

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium channel protein type 1/2/3 subunit alpha (Rattus norvegicus) | BDBM50082319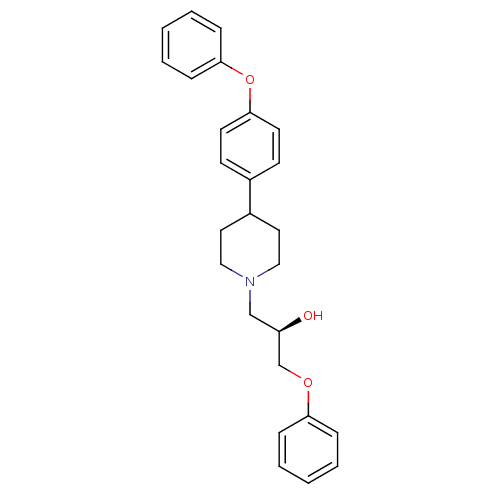 ((R)-1-Phenoxy-3-[4-(4-phenoxy-phenyl)-piperidin-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of veratridine-induced depolarization in rat cerebrocortical synaptosomes using the voltage sensitive fluorescent dye Rhodamine 6G | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 1/2/3 subunit alpha (Rattus norvegicus) | BDBM50082324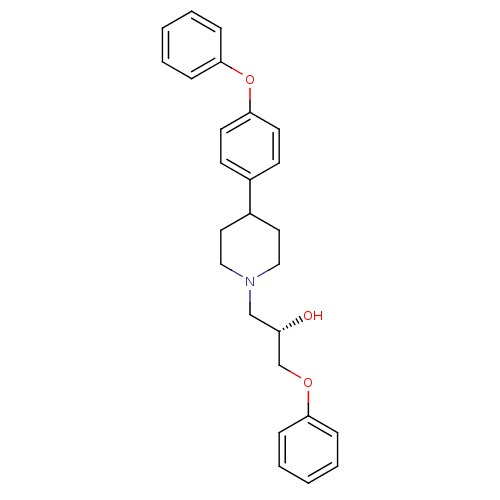 ((S)-1-Phenoxy-3-[4-(4-phenoxy-phenyl)-piperidin-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of veratridine-induced depolarization in rat cerebrocortical synaptosomes using the voltage sensitive fluorescent dye Rhodamine 6G | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 1/2/3 subunit alpha (Rattus norvegicus) | BDBM50082323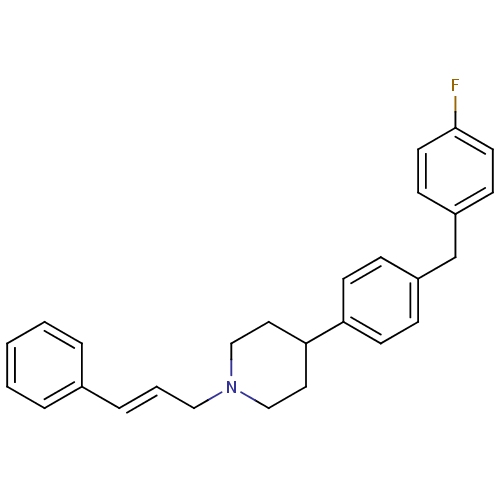 (4-[4-(4-Fluoro-benzyl)-phenyl]-1-((E)-3-phenyl-all...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of veratridine-induced depolarization in rat cerebrocortical synaptosomes using the voltage sensitive fluorescent dye Rhodamine 6G | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 1/2/3 subunit alpha (Rattus norvegicus) | BDBM50082319 ((R)-1-Phenoxy-3-[4-(4-phenoxy-phenyl)-piperidin-1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of veratridine-induced depolarization in rat cerebrocortical synaptosomes using the voltage sensitive fluorescent dye Rhodamine 6G | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017702 (1-(bis(4-fluorophenyl)methyl)-4-cinnamylpiperazine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 228 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to Dopamine receptor D2 of rat striatum membranes | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 1/2/3 subunit alpha (Rattus norvegicus) | BDBM50017702 (1-(bis(4-fluorophenyl)methyl)-4-cinnamylpiperazine...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of veratridine-induced depolarization in rat cerebrocortical synaptosomes using the voltage sensitive fluorescent dye Rhodamine 6G | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 1/2/3 subunit alpha (Rattus norvegicus) | BDBM50082320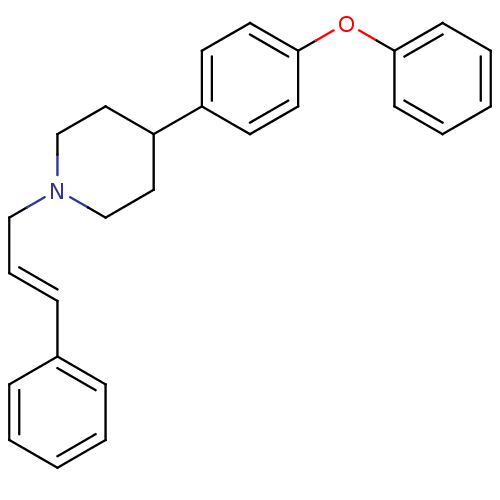 (4-(4-Phenoxy-phenyl)-1-((E)-3-phenyl-allyl)-piperi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of veratridine-induced depolarization in rat cerebrocortical synaptosomes using the voltage sensitive fluorescent dye Rhodamine 6G | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 1/2/3 subunit alpha (Rattus norvegicus) | BDBM50082322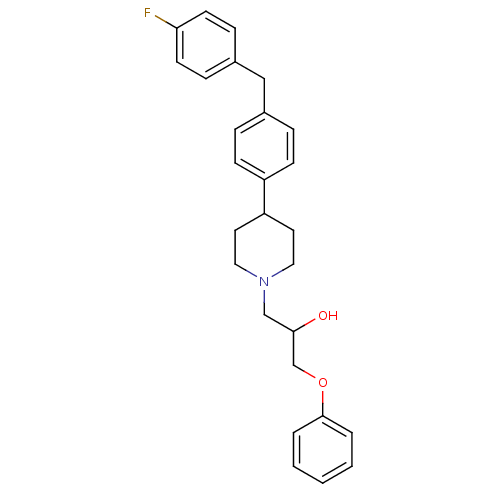 (1-{4-[4-(4-Fluoro-benzyl)-phenyl]-piperidin-1-yl}-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of veratridine-induced depolarization in rat cerebrocortical synaptosomes using the voltage sensitive fluorescent dye Rhodamine 6G | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50082320 (4-(4-Phenoxy-phenyl)-1-((E)-3-phenyl-allyl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to Dopamine receptor D2 of rat striatum membranes | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50263669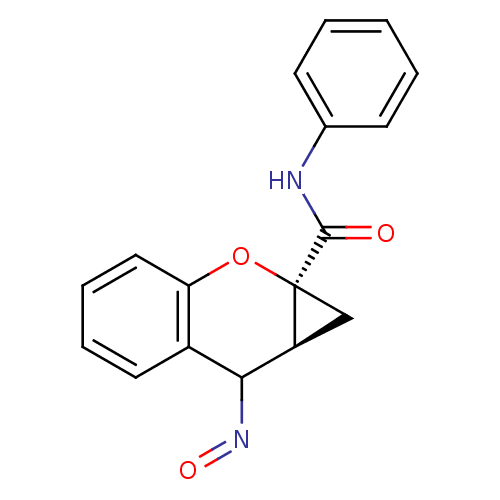 ((1aS,7aS)-7-(hydroxyimino)-N-phenyl-1,1a,7,7a-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of glutamate-evoked (10 uM) [Ca2+] mobilization in mGluR1-alpha expressed-CHO cells. | Bioorg Med Chem Lett 6: 763-766 (1996) Article DOI: 10.1016/0960-894X(96)00104-7 BindingDB Entry DOI: 10.7270/Q2WS8T7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50082323 (4-[4-(4-Fluoro-benzyl)-phenyl]-1-((E)-3-phenyl-all...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to Dopamine receptor D2 of rat striatum membranes | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50082319 ((R)-1-Phenoxy-3-[4-(4-phenoxy-phenyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to Dopamine receptor D2 of rat striatum membranes | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50082324 ((S)-1-Phenoxy-3-[4-(4-phenoxy-phenyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to Dopamine receptor D2 of rat striatum membranes | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50082319 ((R)-1-Phenoxy-3-[4-(4-phenoxy-phenyl)-piperidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to Dopamine receptor D2 of rat striatum membranes | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50289106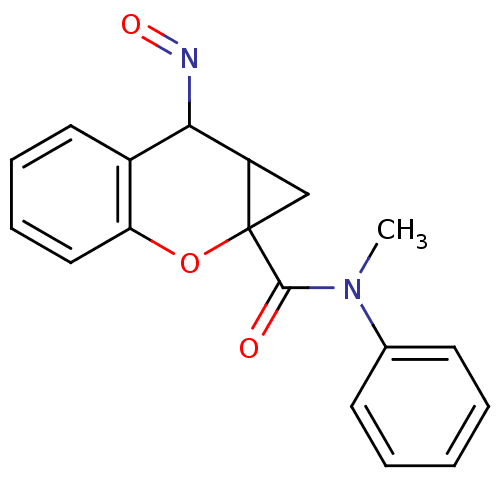 (2-[(E)-Hydroxyimino]-1a,2-dihydro-1H-7-oxa-cyclopr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of glutamate-evoked (10 uM) [Ca2+] mobilization in mGluR1-alpha expressed-CHO cells. | Bioorg Med Chem Lett 6: 763-766 (1996) Article DOI: 10.1016/0960-894X(96)00104-7 BindingDB Entry DOI: 10.7270/Q2WS8T7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50082322 (1-{4-[4-(4-Fluoro-benzyl)-phenyl]-piperidin-1-yl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to Dopamine receptor D2 of rat striatum membranes | Bioorg Med Chem Lett 9: 2999-3002 (1999) BindingDB Entry DOI: 10.7270/Q2D21Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM86213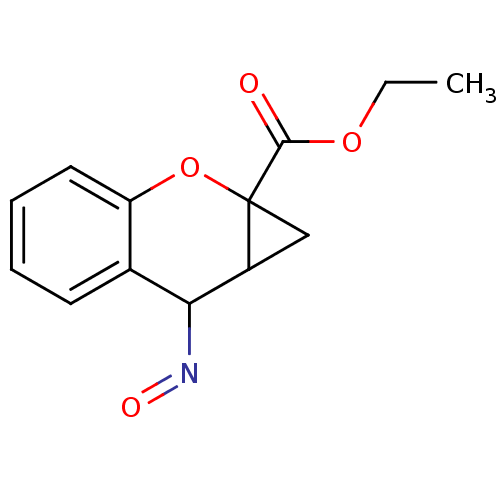 (CAS_5126051 | CHEMBL327783 | CPCCOEt | NSC_5126051) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of glutamate-evoked (10 uM) [Ca2+] mobilization in mGluR1-alpha expressed-CHO cells. | Bioorg Med Chem Lett 6: 763-766 (1996) Article DOI: 10.1016/0960-894X(96)00104-7 BindingDB Entry DOI: 10.7270/Q2WS8T7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50030628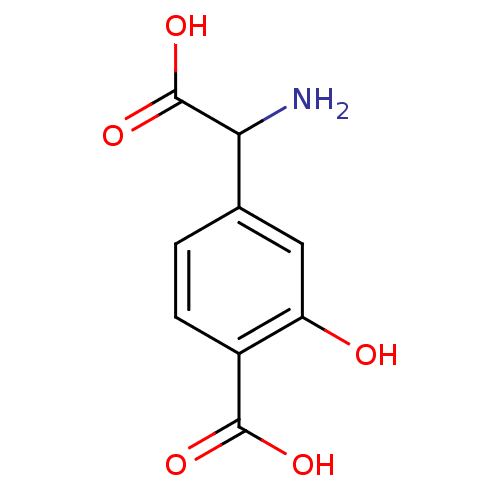 ((S)-4C3HPG | 4-(Amino-carboxy-methyl)-2-hydroxy-be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of glutamate-evoked (10 uM) [Ca2+] mobilization in mGluR1-alpha expressed-CHO cells. | Bioorg Med Chem Lett 6: 763-766 (1996) Article DOI: 10.1016/0960-894X(96)00104-7 BindingDB Entry DOI: 10.7270/Q2WS8T7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50030627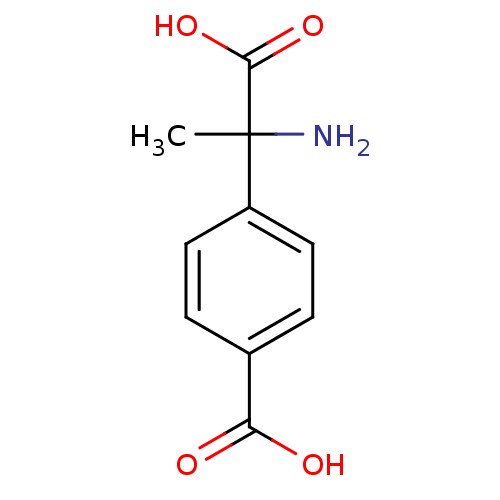 ((+-)-MCPG | (R,S)-alpha-Methyl-4-carboxyphenylglyc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of glutamate-evoked (10 uM) [Ca2+] mobilization in mGluR1-alpha expressed-CHO cells. | Bioorg Med Chem Lett 6: 763-766 (1996) Article DOI: 10.1016/0960-894X(96)00104-7 BindingDB Entry DOI: 10.7270/Q2WS8T7Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||