

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ecdysone receptor (Aedes aegypti) | BDBM50128466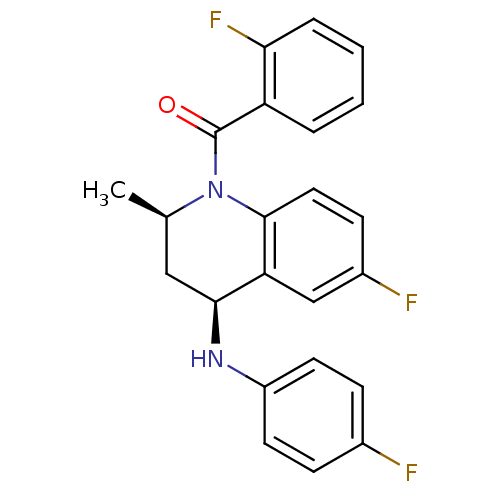 (CHEMBL291513 | [(2R,4S)-6-Fluoro-4-(4-fluoro-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128468 ((4-Chloro-phenyl)-[(2R,4S)-6-fluoro-4-(4-fluoro-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128467 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128469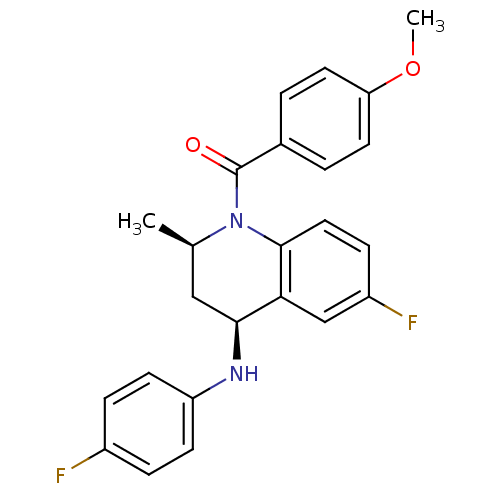 (CHEMBL56663 | [(2R,4S)-6-Fluoro-4-(4-fluoro-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128471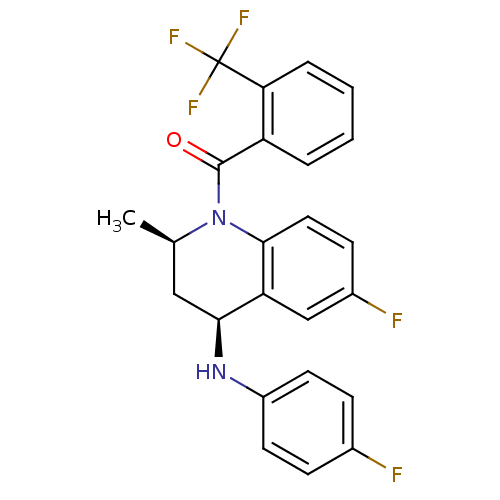 (CHEMBL59557 | [(2R,4S)-6-Fluoro-4-(4-fluoro-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128470 (CHEMBL416140 | [(2R,4S)-6-Fluoro-4-(4-fluoro-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.19E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128472 (CHEMBL294491 | [(2R,4S)-6-Fluoro-4-(4-fluoro-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128473 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128474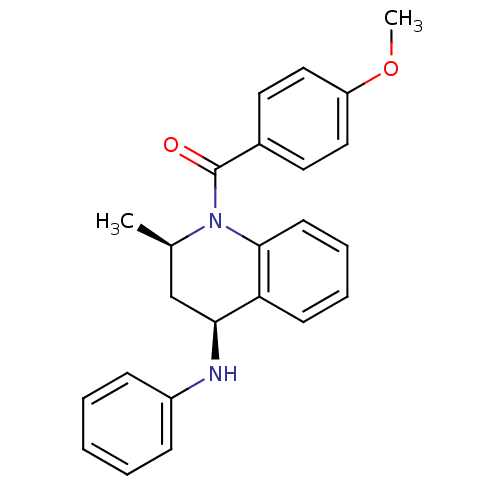 ((4-Methoxy-phenyl)-((2R,4S)-2-methyl-4-phenylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128475 ((3-Fluoro-phenyl)-((2R,4S)-2-methyl-4-phenylamino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128476 (CHEMBL61369 | [(2R,4S)-6-Fluoro-4-(4-fluoro-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128479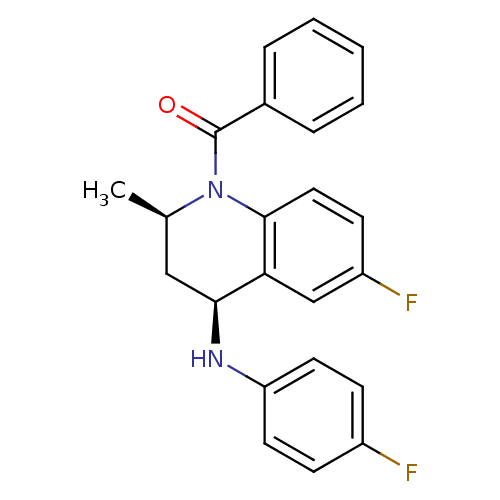 (CHEMBL57186 | [(2R,4S)-6-Fluoro-4-(4-fluoro-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128478 (CHEMBL301460 | [(2R,4S)-6-Fluoro-4-(4-fluoro-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128477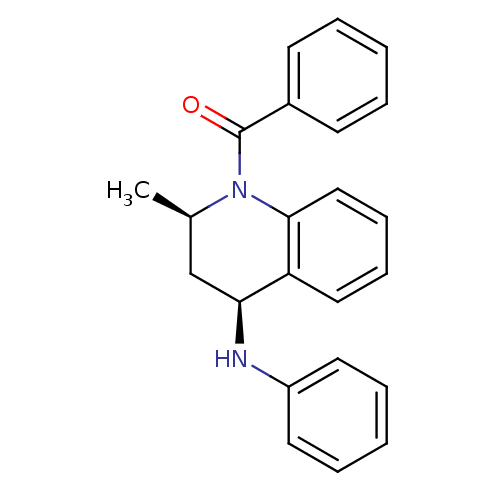 (((2R,4S)-2-Methyl-4-phenylamino-3,4-dihydro-2H-qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128480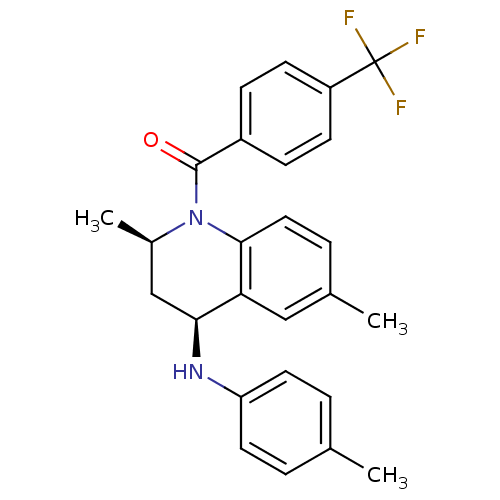 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.41E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128481 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 5.32E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128309 (3,5-Dimethyl-benzoic acid N-tert-butyl-N'-(2-ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128484 (CHEMBL58731 | [(2R,4S)-6-Fluoro-4-(4-fluoro-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 990 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128483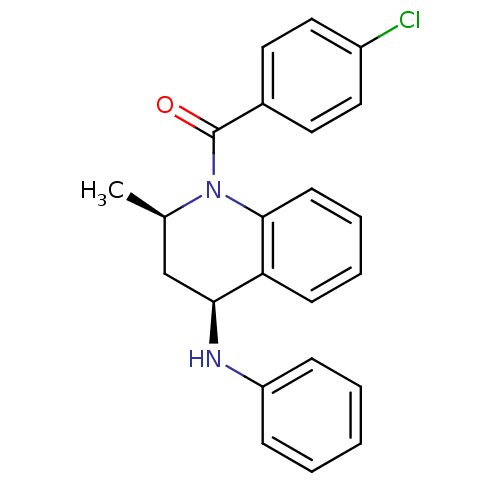 ((4-Chloro-phenyl)-((2R,4S)-2-methyl-4-phenylamino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 980 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128482 (CHEMBL58694 | [(2R,4S)-6-Fluoro-4-(4-fluoro-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 920 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128485 (CHEMBL291343 | [(2R,4S)-6-Fluoro-4-(4-fluoro-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128486 (((2R,4S)-2-Methyl-4-phenylamino-3,4-dihydro-2H-qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128493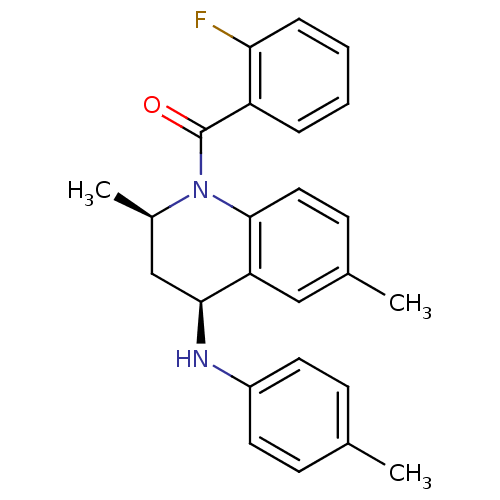 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128494 ((3-Methoxy-phenyl)-((2R,4S)-2-methyl-4-phenylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128488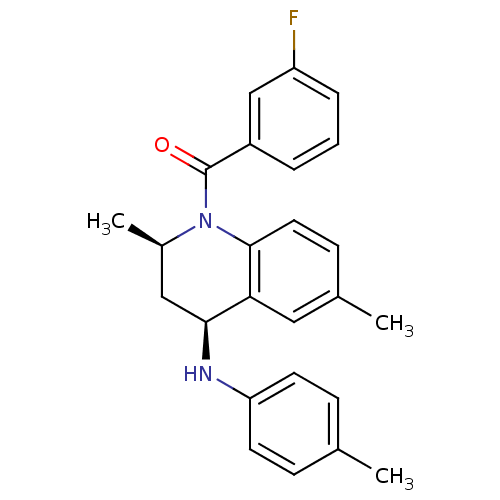 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128490 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128489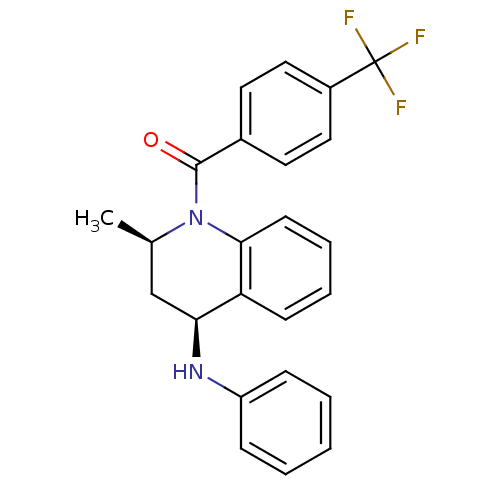 (((2R,4S)-2-Methyl-4-phenylamino-3,4-dihydro-2H-qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128487 (CHEMBL57114 | [(2R,4S)-6-Fluoro-4-(4-fluoro-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 870 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128491 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.33E+4 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128492 ((4-Chloro-phenyl)-((2R,4S)-2,6-dimethyl-4-p-tolyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128495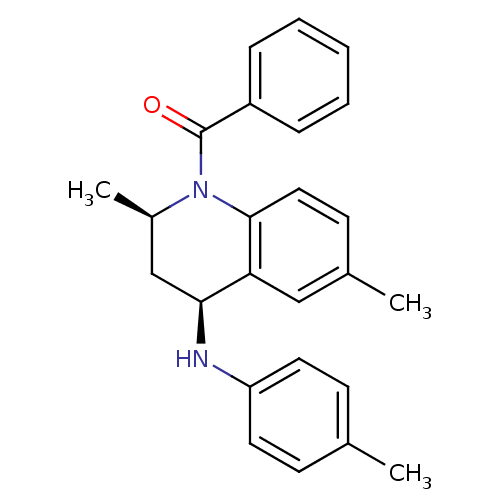 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128497 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128496 (((2R,4S)-2-Methyl-4-phenylamino-3,4-dihydro-2H-qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 990 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Aedes aegypti) | BDBM50128498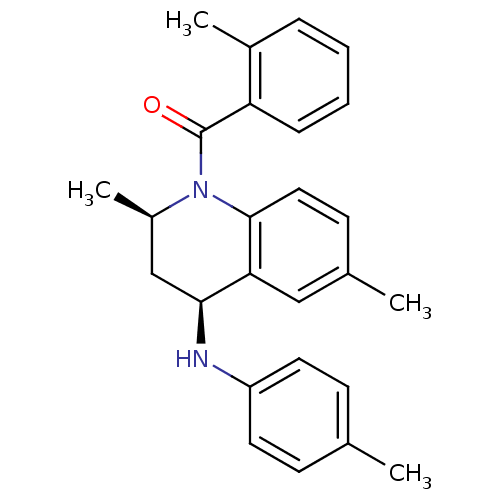 (((2R,4S)-2,6-Dimethyl-4-p-tolylamino-3,4-dihydro-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8.71E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Effective concentration for ecdysone-dependent transactivation in mammalian cell line expressing Aedes aegypti ecdysone receptor | Bioorg Med Chem Lett 13: 1943-6 (2003) BindingDB Entry DOI: 10.7270/Q2NV9HN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Choristoneura fumiferana) | BDBM50128297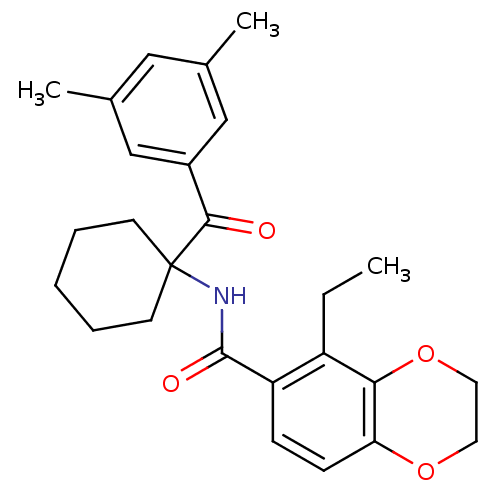 (5-Ethyl-2,3-dihydro-benzo[1,4]dioxine-6-carboxylic...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Choristoneura fumiferana (CfEcR) and a luciferase reporter gene in CHO cells | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Choristoneura fumiferana) | BDBM50128298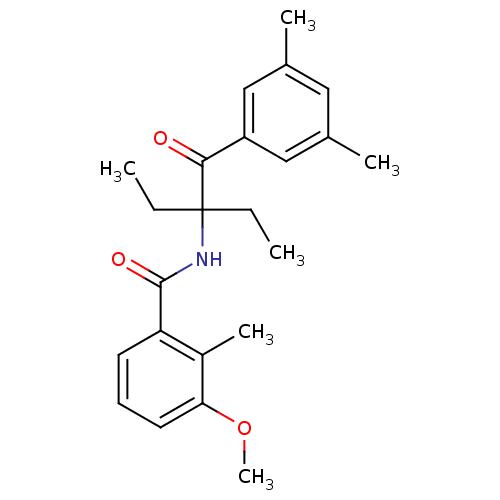 (CHEMBL299671 | N-[1-(3,5-Dimethyl-benzoyl)-1-ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.23E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Choristoneura fumiferana (CfEcR) and a luciferase reporter gene in CHO cells | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50128299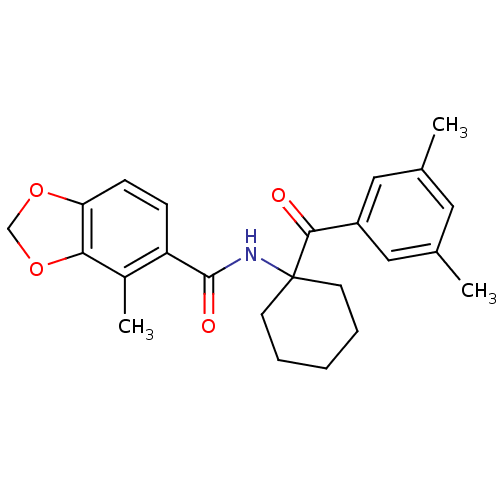 (4-Methyl-benzo[1,3]dioxole-5-carboxylic acid [1-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Bombyx mori (BmEcR) and a beta-galactosidase reporter gene in HEK-293 cell-l... | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50128300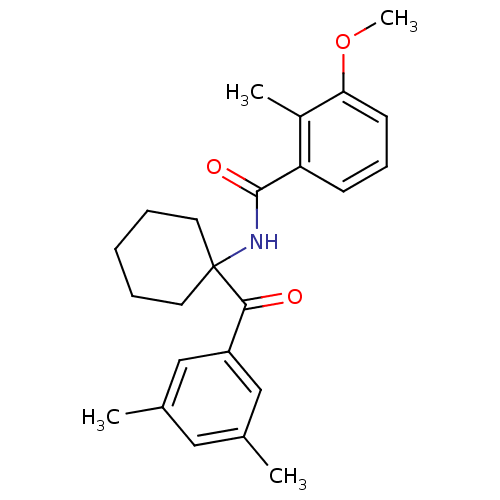 (CHEMBL55869 | N-[1-(3,5-Dimethyl-benzoyl)-cyclohex...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Bombyx mori (BmEcR) and a beta-galactosidase reporter gene in HEK-293 cell-l... | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Choristoneura fumiferana) | BDBM50128301 (CHEMBL54575 | N-[1-(3,5-Dimethyl-benzoyl)-cyclobut...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Choristoneura fumiferana (CfEcR) and a luciferase reporter gene in CHO cells | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50128301 (CHEMBL54575 | N-[1-(3,5-Dimethyl-benzoyl)-cyclobut...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8.52E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Bombyx mori (BmEcR) and a beta-galactosidase reporter gene in HEK-293 cell-l... | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50128302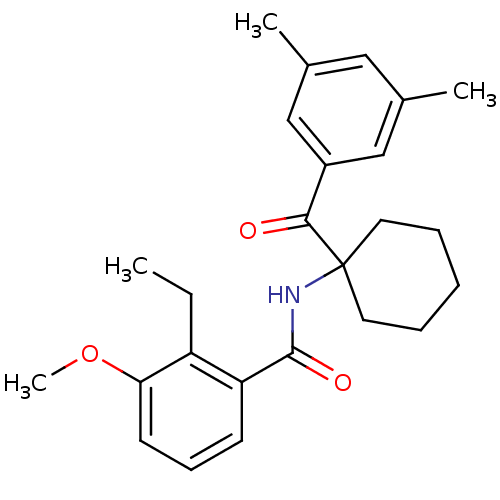 (CHEMBL59044 | N-[1-(3,5-Dimethyl-benzoyl)-cyclohex...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Bombyx mori (BmEcR) and a beta-galactosidase reporter gene in HEK-293 cell-l... | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Choristoneura fumiferana) | BDBM50128303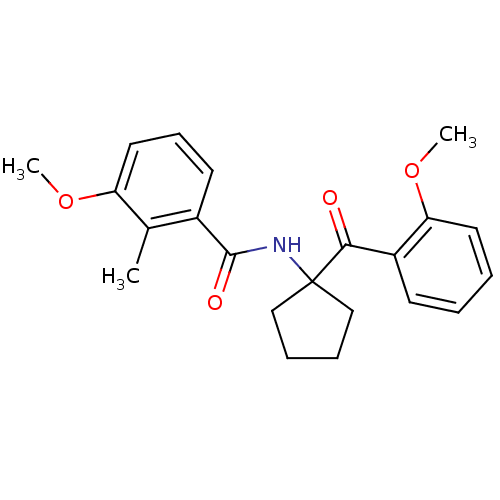 (3-Methoxy-N-[1-(2-methoxy-benzoyl)-cyclopentyl]-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.61E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Choristoneura fumiferana (CfEcR) and a luciferase reporter gene in CHO cells | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Choristoneura fumiferana) | BDBM50128304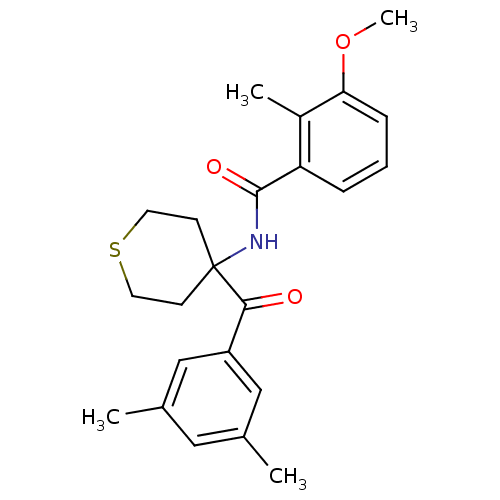 (CHEMBL52252 | N-[4-(3,5-Dimethyl-benzoyl)-tetrahyd...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Choristoneura fumiferana (CfEcR) and a luciferase reporter gene in CHO cells | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Choristoneura fumiferana) | BDBM50128305 (3-methoxy-2-methylbenzoic acid 2-(3,5-dimethylbenz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Choristoneura fumiferana (CfEcR) and a luciferase reporter gene in CHO cells | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50128305 (3-methoxy-2-methylbenzoic acid 2-(3,5-dimethylbenz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Bombyx mori (BmEcR) and a beta-galactosidase reporter gene in HEK-293 cell-l... | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50128306 (3-Methoxy-N-[1-(2-methoxy-benzoyl)-cycloheptyl]-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Bombyx mori (BmEcR) and a beta-galactosidase reporter gene in HEK-293 cell-l... | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50128297 (5-Ethyl-2,3-dihydro-benzo[1,4]dioxine-6-carboxylic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 740 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Bombyx mori (BmEcR) and a beta-galactosidase reporter gene in HEK-293 cell-l... | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Choristoneura fumiferana) | BDBM50128302 (CHEMBL59044 | N-[1-(3,5-Dimethyl-benzoyl)-cyclohex...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Choristoneura fumiferana (CfEcR) and a luciferase reporter gene in CHO cells | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50128307 (CHEMBL56411 | N-[4-(3,5-Dimethyl-benzoyl)-tetrahyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Bombyx mori (BmEcR) and a beta-galactosidase reporter gene in HEK-293 cell-l... | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ecdysone receptor (Bombyx mori) | BDBM50128298 (CHEMBL299671 | N-[1-(3,5-Dimethyl-benzoyl)-1-ethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a |
RHeoGene Curated by ChEMBL | Assay Description Dose affording 50% of maximum transactivation for ecdysone receptor from Bombyx mori (BmEcR) and a beta-galactosidase reporter gene in HEK-293 cell-l... | Bioorg Med Chem Lett 13: 1883-6 (2003) BindingDB Entry DOI: 10.7270/Q2TM79HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 71 total ) | Next | Last >> |