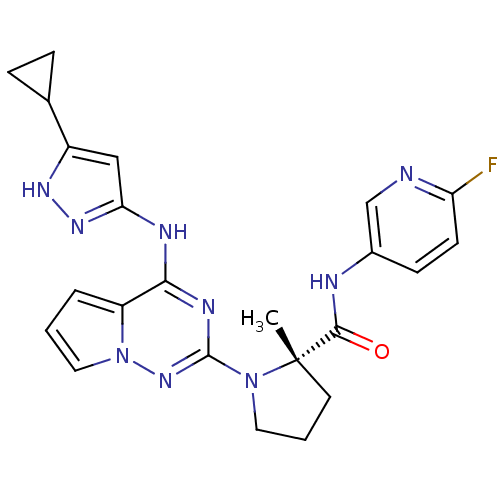
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299143
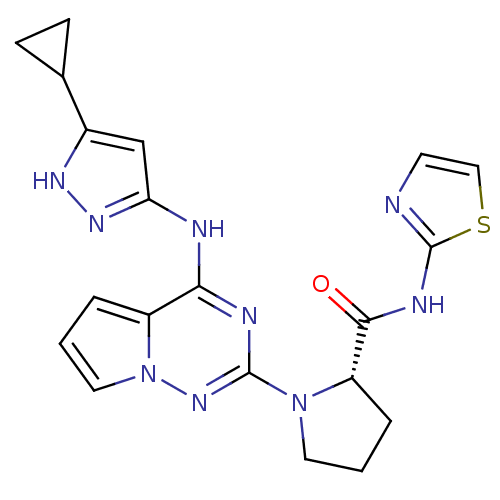
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES O=C(Nc1nccs1)[C@@H]1CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1 |r| Show InChI InChI=1S/C20H21N9OS/c30-18(24-20-21-7-10-31-20)15-4-1-8-28(15)19-23-17(14-3-2-9-29(14)27-19)22-16-11-13(25-26-16)12-5-6-12/h2-3,7,9-12,15H,1,4-6,8H2,(H,21,24,30)(H2,22,23,25,26,27)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107026
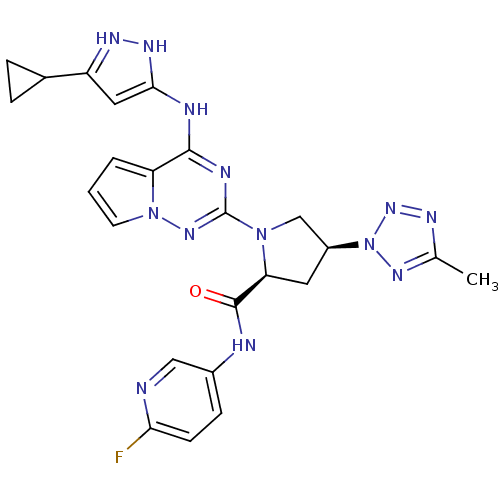
(US8592579, 301)Show SMILES Cc1nnn(n1)[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C24H24FN13O/c1-13-30-35-38(33-13)16-9-19(23(39)27-15-6-7-20(25)26-11-15)36(12-16)24-29-22(18-3-2-8-37(18)34-24)28-21-10-17(31-32-21)14-4-5-14/h2-3,6-8,10-11,14,16,19H,4-5,9,12H2,1H3,(H,27,39)(H2,28,29,31,32,34)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107024

(US8592579, 293)Show SMILES F[C@@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ncns1 |r| Show InChI InChI=1S/C19H19FN10OS/c20-11-6-14(17(31)25-19-21-9-22-32-19)29(8-11)18-24-16(13-2-1-5-30(13)28-18)23-15-7-12(26-27-15)10-3-4-10/h1-2,5,7,9-11,14H,3-4,6,8H2,(H,21,22,25,31)(H2,23,24,26,27,28)/t11-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107021

(US8592579, 259)Show SMILES CC1(CC1)c1cc(Nc2nc(nn3cccc23)N2C[C@@H](O)C[C@H]2C(=O)Nc2cnccn2)[nH]n1 |r| Show InChI InChI=1S/C22H24N10O2/c1-22(4-5-22)16-10-17(29-28-16)25-19-14-3-2-8-32(14)30-21(27-19)31-12-13(33)9-15(31)20(34)26-18-11-23-6-7-24-18/h2-3,6-8,10-11,13,15,33H,4-5,9,12H2,1H3,(H,24,26,34)(H2,25,27,28,29,30)/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107020

(US8592579, 256)Show SMILES CO[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ncns1 |r| Show InChI InChI=1S/C20H22N10O2S/c1-32-12-7-15(18(31)25-20-21-10-22-33-20)29(9-12)19-24-17(14-3-2-6-30(14)28-19)23-16-8-13(26-27-16)11-4-5-11/h2-3,6,8,10-12,15H,4-5,7,9H2,1H3,(H,21,22,25,31)(H2,23,24,26,27,28)/t12-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107012

(US8592579, 209)Show SMILES O[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1cnccn1 |r| Show InChI InChI=1S/C21H22N10O2/c32-13-8-16(20(33)25-18-10-22-5-6-23-18)30(11-13)21-26-19(15-2-1-7-31(15)29-21)24-17-9-14(27-28-17)12-3-4-12/h1-2,5-7,9-10,12-13,16,32H,3-4,8,11H2,(H,23,25,33)(H2,24,26,27,28,29)/t13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50404835

(CHEMBL5284599)Show InChI InChI=1S/C11H18O3S/c1-7(6-15)10(12)8-4-2-3-5-9(8)11(13)14/h7-9,15H,2-6H2,1H3,(H,13,14)/t7-,8-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107000

(US8592579, 104)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ccc(F)nc1 |r| Show InChI InChI=1S/C23H24FN9O/c1-23(21(34)26-15-7-8-18(24)25-13-15)9-3-10-32(23)22-28-20(17-4-2-11-33(17)31-22)27-19-12-16(29-30-19)14-5-6-14/h2,4,7-8,11-14H,3,5-6,9-10H2,1H3,(H,26,34)(H2,27,28,29,30,31)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252296
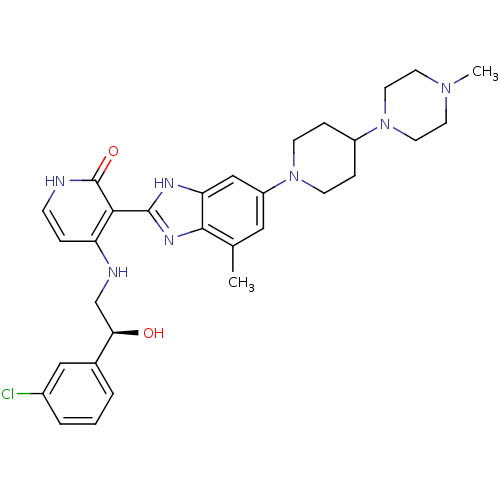
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CN1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C31H38ClN7O2/c1-20-16-24(38-10-7-23(8-11-38)39-14-12-37(2)13-15-39)18-26-29(20)36-30(35-26)28-25(6-9-33-31(28)41)34-19-27(40)21-4-3-5-22(32)17-21/h3-6,9,16-18,23,27,40H,7-8,10-15,19H2,1-2H3,(H,35,36)(H2,33,34,41)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252302

((S)-2-methoxyethyl 4-(1-(2-(4-(2-(3-chlorophenyl)-...)Show SMILES COCCOC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C34H42ClN7O5/c1-22-18-26(40-10-7-25(8-11-40)41-12-14-42(15-13-41)34(45)47-17-16-46-2)20-28-31(22)39-32(38-28)30-27(6-9-36-33(30)44)37-21-29(43)23-4-3-5-24(35)19-23/h3-6,9,18-20,25,29,43H,7-8,10-17,21H2,1-2H3,(H,38,39)(H2,36,37,44)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252194
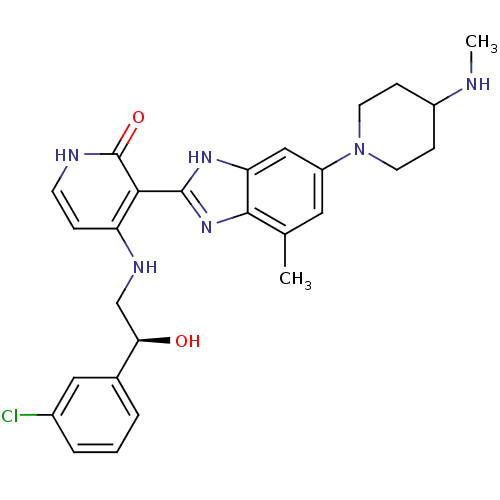
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CNC1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C27H31ClN6O2/c1-16-12-20(34-10-7-19(29-2)8-11-34)14-22-25(16)33-26(32-22)24-21(6-9-30-27(24)36)31-15-23(35)17-4-3-5-18(28)13-17/h3-6,9,12-14,19,23,29,35H,7-8,10-11,15H2,1-2H3,(H,32,33)(H2,30,31,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405184
(CHEMBL5290743)Show SMILES CCCCC[C@@H](O)\C=C\[C@H]1C2CCC(O2)[C@@H]1C\C=C/CCCC(O)=O |TLB:16:15:14:12.11| Show InChI InChI=1S/C21H34O4/c1-2-3-6-9-16(22)12-13-18-17(19-14-15-20(18)25-19)10-7-4-5-8-11-21(23)24/h4,7,12-13,16-20,22H,2-3,5-6,8-11,14-15H2,1H3,(H,23,24)/b7-4-,13-12+/t16-,17-,18-,19?,20?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107028

(US8592579, 318)Show SMILES F[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ncns1 |r| Show InChI InChI=1S/C19H19FN10OS/c20-11-6-14(17(31)25-19-21-9-22-32-19)29(8-11)18-24-16(13-2-1-5-30(13)28-18)23-15-7-12(26-27-15)10-3-4-10/h1-2,5,7,9-11,14H,3-4,6,8H2,(H,21,22,25,31)(H2,23,24,26,27,28)/t11-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252299

((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES COCCOCC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C35H44ClN7O5/c1-23-18-27(41-10-7-26(8-11-41)42-12-14-43(15-13-42)31(45)22-48-17-16-47-2)20-29-33(23)40-34(39-29)32-28(6-9-37-35(32)46)38-21-30(44)24-4-3-5-25(36)19-24/h3-6,9,18-20,26,30,44H,7-8,10-17,21-22H2,1-2H3,(H,39,40)(H2,37,38,46)/t30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107022
(US8592579, 287)Show SMILES O[C@H]1C[C@H](N(C1)c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1ncns1 |r| Show InChI InChI=1S/C19H20N10O2S/c30-11-6-14(17(31)24-19-20-9-21-32-19)28(8-11)18-23-16(13-2-1-5-29(13)27-18)22-15-7-12(25-26-15)10-3-4-10/h1-2,5,7,9-11,14,30H,3-4,6,8H2,(H,20,21,24,31)(H2,22,23,25,26,27)/t11-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50404839
(CHEMBL5286021)Show InChI InChI=1S/C8H12O3S/c9-7(3-4-12)5-1-2-6(5)8(10)11/h5-6,12H,1-4H2,(H,10,11)/t5-,6+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107003

(CHEMBL575447 | US8592579, 110)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1cnccn1 |r| Show InChI InChI=1S/C22H24N10O/c1-22(20(33)26-18-13-23-8-9-24-18)7-3-10-31(22)21-27-19(16-4-2-11-32(16)30-21)25-17-12-15(28-29-17)14-5-6-14/h2,4,8-9,11-14H,3,5-7,10H2,1H3,(H,24,26,33)(H2,25,27,28,29,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252294
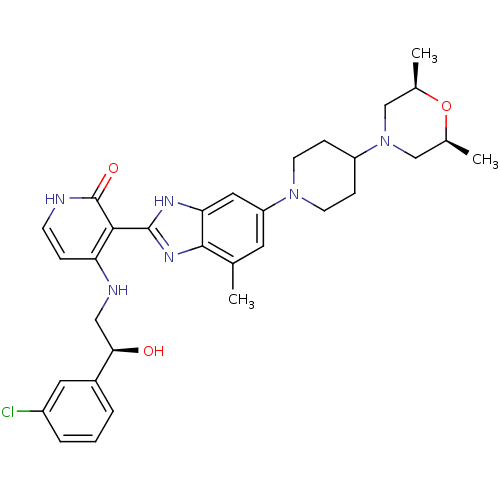
(4-((S)-2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES C[C@H]1CN(C[C@@H](C)O1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H39ClN6O3/c1-19-13-25(38-11-8-24(9-12-38)39-17-20(2)42-21(3)18-39)15-27-30(19)37-31(36-27)29-26(7-10-34-32(29)41)35-16-28(40)22-5-4-6-23(33)14-22/h4-7,10,13-15,20-21,24,28,40H,8-9,11-12,16-18H2,1-3H3,(H,36,37)(H2,34,35,41)/t20-,21+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252301
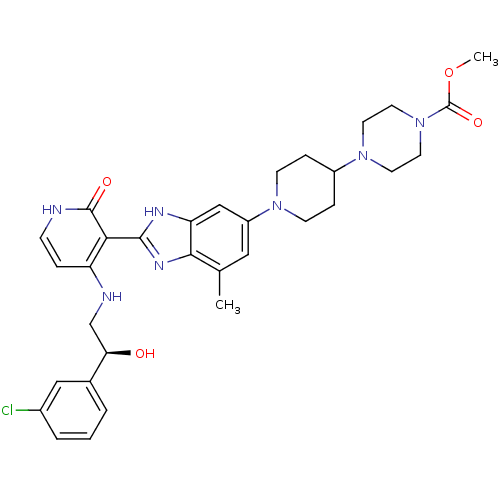
((S)-methyl 4-(1-(2-(4-(2-(3-chlorophenyl)-2-hydrox...)Show SMILES COC(=O)N1CCN(CC1)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C32H38ClN7O4/c1-20-16-24(38-10-7-23(8-11-38)39-12-14-40(15-13-39)32(43)44-2)18-26-29(20)37-30(36-26)28-25(6-9-34-31(28)42)35-19-27(41)21-4-3-5-22(33)17-21/h3-6,9,16-18,23,27,41H,7-8,10-15,19H2,1-2H3,(H,36,37)(H2,34,35,42)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252195
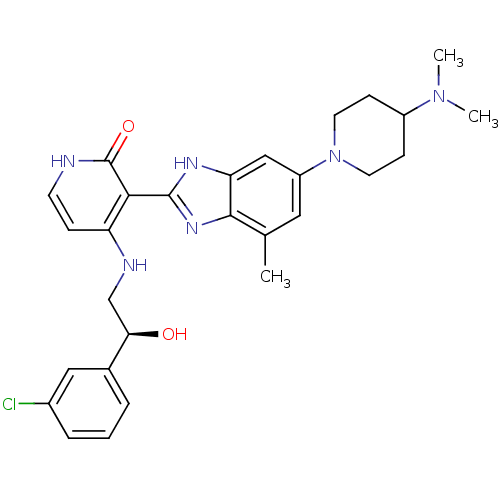
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES CN(C)C1CCN(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O |r| Show InChI InChI=1S/C28H33ClN6O2/c1-17-13-21(35-11-8-20(9-12-35)34(2)3)15-23-26(17)33-27(32-23)25-22(7-10-30-28(25)37)31-16-24(36)18-5-4-6-19(29)14-18/h4-7,10,13-15,20,24,36H,8-9,11-12,16H2,1-3H3,(H,32,33)(H2,30,31,37)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405189
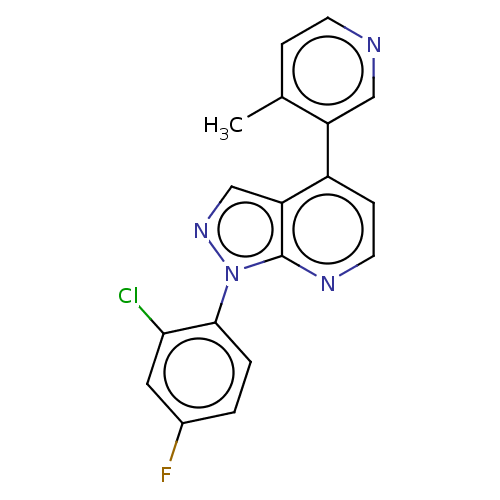
(CHEMBL5284859)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)CC[C@H]4N)ccc5O |r| Show InChI InChI=1S/C17H22N2O3/c1-19-7-6-16-13-9-2-3-11(20)14(13)22-15(16)10(18)4-5-17(16,21)12(19)8-9/h2-3,10,12,15,20-21H,4-8,18H2,1H3/t10-,12-,15+,16+,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity by the displacement of [3H]NMS binding to muscarinic M1 receptor of rat brain |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50405217
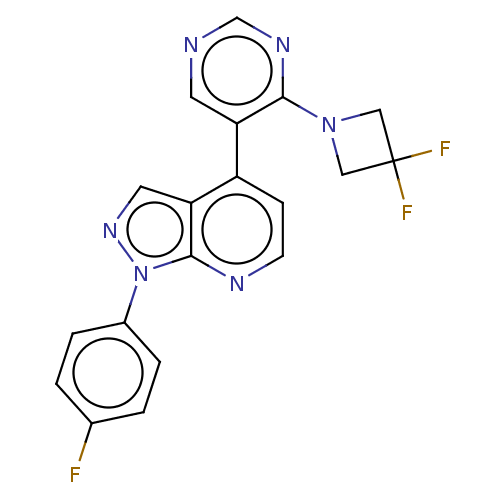
(CHEMBL5265986)Show InChI InChI=1S/C15H19N5O2/c16-15-18-13(12(20-22)14(21)19-15)17-10-6-2-5-9-11-7-3-1-4-8-11/h1,3-4,7-8H,2,5-6,9-10H2,(H4,16,17,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity by the displacement of [3H]NMS binding to muscarinic M1 receptor of rat brain |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50299145
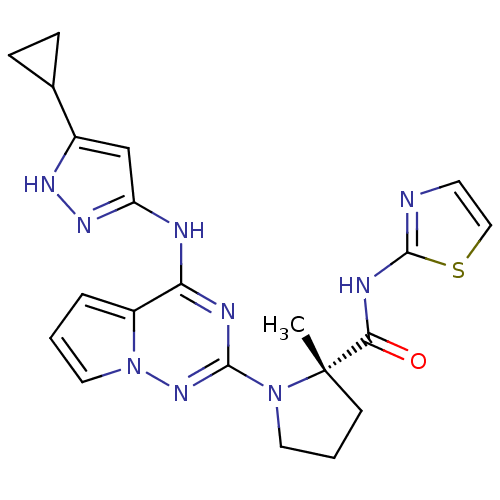
((S)-1-(4-(5-cyclopropyl-1H-pyrazol-3-ylamino)pyrro...)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc([nH]n2)C2CC2)c2cccn2n1)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C21H23N9OS/c1-21(18(31)25-20-22-8-11-32-20)7-3-9-29(21)19-24-17(15-4-2-10-30(15)28-19)23-16-12-14(26-27-16)13-5-6-13/h2,4,8,10-13H,3,5-7,9H2,1H3,(H,22,25,31)(H2,23,24,26,27,28)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R after 60 mins by fluorescence electrophoresis |
J Med Chem 52: 7360-3 (2009)
Article DOI: 10.1021/jm900786r
BindingDB Entry DOI: 10.7270/Q2P27022 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM107001

(US8592579, 105)Show SMILES C[C@]1(CCCN1c1nc(Nc2cc(n[nH]2)C2CC2)c2cccn2n1)C(=O)Nc1nccs1 |r| Show InChI InChI=1S/C21H23N9OS/c1-21(18(31)25-20-22-8-11-32-20)7-3-9-29(21)19-24-17(15-4-2-10-30(15)28-19)23-16-12-14(26-27-16)13-5-6-13/h2,4,8,10-13H,3,5-7,9H2,1H3,(H,22,25,31)(H2,23,24,26,27,28)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
Kinase assay using IGF1-receptor. |
US Patent US8592579 (2013)
BindingDB Entry DOI: 10.7270/Q2SQ8Z12 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252293
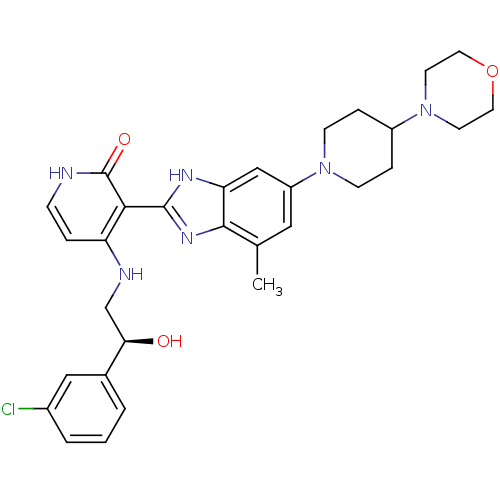
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1CCOCC1 |r| Show InChI InChI=1S/C30H35ClN6O3/c1-19-15-23(36-9-6-22(7-10-36)37-11-13-40-14-12-37)17-25-28(19)35-29(34-25)27-24(5-8-32-30(27)39)33-18-26(38)20-3-2-4-21(31)16-20/h2-5,8,15-17,22,26,38H,6-7,9-14,18H2,1H3,(H,34,35)(H2,32,33,39)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50252235
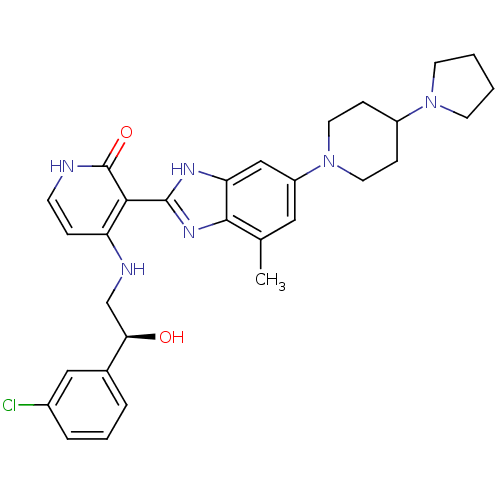
((S)-4-(2-(3-chlorophenyl)-2-hydroxyethylamino)-3-(...)Show SMILES Cc1cc(cc2[nH]c(nc12)-c1c(NC[C@@H](O)c2cccc(Cl)c2)cc[nH]c1=O)N1CCC(CC1)N1CCCC1 |r| Show InChI InChI=1S/C30H35ClN6O2/c1-19-15-23(37-13-8-22(9-14-37)36-11-2-3-12-36)17-25-28(19)35-29(34-25)27-24(7-10-32-30(27)39)33-18-26(38)20-5-4-6-21(31)16-20/h4-7,10,15-17,22,26,38H,2-3,8-9,11-14,18H2,1H3,(H,34,35)(H2,32,33,39)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 18: 4075-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.104
BindingDB Entry DOI: 10.7270/Q2V40V03 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data