

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucagon receptor (Homo sapiens (Human)) | BDBM50122102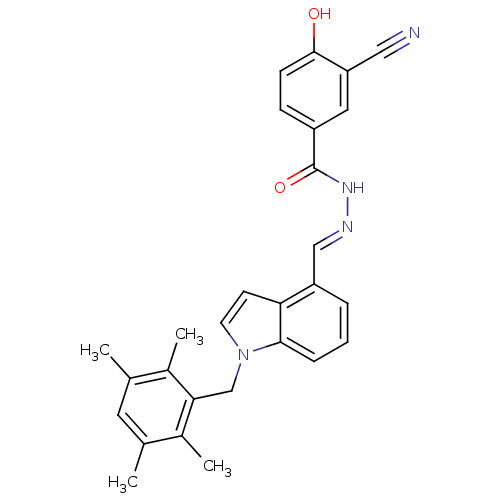 (3-Cyano-4-hydroxy-benzoic acid [1-(2,3,5,6-tetrame...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Antagonist activity against human glucagon receptor | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057796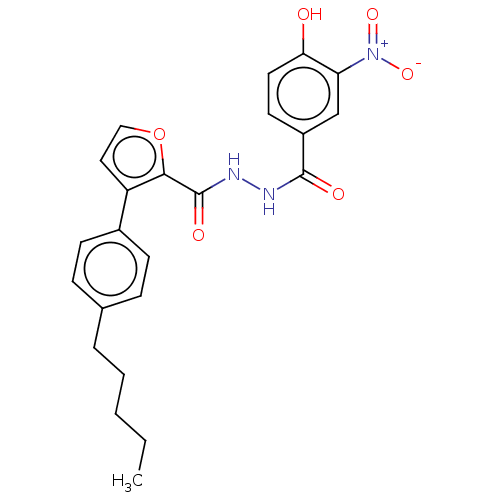 (CHEMBL3326188) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Inhibition of glucagon-induced glucagon receptor-mediated cAMP production in rat hepatocytes after 15 mins by cAMP dynamic2 assay | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116666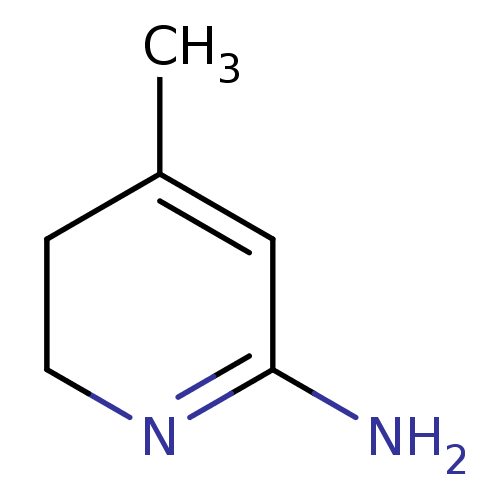 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116674 (6-Allyl-4-methyl-5,6-dihydro-1H-pyridin-(2Z)-ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116674 (6-Allyl-4-methyl-5,6-dihydro-1H-pyridin-(2Z)-ylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116668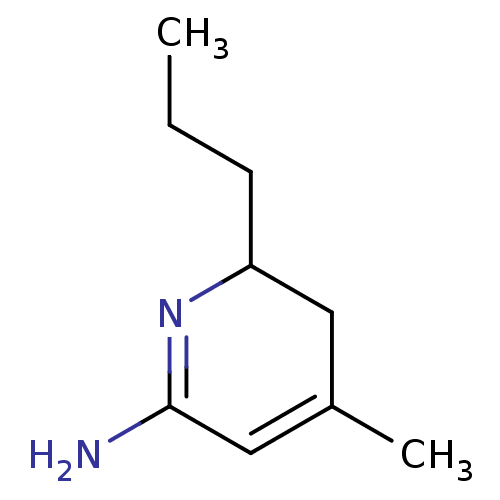 (4-Methyl-6-propyl-5,6-dihydro-1H-pyridin-(2Z)-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057824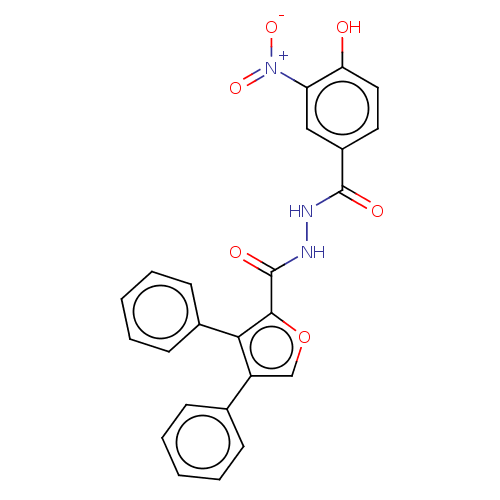 (CHEMBL3326175) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116668 (4-Methyl-6-propyl-5,6-dihydro-1H-pyridin-(2Z)-ylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116667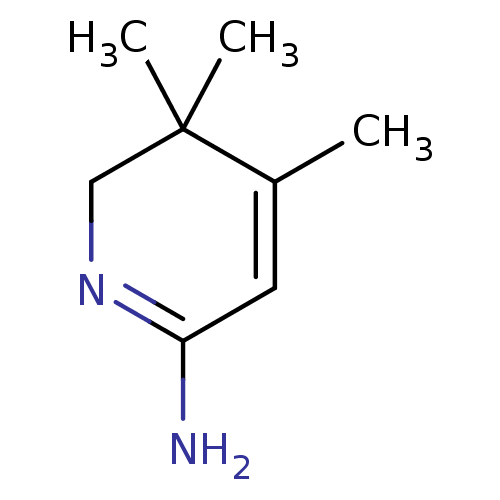 (4,5,5-Trimethyl-5,6-dihydro-1H-pyridin-(2Z)-yliden...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057820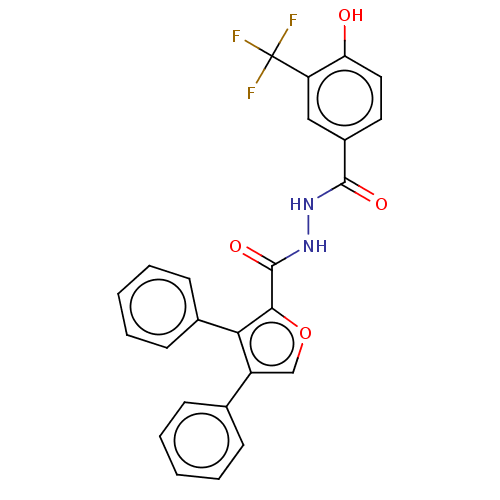 (CHEMBL3326174) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116670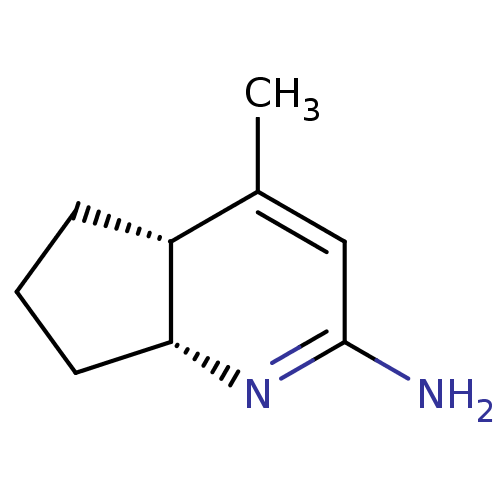 ((4aR,7aR)-4-Methyl-1,4a,5,6,7,7a-hexahydro-[1]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116676 (4,6-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116676 (4,6-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057813 (CHEMBL3326173) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50057796 (CHEMBL3326188) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 292 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Inhibition of glucagon-induced glucagon receptor-mediated cAMP production in human hepatocytes after 15 mins by cAMP dynamic2 assay | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116673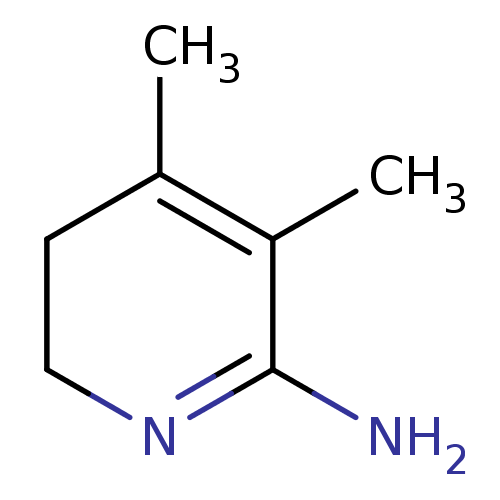 (3,4-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116673 (3,4-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116674 (6-Allyl-4-methyl-5,6-dihydro-1H-pyridin-(2Z)-ylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116667 (4,5,5-Trimethyl-5,6-dihydro-1H-pyridin-(2Z)-yliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057806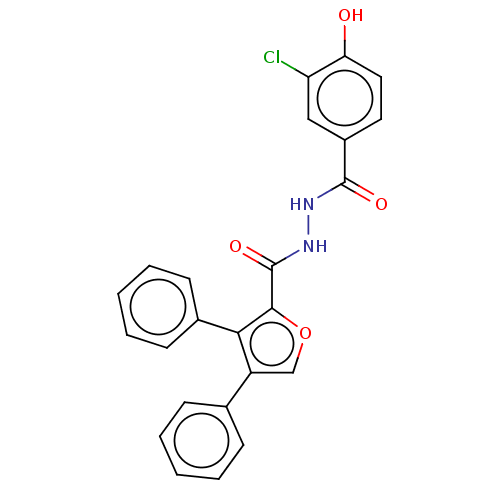 (CHEMBL3326172) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116670 ((4aR,7aR)-4-Methyl-1,4a,5,6,7,7a-hexahydro-[1]pyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116675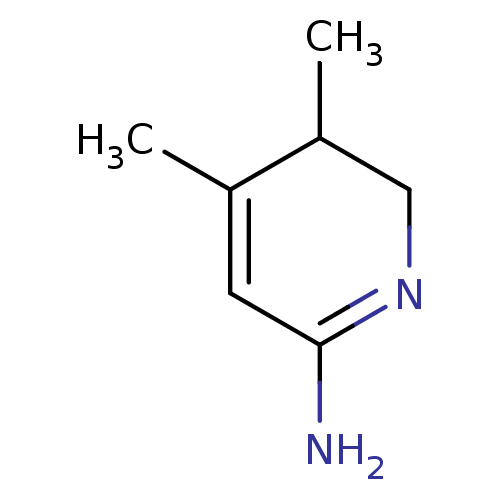 (4,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116675 (4,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116668 (4-Methyl-6-propyl-5,6-dihydro-1H-pyridin-(2Z)-ylid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057798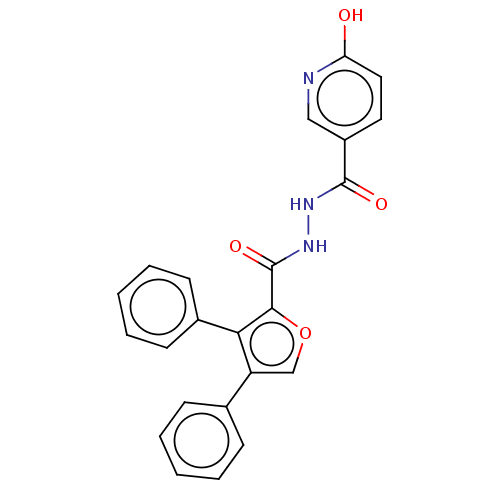 (CHEMBL3326170) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50057796 (CHEMBL3326188) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Inhibition of GLP-1R (unknown origin) | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50057796 (CHEMBL3326188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human ERG | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50237936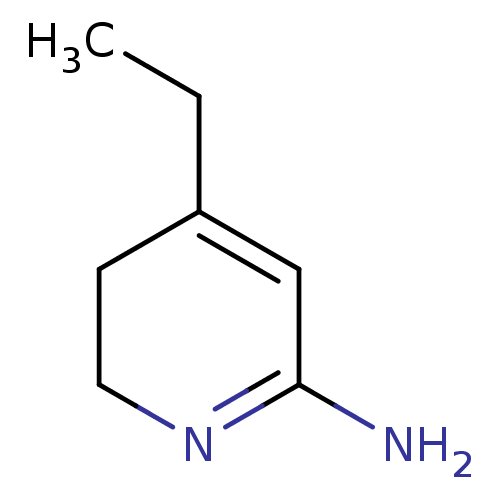 (4-Ethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneamine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116677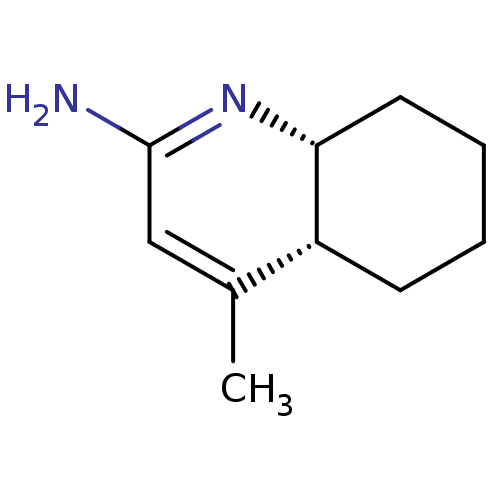 ((4aR,8aR)-4-Methyl-4a,5,6,7,8,8a-hexahydro-1H-quin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057800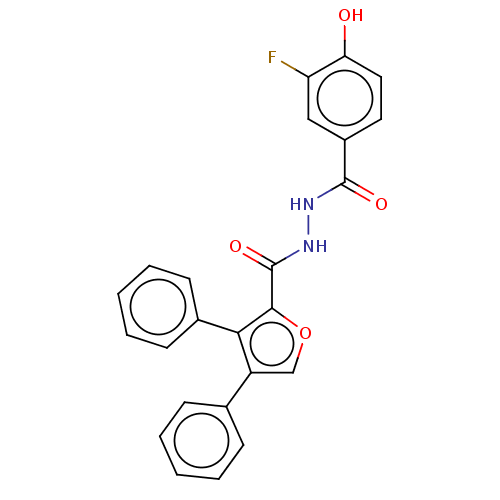 (CHEMBL3326171) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50237936 (4-Ethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneamine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116667 (4,5,5-Trimethyl-5,6-dihydro-1H-pyridin-(2Z)-yliden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116669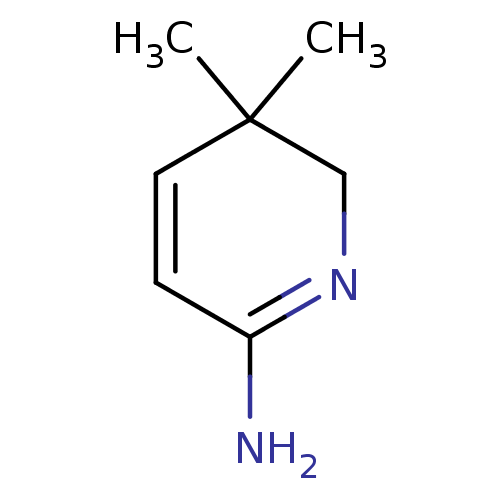 (5,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057864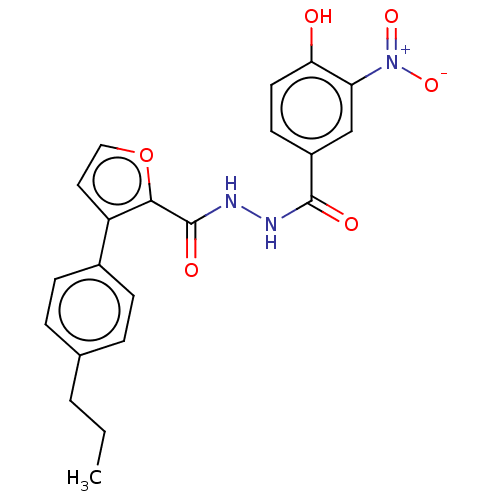 (CHEMBL3325456) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057817 (CHEMBL3326187) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116677 ((4aR,8aR)-4-Methyl-4a,5,6,7,8,8a-hexahydro-1H-quin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057797 (CHEMBL3326165) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057825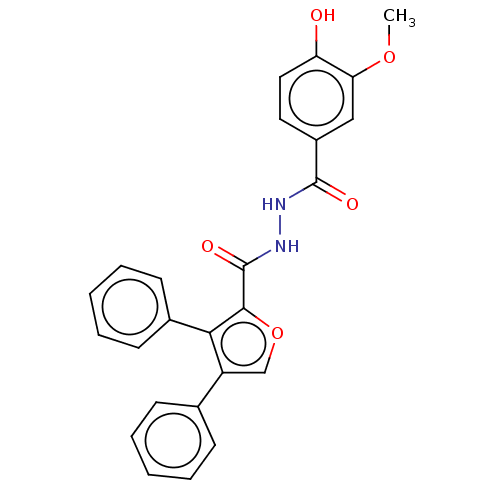 (CHEMBL3326176) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057796 (CHEMBL3326188) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057831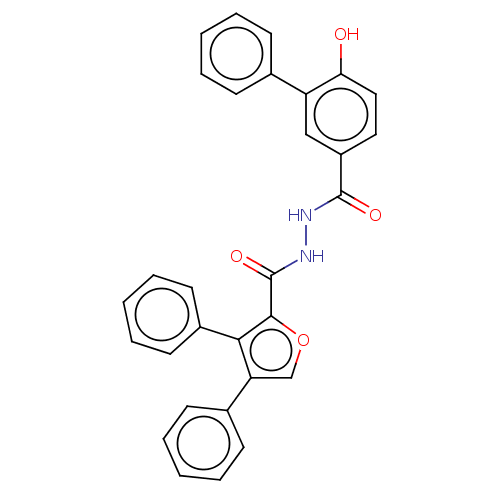 (CHEMBL3326177) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50058003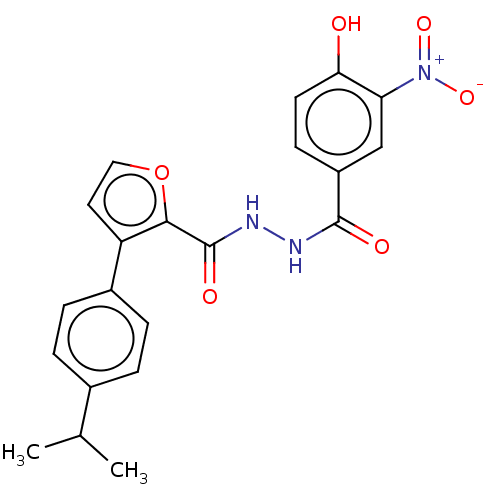 (CHEMBL3326186) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057859 (CHEMBL3326181) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116669 (5,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057863 (CHEMBL3326185) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057861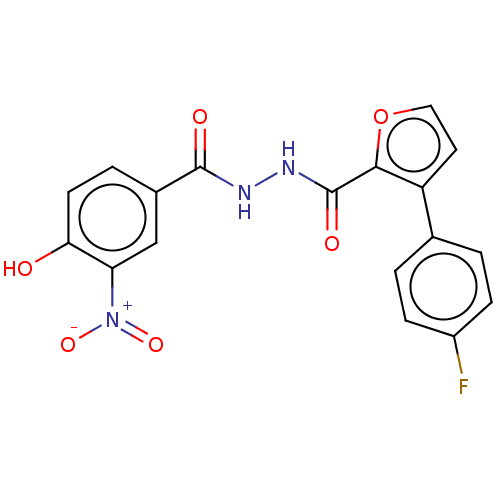 (CHEMBL3326183) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Rattus norvegicus) | BDBM50057834 (CHEMBL3326178) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma. Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]glucagon from glucagon receptor in rat hepatocyte membranes after 30 mins by gamma-counting | Bioorg Med Chem Lett 24: 4266-70 (2014) Article DOI: 10.1016/j.bmcl.2014.07.025 BindingDB Entry DOI: 10.7270/Q2ZW1NK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |